Abstract
Objective(s):
The current study aimed to assess cisplatin-mediated ovarian apoptosis in a rat model by Royal jelly (RJ).
Materials and Methods:
Thirty female adult albino rats (180-200 g) were divided into three groups (n=10): saline (0.9% NaCl, IP) was given to the control group, the cisplatin group: received (5 mg/kg/once a week IP) for 5 successive weeks, the RJ+Cis. group: received RJ (100 mg/kg/ day PO daily), and Cisplatin (5 mg/kg/once per week IP) for 5 successive weeks. At the end of the experiment, rats were sacrificed and their ovaries were isolated and used for biochemical analysis, molecular investigations and morphometric assessment as well as histological study. Moreover, blood samples were collected for determination of follicle-stimulating hormone (FSH), luteinizing hormone (LH), Estradiol, progesterone and anti-mullerian hormone (AMH).
Results:
The current study clarified that RJ given to rats prior to cisplatin significantly increased the ovarian and uterine weights, in addition to follicular count at P˂0.05 compared to rats injected only with cisplatin. Moreover, it restored normal ovarian histological structure with a concurrent reduction in FSH, and LH levels, and increased AMH and ovarian hormone concentrations at P˂0.05 compared to cisplatin group. Also, RJ decreased the ovarian antioxidant/oxidative imbalance harmonized with significant suppression of inducible nitric oxide synthase and increase of quinone oxidoreductase 1 mRNA expression at P˂0.05 compared to cisplatin group.
Conclusion:
We concluded that RJ could alleviate mitochondrial-induced ovarian apoptosis caused by cisplatin via increasing anti-apoptotic Bcl2, and diminishing pro-apoptotic Bax with a concomitant increase of Mfn2 mRNA and protein expressions.
Key Words: Bax, Bcl2, Mfn2, Mitochondrial viability, Ovaries
Introduction
The reduced oocyte reserve, ovarian complications and infertility are regular adverse events of several cancer therapeutic approaches such as irradiation and chemotherapy drugs (1, 2). Among these agents, cisplatin [CDDP, cis-diamminedichloroplatinum (II)] is increasingly used in the management of various types of cancer especially bladder, head, neck, and lung cancers (3).
Cisplatin has various adverse effects such as nephrotoxicity, ototoxicity, cardiotoxicity, and hepatotoxicity, and causes gastrointestinal and reproductive dysfunction (4, 5). Many theories explained the mechanism of cisplatin-induced tissue damage including, chemical bonding and crosslinking of DNA, which induces cell apoptosis (6, 7), induction of the cell membrane lipid peroxidation (8), disturbance of mitochondrial function (9), and protein synthesis inhibition (10). Moreover, increasing production of reactive oxygen species (ROS) and suppression of antioxidant capacity are the most detectable adverse impacts of cisplatin (11-13). These toxic effects extend also to involve the ovarian tissue, and the follicular granulosa cells, causing infertility due to acute and chronic ovarian failure (14). Exhaustion of the ovarian reserve and the resultant premature ovarian failure attributed to cisplatin-induced ovarian dysfunction may lead to disturbance in the menstrual cycle and damage of primordial follicles due to apoptosis in human females (15, 16). Biochemical variations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), Estradiol 2 (E2) and anti-mullerian hormone (AMH) levels are considered a direct indicator for ovarian damage and reduced follicular reserve following chemotherapy (17-19).
Mitochondria, the main drive-provider to the cell, play an essential role in the process of cellular apoptosis. It controls cellular metabolism, cell cycle, and signal transition (20). Many studies proved that decreased mitochondrial viability is involved in the pathogenesis of ovarian damage and apoptosis (21). Mitochondrial-induced granular cell disturbance and disruption of oocyte development could be confined to the ability of mitochondria to alter ATP production, increase the production of reactive oxygen species (ROS), and then modify granular cell function as well as the development of oocytes. This extends even to the point of inducing cell death (22).
