The presence of a systemic autoimmune rheumatic disease (ARD) is a well-known risk factor for interstitial lung disease (ILD). For example, 33% of adults with rheumatoid arthritis (RA) have subclinical ILD.[1] Higher serum levels of IgM rheumatoid factor (RF), IgA RF, and anti-cyclic citrullinated peptide antibody 2 are associated with subclinical ILD in community-dwelling adults.[2] It is unknown whether this relationship between autoimmunity and subclinical ILD is limited to RA-related autoantibodies, or extends more broadly to other epitopes. High attenuation areas (HAA) and interstitial lung abnormalities (ILA) are validated quantitative and qualitative subclinical ILD phenotypes, respectively. In community-dwelling adults, greater HAA is associated with reduced forced vital capacity, reduced exercise capacity, elevated serum levels of matrix metalloproteinase-7 and interleukin-6, higher prevalence of ILA on computed tomography (CT) scans of the chest, higher all-cause mortality rate, and an increased risk of developing clinically evident ILD and ILD-specific mortality at 12-year follow-up.[3, 4] ILA has been associated with all-cause mortality in 4 different longitudinal cohorts.[5] The purpose of this study was to examine the association between antinuclear antibody (ANA) and both HAA and ILA in community-dwelling adults enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA).
MESA is a population-based cohort study of 6,814 adults aged 45–84 when they were enrolled from 2000–2002 without regard to lung disease or ARD.[6] Cardiac CT scans were performed in 6,812 participants at Exam 1 (2000–2002)[7] and full lung CT scans in 2,907 participants at Exam 5 (2010–2012). Measurement of HAA, defined as the percentage of lung volume with attenuation values between −600 and −250 Hounsfield Units, and ILA, defined as ground glass abnormalities, reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, and traction bronchiectasis affecting >5% of a lung zone in a non-dependent manner,[8] has been previously described.[3] HAA was quantified on the 6,812 Exam 1 cardiac CT scans. Each of the 2,907 Exam 5 full lung CT scans was visually inspected by one expert radiologist for the presence or absence of ILA. ANA was measured in frozen Exam 1 sera from 6,626 participants using indirect immunofluorescence with HEp-2 cell substrate at TheraTest Labs (TheraTest Labs Inc, Lombard, IL, USA).[9] Intra-assay coefficient of variation was <10%.[9] ANA level was expressed in Units. An ANA value >10 Units was defined as positive. We examined the linearity of the associations between ANA and both HAA and ILA using generalized additive models with loess smoothing functions. We used multiple linear regression to examine associations between natural log-transformed ANA and natural log-transformed HAA, controlling for age, sex, race/ethnicity, BMI, height, waist circumference, pack-years of smoking, current smoking status, estimated glomerular filtration rate, study site, education, total imaged lung volume, percent emphysema, and tube current. To ease interpretation of our beta coefficients of natural log transformed ANA, we have presented base 2 exponentiated beta coefficients, which are the percent differences in HAA per doubling of ANA. We estimated prevalence ratios (PR) for the associations between log2-transformed ANA and ILA using Poisson regression with robust standard error estimation, controlling for age, sex, race/ethnicity, pack-years of smoking, and current smoking status. We performed analyses stratified by age, sex, race/ethnicity, smoking status, and BMI. We used likelihood ratio tests to test for effect modification, and multiple imputation by chained equations to account for missing covariate data.[10] Only 0.4% of participants had any missing data. Analyses were performed in STATA, version 15.1 (College Station, TX) and R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
The baseline characteristics of the MESA cohort have been previously published.[3] Of the 2,430 participants with non-equivocal measurements of ILA at Exam 5, ANA was measured in 2,366 at Exam 1. Thus, 6,626 and 2,366 participants were included in the HAA and ILA analyses, respectively. Mean age at Exam 1 was 62±10 years; 53% (3,516/6,626) were female. Of the 6,626 participants included in the HAA analyses, 39% identified themselves as White, 27% as African American, 12% as Chinese American, and 22% as Hispanic. Forty-one percent (2,688/6,623) were former smokers; 14% (927/6,623) were current smokers. Eleven percent (741/6,626) were ANA positive. Median ANA was 4 Units (IQR 3–7). Median HAA was 5.62% (IQR 4.55–7.19%). ILA prevalence was 12.4% (293/2,366).