Previous studies have clarified that Bcl-2 acts on the mitochondria. It has obvious anti-apoptotic effect attributed to its ability to modify the intracellular Ca2+ balance, which is a second messenger involved in regulating cell survival and apoptosis. The release of Ca2+ to the cytoplasm and mitochondria from endoplasmic reticulum (ER) is facilitated by inositol 1,4,5-trisphosphate receptor (IP3R) that resulted in mitochondrial Ca2+overload (23). Stary et al., reported that voltage-dependent anion channel 1 (VDAC1), an outer mitochondrial membrane protein, controls Ca2+ entry into the mitochondria (24). This Ca2+ overload opens the mitochondrial permeability transition pore (mPTP) and causes the loss of mitochondrial membrane potential (Δψm) that facilitates the mitochondrial-mediated programmed cell death (25). The anti-apoptotic mechanism of Bcl2 could be described by its ability to interact with IP3R preventing Ca2+ transfer to the cytoplasm and mitochondria (26) and also binding Bcl2 with VDAC1 to prevent the Ca2+ entry to mitochondria and thus preventing mitochondrial-induced cellular apoptosis (27). Moreover, Yuan et al. reported that Bcl2 has the ability to increase the expression of fusion mitochondrial proteins Mitofusin 2 (Mfn2) that could be another mechanism explaining the anti-apoptotic effect of Bcl2 (28). Mfn2 is a trans-membrane GTPase protein, which presents not only in the outer mitochondrial membrane but also in mitochondrial-associated membranes. This protein, beside its ability to control ER morphology, is also incorporated in mitochondrial fusion and controls the transfer of calcium from the ER to mitochondria (29-31). Furthermore, Mfn2 exerts a vital role in metabolic homeostasis, energy metabolism, mitochondrial morphology, ER stress, signal transduction (32) and mitochondrial integrity thus favors the survival of the cell (28). Nearby, it has an indispensable role in the synthesis of the blastocyst and early embryonic development (33, 34).
Royal jelly (RJ) is a white and thick jelly-like substance, which is a type of the worker bees’ hypopharyngeal and mandibular gland secretion components (35). It possesses many beneficial effects including antitumor, and antioxidant activities in addition to improvement of menopausal symptoms in different animal models (36, 37). The most elevated constituents of RJ are water (50% to 60%), proteins (18%), carbohydrates (15%), lipids (3%–6%), mineral salts (1.5%), and vitamins (38). Also, RJ contains numerous bioactive mixes including fatty acid, proteins, adenosine monophosphate (AMP), polyphenols, and hormones such as testosterone, progesterone, prolactin, and also estradiol (39). Furthermore, the antioxidant role and free radicals scavenging effect of RJ (40) could be attributed to the flavonoids, phenolic compounds (41), and free amino acids such as aspartic acid, cysteine, cystine, tyrosine, glycine, lysine, leucine, valine, and isoleucine (42). In addition, RJ possesses an immune-stimulatory effect due to the presence of 10-hydroxy-2-decenoic acid (HAD) (43).
Ibrahim et al. described that RJ exerts a protective role against cisplatin-induced kidney injuries via suppression of fibrogenic factors, α smooth muscle actin (α -SMA) and transforming growth factor β1(TGF- β1) (44). Recently, mitochondria is considered as a target of RJ components (45), particularly leucine (46), and 10-hydroxy-2-decenoic acid (47), which is the exclusive fatty acid that induces the activation of AMP-activated protein kinase (AMPK) that is considered as the most essential mediator of the mitochondrial biogenesis in many tissues like skeletal muscles (48).
For these reasons, we suggest that RJ might apply a protective role on mitochondrial-mediated ovarian apoptosis caused by cisplatin. The protective effects of RJ have been previously studied, but the suggested protective mechanism of RJ on mitochondria-induced ovarian toxicity by cisplatin has not been developed yet. Therefore, this study aimed to design a rat model for evaluation of the potential protective effects of RJ on mitochondrial-mediated ovarian apoptosis by cisplatin.
Materials and Methods
Chemicals
Cisplatin was obtained from Sigma-Aldrich Corporation (St. Louis, MO), CASE number: 15663-27-1. RJ soft capsules were purchased from Pharco Pharmaceuticals Co. (Alexandria, Egypt). All chemicals used in this experiment were of analytical grade.
Animals
In this study, 30 female adult albino rats with average weight of 180- 200 g were used. The animals were accommodated at the animal house, Faculty of Medicine and were provided with a standard pallet diet and water ad libitum and kept under conditions of adequate ventilation and temperature. Animals were divided into four groups (n=10) including:
- Control: Rats given normal saline (0.9% NaCl 1 ml IP)
- Cisplatin: Rats received cisplatin (5 mg/kg/once a week IP) for 5 successive weeks (49).
- Cis and RJ: Rats were given RJ (100 mg/kg/ day PO) (50) daily for 5 consecutive weeks half an hour prior to cisplatin (5 mg/kg/once a week IP) for 5 consecutive weeks.
All animal dealings were constructed following the strict guidelines assigned by Institutional Animal Care and Use Committee (IACUC-Beni-Suef University) at Beni-Suef University, faculty of veterinary medicine, Beni-Suef, Egypt.
Sampling
Specimen collection
At the end of the experiment, all animals were sacrificed under light anesthesia. Ovaries were isolated, weighted by using single-pan electronic balance (Leyte, Guangdong, China (Mainland)) and divided into three parts. The first one was dissected for morphometric analysis and histological study, the second part was prepared for biochemical evaluation and the third part was used for molecular assessment. In addition, the collected blood samples were subjected to centrifugation to obtain serum that was kept frozen and used for hormonal analysis (determination of serum FSH, LH, estradiol, progesterone and AMH)
Morphometric analysis of ovarian tissue and quantification study of folliculogenesis
For fixation of the ovary and uterine horn, Bouin’s fluid was used. Graded dehydration of the tissue was performed by 70 to 100% alcohol in successive steps. Xylene was used as the clearing agent. The tissues were embedded in paraffin (58.6°C). Sections of paraffin blocks were cut by a rotatory microtome (CRAFTEK, China) into 5 µm-thick paraffin sections and were processed to prepare for hematoxylin and eosin (H&E) staining (51) and were then inspected under a microscope (Sanli, China Mainland). The quantification study of folliculogenesis was assessed consistent with Patil et al. (52) with minor modifications. According to follicles diameters and morphologies, they were classified as follow:
Class I: Small pre-antral follicle (SPAF) (<94 µm);
Class II: Large pre-antral follicle (LPAF) (94–260 µm);
Class III: Small antral follicle (SAF) (261–350 µm);
Class IV: Medium antral follicle (MAF) (351–430 µm);
Class V: Large antral follicle (LAF) (431–490 µm);
Class VI: Graafian follicles (GF) (<491 µm).
For histological evaluation, method of Li et al. was used (53) with some modifications. The histological sections, stained with H&E, and were inspected for the existence of vascular congestion, hemorrhage, follicular degeneration, hyalinosis and interstitial edema. According to the obtained histological findings, changes were scored from 0 to 3, where 0 indicates no pathological changes of the ovary, while 1, 2 and 3 represent pathological changes of <33%, 33–66% and >66%, respectively. The scores for each parameter were calculated and the total scores were obtained and presented as means±SEM.
Measurement of oxidative/antioxidant parameters
For tissue homogenate preparation, 0.5 gram of ovarian tissue was homogenized in 5 ml saline (NaCl 0.9%) by using homogenizer (Ortoalresa, Spain). The homogenates were centrifuged at 1000 X g for 15 min. The supernatant was collected in Eppendorf tubes that were kept in the deep freezer (at -80 °C) for further biochemical evaluation according to the directions of the biochemical assay kits.
Biochemical assays (Colorimetric method)
The supernatant of ovarian tissue homogenates were used for measurement of reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), total antioxidant capacity (TAC) and total oxidative stress (TOS) by spectrophotometry (UV-1700; Shimadzu Corporation, Kyoto, Japan). All colorimetric kits were obtained from Biodiagnostic Company for chemicals, Egypt.
GSH concentration was measured according to the method of Beutler et al. ( 54), CAT was determined according to the method of Aebi (55), SOD was measured according to the method of Nishikimi et al. (56), and TAC was determined by the reaction of antioxidants in the sample with a distinct amount of exogenously provide hydrogen peroxide (H2O2). The antioxidants in the sample abolish a definite amount of the provided hydrogen peroxide. The residual H2O2 is determined colorimetrically (57).
Determination of TOS level was performed using a colorimetric measurement method, which was described by Erel (58), and the oxidative stress index (OSI) was calculated as the percentage ratio of TOS to TAC levels according to the following formula:
OSI (arbitrary unit)=TOS (micromolar H2O2 equivalent/liter)/TAC (micromolar equivalent/liter) (59).
Ovarian mitochondria enriched fraction
0.5 gram of ovarian homogenate was kept on ice and added to isolation medium (10 mM HEPES buffer pH 7.0 containing 220 mM mannitol, 68 mM sucrose, 10 mM KCl and 0.1% serum albumin) in a ratio 1:10. Centrifugation was performed for 10 min at 1000 X g, and then the supernatant was re-centrifuged at 11,500 X g for 10 min. The supernatant was discarded, and the pellet was re-suspended in the isolation medium but without albumin (60).