In an unadjusted model, HAA at Exam 1 increased by 3.50% (95% CI 2.25 to 4.77%, p-value<0.001) per doubling of ANA at Exam 1. In a fully adjusted model, HAA increased by 1.83% (95% CI 1.12 to 2.55%, p-value<0.001) per doubling of ANA (Panel A). In a fully adjusted model, the p-value for the interaction between ANA and race/ethnicity was 0.04. In fully adjusted models, HAA increased by 2.82% (95% CI 1.49 to 4.16%, p-value<0.001) among African Americans, 2.93% (95% CI 0.85 to 5.06%, p-value=0.006) among Chinese Americans, and 2.14% (95% CI 0.65 to 3.66%, p-value=0.005) among Hispanics per doubling of ANA. There was no statistically significant association between ANA and HAA among Whites (0.65% increase per doubling of ANA, 95% CI −0.46 to 1.76%, p-value=0.25). There was no statistically significant interaction between ANA and age, sex, smoking status, or BMI (p-value for interaction>0.50 for each).
Panel A:
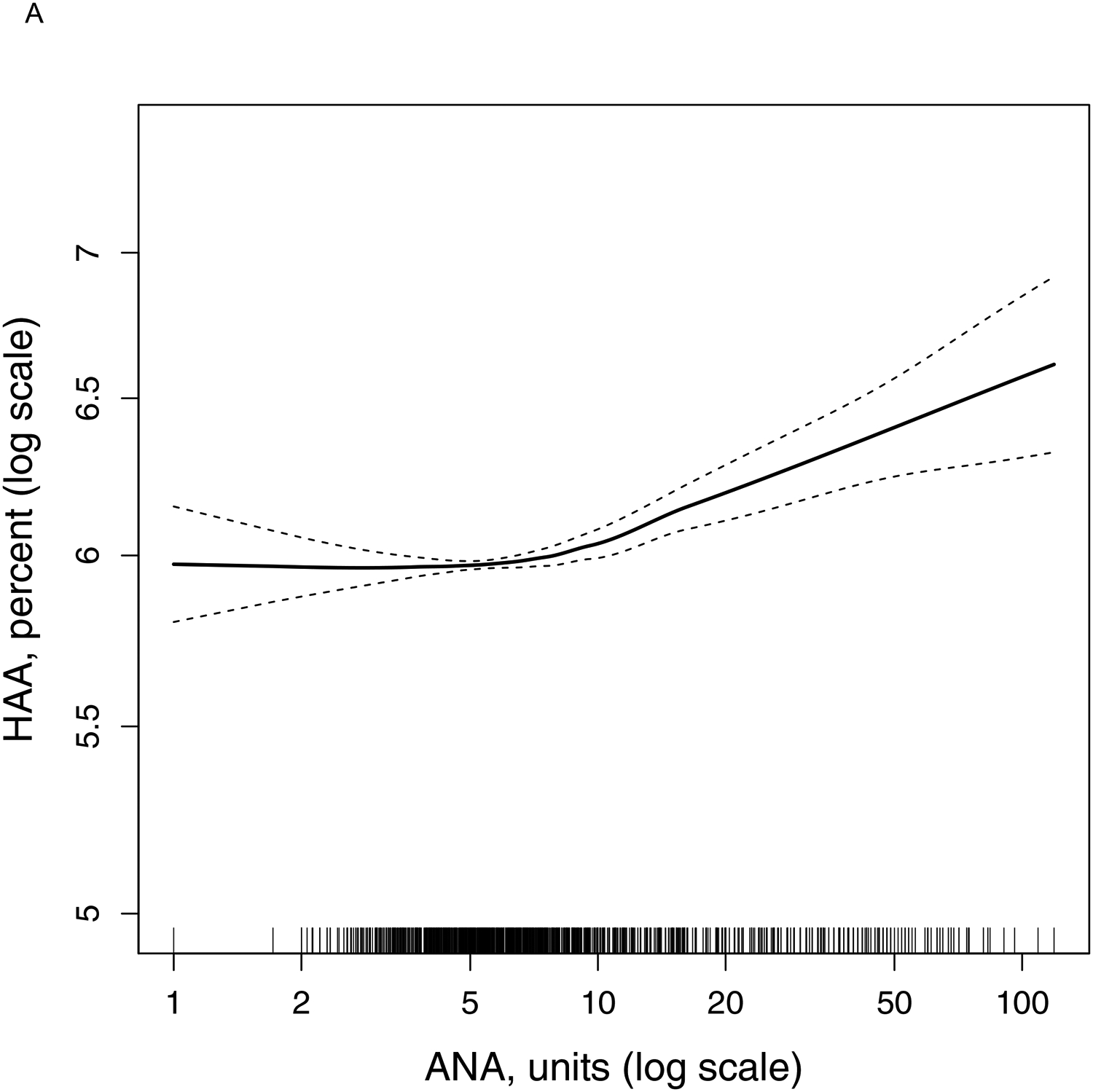
Continuous relationship of antinuclear antibody at Exam 1 with predicted (adjusted) percent high attenuation areas at Exam 1 (N = 6,626). Smoothed regression line (solid black line) is adjusted for age, sex, race/ethnicity, body mass index, height, waist circumference, pack-years of smoking, current smoking status, estimated glomerular filtration rate, study site, education, total imaged lung volume, percent emphysema, and tube current. Thin dashed lines are the 95% confidence bands. Each vertical tick mark on the rug plot along the internal border of the x-axis represents one study participant. Overall p-value for association < 0.001, p-value for non-linearity = 0.002.
In an unadjusted model, the prevalence of ILA at Exam 5 increased by 17% per doubling of ANA at Exam 1 (PR 1.17, 95% CI 1.04–1.33, p-value=0.01). However, in a fully adjusted model, there was no statistically significant association between ANA and ILA prevalence (PR 1.07, 95% CI 0.94–1.22, p-value=0.29). In a fully adjusted model, the p-value for the interaction between ANA and age was 0.003. In a fully adjusted model, ILA increased by 33% per doubling of ANA (PR 1.33, 95% CI 1.09–1.63, p-value=0.006) among younger participants (age 45–59 at Exam 1; Panel B). There was no statistically significant association between ANA and ILA prevalence among older participants (age 60–84 at Exam 1) (PR 0.97, 95% CI 0.83–1.13, p-value=0.68). There was no statistically significant interaction between ANA and sex, race/ethnicity, or smoking status (p-value for interaction>0.10 for each).
Panel B:
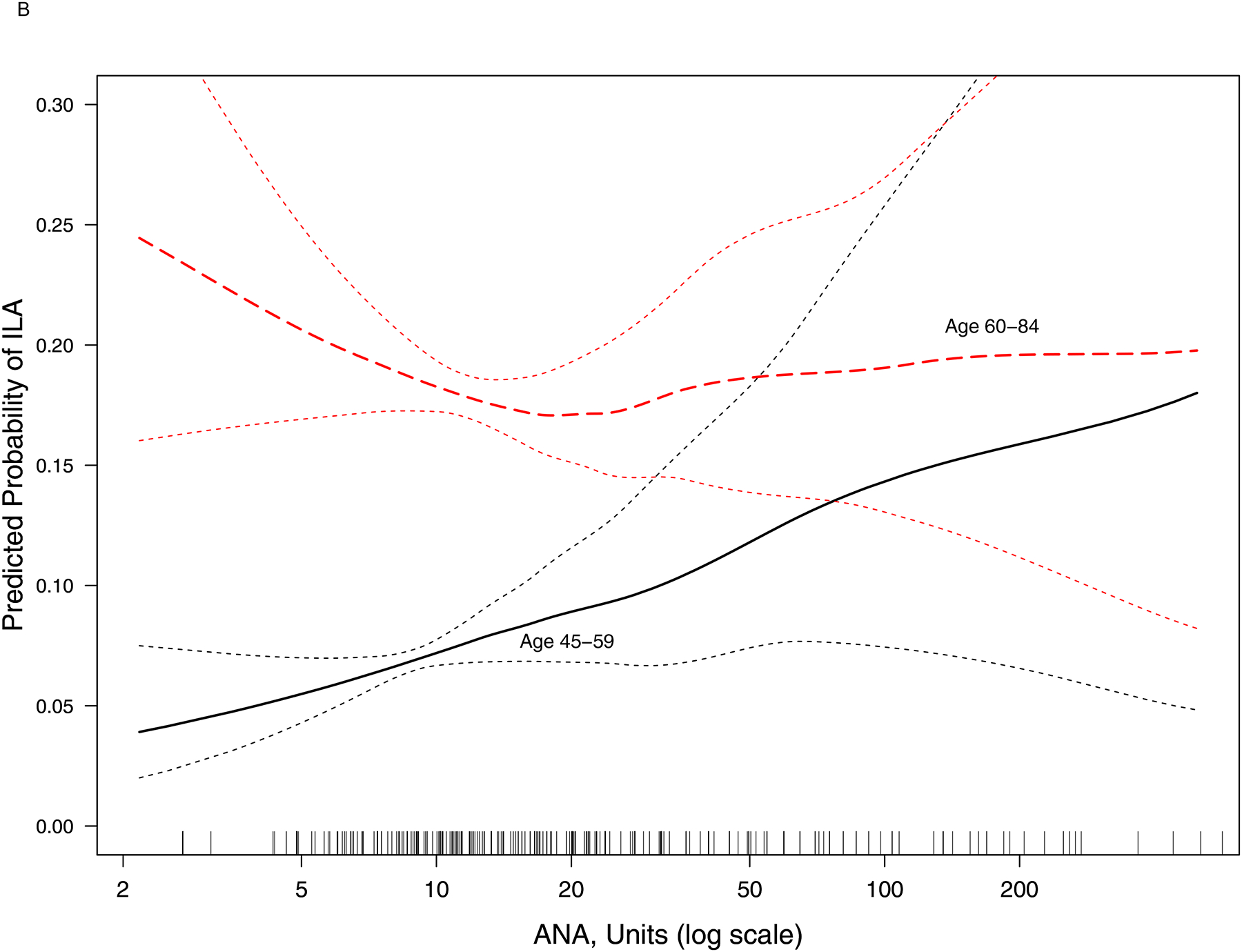
Continuous relationship of antinuclear antibody at Exam 1 with the predicted probability of interstitial lung abnormalities at Exam 5 (N = 2,366). Smoothed regression lines (black solid line: age 45–59 years at Exam 1; red dashed line: age 60–84 years at Exam 1) are adjusted for age, sex, race/ethnicity, pack-years of smoking, and current smoking status. Thin dashed lines are the 95% confidence bands. Each vertical tick mark on the rug plot along the internal border of the x-axis represents one study participant. In participants aged 45–59 (n = 1,256), overall p-value for association = 0.009, p-value for non-linearity = 0.58. In participants aged 60–84 (n = 1,110), overall p-value for association = 0.68, p-value for non-linearity = 0.12.
In this study, we demonstrated a positive association between levels of ANA, a marker of autoimmunity, and HAA, a quantitative CT biomarker of subclinical ILD. The association between ANA and HAA was strongest among non-White participants and the association between ANA and ILA was stronger among younger participants. Our results indicate that the relationship between autoimmunity and subclinical ILD is not limited to RA-related autoantibodies, but includes other epitopes as well.
It is well-established that autoantibodies precede the development of clinical manifestations of ARDs by several years.[11, 12] ANA was detected in the serum of 77% (89/115) of military personnel with systemic lupus erythematosus (SLE)[11] and in the serum of 44% (23/44) of Swedish patients with Sjogren’s Syndrome prior to symptom onset.[12] Our findings suggest that higher serum ANA levels may also be a risk factor for subclinical ILD.