Assessment of ovarian mitochondrial function
Mitochondrial function was evaluated using MTT reduction assay. This assay is linked to the ability of the mitochondrial dehydrogenases to metabolize 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan, a reaction that occurs if the mitochondrial preparation is functionally intact.
DNA fragmentation %
The ovarian tissues were added to 0.5 ml lysis buffer (10 mM Tris-HCl (PH 8), 1 mM EDTA, 0.2% triton X 100), then centrifuged at 10000 rpm for 20 min at 4 °C. Supernatant and sediment were collected in separate Eppendorf tubes. 0.5 ml of 25 % Trichloroacetic acid was added to the sediment and supernatant and incubated at 4 °C for 24 hr. The samples were centrifuged for 20 minutes at 10000 rpm at 4 °C, and then incubate at 83 °C for 20 min. Subsequently, 160 µl of Diphenylamine (DPA) solution (150 gram DPA in 10 ml glacial acetic acid, 150 µl sulphuric acid and 50 µl acetaldehyde (16 mg/ ml)) was added and incubated at room temperature for 24 hr (61). The portion of fragmented DNA was calculated from the absorbance reading at 600 nm using the following formula:
Detection of iNOS, NQO1 and Mfn2 mRNA expression by RT-PCR
Based on the instruction of the kit and by using RNeasy Purification Reagent (Qiagen, Valencia, CA), the total RNA was isolated from ovarian homogenates. The concentration of RNA was measured using a UV spectrophotometer. Afterwards, mRNA expressions of inducible nitric oxide synthase (iNOS), quinone oxidoreductase 1(NQO1) and Mfn2 were evaluated by RT-PCR.
cDNA synthesis
Five microgram RNA was reverse transcribed and denatured by using oligonucleotide (dT) 18 primer (final concentration, 0.2 mM) and keeping at 70 °C for 2 min, respectively. Denatured RNA was kept on ice and in the reverse transcription mixture containing 50 mM KCl, 50 mM Tris HCl (pH 8.3), 0.5 mM of deoxyribonucleotide triphosphate (dNTP), 3 mM MgCl2, 1 U/ml RNase inhibitor, and 200 units of murine leukemia virus reverse transcriptase. The reaction tube was exposed at 42 °C for 1 hr, followed by heating to 92 °C to stop the reaction.
Real-time quantitative polymerase chain reaction
Five microliter of the first-strand cDNA was used in a total volume of 25 μl, containing 12.5 μl 2x SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and 200 ng of each primer as shown in Table 1. PCR program was 1 cycle at 95 °C/ 10 min, 94 °C/ 15 sec and 40 cycles at 60 °C/ 1 min, by using step one plus Real Time PCR system (Applied Biosystems). Data analysis was performed by the ABI Prism 7500 sequence detection system software and quantified using the v 1.7 Sequence Detection Software from PE Biosystems (Foster City, CA). Relative expression of studied genes was calculated using the comparative threshold cycle method. All values were normalized to the beta actin genes, and all these steps were described by Livak and Schmittgen (62).
Table 1.
Primer sequences used for RT-PCR
| Gene name | Primer sequence |
|---|---|
| -actin |
Forward primer (5′-3′): GGGAAATCGTGCGTGACATT
Reverse primer (5′-3′): GCGGCAGTGGCCATCTC |
| iNOS |
Forward(5′-3′)
:
GACCAGAAACTGTCTCACCTG
Reverse(5′-3′) : CGAACATCGAACGTCTCACA |
| NQO1 |
Forward (5′-3′): -AGGCTGGTTTGAGCGAGT
Reverse (5′-3′): ATTGAATTCGGGCGTCTGCTG |
| Mfn2 |
Forward primer (5′-3′): CTTGAAGACACCCACAG-GAACA
Reverse primer (5′-3′): GGCCAGCACTTCGCTGATAC |
(iNOS): Inducible nitric oxide synthase, (NQO1): Quinone oxidoreductase 1, (Mfn2): Mitofusin 2
Western blotting analysis
Thirty microgram proteins were separated by SDS-PAGE, and moved to PVDF membranes (Invitrogen, USA). 5% non-fat milk was used for 1 hr at 37 °C to block the non-specific binding. Overnight hybridization of nitrocellulose membranes was performed with the rabbit polyclonal anti-Mfn2 antibody (Abcam, USA), the rabbit polyclonal anti-Bcl-2 antibody (Cell Signaling Technology, USA), the rabbit polyclonal anti-Bax antibody (Cell Signaling Technology, USA), and the rabbit polyclonal anti-β-actin antibody (Santa cruz Biotechnology Inc, USA) in the Primary Antibody Dilution Buffer at 4 °C. After four times washing of the bands with TBS-T, each time for 10 min at 37 °C, the membranes were incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hr at 37 °C (AntGene, USA). Finally, the immune reactive bands were detected by the enhanced chemiluminescence system (Beyotime Institute of Biotechnology, China). The intensity of the band was quantified by densitometry using the Quantity One 4.62 analysis software, and all results were normalized to β-actin signal intensity.
Statistical analysis
All results were analyzed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). The obtained results were expressed as means±SD. Significant differences between means were verified by one-way ANOVA. The calculated data were determined to be significant if the P<0.05.
Results
Ovarian and uterine weight
Results in Table 2 showed that administration of cisplatin significantly (P<0.001) reduced ovarian (50.1%) and uterine weight (43.7%) in comparison with the control group. On the other side, RJ significantly (P<0.001) increased ovarian (89.5%) and uterine weight (75.4%) compared to the animal group injected with cisplatin (Table 2).
Table 2.
Effect of Royal jelly on ovarian and uterine weight in cisplatin-treated female rats
| Ovarian weight in pair (mg) | Uterine weight (mg) | |
|---|---|---|
| Control | 40.3 ± 0.22 | 142.28 ±. 4.46 |
| Cisplatin | 20.12± 0.5a | 80.12± 1.28a |
| RJ + Cis. | 38.12 ± 0.92b | 140.6 ± 2.42b |
Values were expressed as mean±SD. RJ mean Royal jelly and Cis. Means cisplatin.
a: express a significant difference when compared to the control group. b: indicates a significant difference when compared to cisplatin group
Ovarian morphometric analysis and quantitation of folliculogenesis
Ovaries subjected to cisplatin showed a significant (P<0.001) decrease of small preantral follicle (SPAF), large preantral follicle (LPAF), small antral follicle (SAF), medium antral follicle (MAF), large antral follicle (LAF) and Graafian follicles (GF) and a significant (P<0.01) decrease of corpus luteum number when compared to control group. Moreover, injection of cisplatin caused a significant (P<0.001) increase in the number of atretic follicles compared to control group. On the contrary, ovaries exposed to RJ in RJ+Cis. group showed a significant (P<0.001) increase in the number of SPAF, LPAF, SAF, MAF, AF (P<0.01) and GF(P<0.01) as well as corpusluteum when compared to cisplatin group (Table 3).
Table 3.
Effect of Royal jelly on ovarian morphometric analysis and quantitation of folliculogenesis in cisplatin-treated female rats
|
Follicular Counts
|
||||||||
|---|---|---|---|---|---|---|---|---|
| SPAF | LPAF | SAF | MAF | LAF | GF | Corpus luteum | Atretic follicles | |
| Control | 26.40±1.4 | 20.50± 2.4 | 16.50± 1.2 | 12.8± 1.6 | 9.62± 2.2 | 7.2±1.4 | 3.8 ±1.2 | 2.8± 0.8 |
| Cisplatin | 3.12± 0.6a | 1.34±0.4a | 2±0.7 a | 2± 0.5a | 1± 0.3a | 1.1±0.2 a | 1±0.2 a | 10.17±0.9 a |
| RJ+Cis. | 25.1± 2b | 18.2±0.6 b | 14.1±0.6b | 11.2± 0.5 b | 8.1± 0.2 b | 5.1±0.3 b | 2±0.4b | 3.17± 0.6b |
Values were expressed as mean±SD. Cis. refers to cisplatin. (RJ): Royal jelly, (SPAF): Small preantral follicle, (LPAF): Large preantral follicle, (SAF): Small antral follicle, (MAF): Medium antral follicle, (LAF): Large antral follicle (LAF) and (GF) Graafian follicles
a: indicates a significant difference (P<0.05) when compared to the control group. b: indicates a significant difference (P<0.05) when compared to the cisplatin group
Histological changes of ovaries
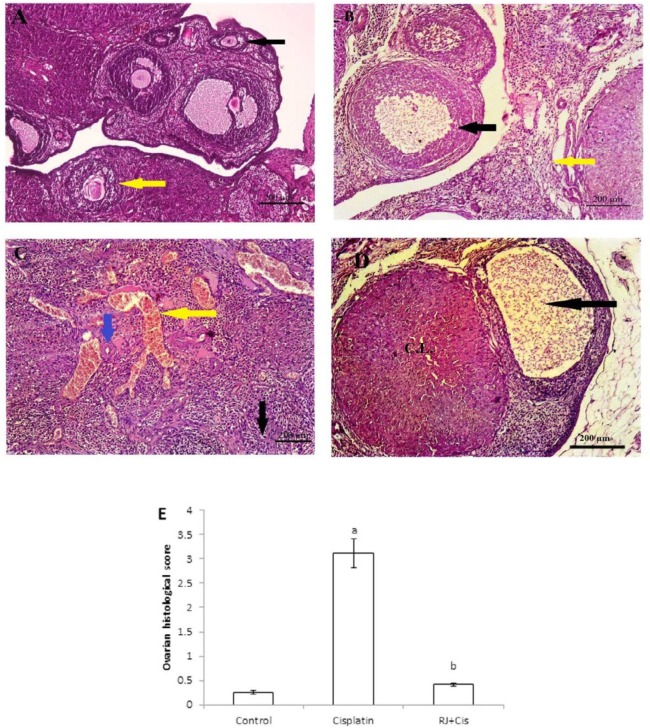