It is perhaps not surprising that race/ethnicity and age modified the effect of ANA on HAA and ILA, respectively. The prevalence of ANA is modestly higher among African Americans than among non-Hispanic Whites in the US,[13] and the prevalence of certain ARDs is higher in non-White populations. For example, African Americans, Asian Americans, and Hispanic Americans have a higher prevalence of SLE than do Whites in the US.[14] Moreover, non-White populations tend to have more severe manifestations of ARDs. Among patients with systemic sclerosis, African Americans have a higher prevalence of ILD and more severe ILD than do Whites.[15] Although the prevalence of ANA increases with age,[13] ARDs such as SLE often present at younger ages.[14]
Our study has some limitations. It was cross-sectional, which limits our ability to make causal inferences. Because it was observational, our results may be confounded by unmeasured or poorly measured potential confounders. However, data in MESA were measured with great precision, and we controlled for potential confounders using multivariate modeling approaches to minimize residual confounding.
In summary, our findings provide additional support for the relationship between autoimmunity and subclinical ILD. They suggest autoimmunity may play a role in the pathogenesis of subclinical ILD, even among individuals without an established ARD. Future studies should characterize the specific autoantibody profiles associated with subclinical ILD.
Acknowledgements:
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dr. Bernstein’s work was supported by K23-AR-075112.
Footnotes
Publisher's Disclaimer: “This is an author-submitted, peer-reviewed version of a manuscript that has been accepted for publication in the European Respiratory Journal, prior to copy-editing, formatting and typesetting. This version of the manuscript may not be duplicated or reproduced without prior permission from the copyright owner, the European Respiratory Society. The publisher is not responsible or liable for any errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final, copy-edited, published article, which is the version of record, is available without a subscription 18 months after the date of issue publication.”
References
- 1.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald SD, Travis WD, Stylianou MP, Rosas IO. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008: 168: 159–166. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein EJ, Barr RG, Austin JHM, Kawut SM, Raghu G, Sell JL, Hoffman EA, Newell JD Jr., Watts JR Jr., Nath PH, Sonavane SK, Bathon JM, Majka DS, Lederer DJ. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax 2016: 71: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, Gochuico BR, Hinckley Stukovsky K, Kaufman JD, Hrudaya Nath P, Newell JD Jr., Palmer SM, Rabinowitz D, Raghu G, Sell JL, Sieren J, Sonavane SK, Tracy RP, Watts JR, Williams K, Kawut SM, Lederer DJ. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 2016: 48: 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, Stukovsky KH, RoyChoudhury A, Michos ED, Raghu G, Kawut SM, Lederer DJ. High-Attenuation Areas on Chest Computed Tomography and Clinical Respiratory Outcomes in Community-Dwelling Adults. Am J Respir Crit Care Med 2017: 196: 1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O’Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O’Connor GT, Washko GR, Rosas IO, Hunninghake GM, Evaluation of CLtIPSEI, Investigators CO. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA 2016: 315: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002: 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, Barr RG. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol 2009: 16: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D’Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Investigators CO. Lung volumes and emphysema in smokers with interstitial lung abnormalities. New Eng J Med 2011: 364: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EL-ANAscr™: An enzyme immunoassay for the screening of human serum to detect antinuclear antibodies (ANAs). [cited 2019 April 4]; Available from: https://docs.wixstatic.com/ugd/c475d7_48e10d5103ec47138e7a182f2650fd15.pdf
- 10.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991: 10: 585–598. [DOI] [PubMed] [Google Scholar]
- 11.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. New Eng J Med 2003: 349: 1526–1533. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjogren syndrome. JAMA 2013: 310: 1854–1855. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, Crockett PW, Pauley BA, Chan JY, Ross SJ, Birnbaum LS, Zeldin DC, Miller FW. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 2012: 64: 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri M Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2002: 16: 847–858. [DOI] [PubMed] [Google Scholar]
- 15.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA Jr. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum 2012: 64: 2986–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]