The alterations of ovarian histology in different groups are presented in Figure 1. The histological sections stained with H&E revealed that those of control had normal ovarian architecture with no considerable pathologic alteration. Normal ovarian follicles in various stages of development were observed in the ovarian cortex (Figure 1A). However, ovaries of cisplatin group showed follicular degeneration (black arrow), interstitial edema (yellow arrow) (Figure 1B), marked vascular congestion (yellow arrow), hyalinosis (blue arrow) as well as stromal edema (black arrow) (Figure 1C). Administration of RJ with cisplatin in RJ+Cis. group restored the normal structure of the ovarian tissue with no significant difference when compared to the control group (Figure 1D).
Figure 1.
Representative photomicrographs of H&E-stained sections of rat ovaries in each experimental group
A) Light microscopy of the ovarian tissue with developing follicles in many different stages in the control group. B) Cisplatin group showed a severe histopathologic injury than in other groups, follicular degeneration (black arrow), interstitial edema (yellow arrow). C) A significant marked vascular congestion (yellow arrow), hyalinosis (blue arrow) as well as stromal edema (black arrow) in cisplatin group when compared to the control group. D) Administration of Royal jelly (RJ) with cisplatin in RJ+Cis. group restored the normal structure of the ovarian tissue with no significant difference from control group. E) Quantification of histopathological scores in the ovarian sections from the experimental groups where data were expressed as means±SD, a indicates a significant difference when compared to control group at P˂0.05 and b: indicates a significant difference when compared to cisplatin group (P˂0.05)
Serum gonadotropins, female sex hormones and AMH
Cisplatin administration caused a significant increase of FSH (P<0.001) and LH (P<0.01) concentrations with respect to control group. In addition, it significantly (P<0.001) decreased estradiol (E2), progesterone and AMH concentration compared to control group. On the other side, RJ administration prior to cisplatin in RJ+Cis group significantly reduced FSH (P<0.001) and LH (P<0.01) concentrations with a simultaneous significant (P<0.001) increase of E2, progesterone and AMH concentration compared to cisplatin group (Table 4).
Table 4.
Effect of Royal jelly on serum gonadotropins, female sex hormones and anti-mullerian hormone in cisplatin treated female rats
| Group | FSH (ng/ml) | LH (ng/ml) | (E2) (ng/ml) | Progesterone (ng/ml) | AMH (ng/ml) |
|---|---|---|---|---|---|
| Control | 8.22 ±1.23 | 4.11 ± 0.65 | 70.98 ± 1.4 | 40.98 ± 0.9 | 9.44 ± 0.66 |
| Cisplatin | 19.98 ± 0.98a | 9.2 ± 1.09 a | 20.65 ± 0.9 a | 12.09 ± 0.32 a | 4.76 ± 0.31 a |
| RJ+Cis | 9.65 ± 0.45 b | 5.25 ± 0.45b | 75.12 ± 1.4b | 35.12 ± 1.07 b | 8.21 ± 0.28b |
Values were expressed as mean±SD. (RJ): Royal jelly, FSH: Follicular stimulating hormone, (LH): Luteinizing hormone, (E2): Estradiol 2, (AMH): Anti-mullerian hormone
a: indicates a significant difference (P<0.05) when compared to the control group. b: indicates a significant difference (P<0.05) when compared to the cisplatin group
Ovarian antioxidant/oxidative redox
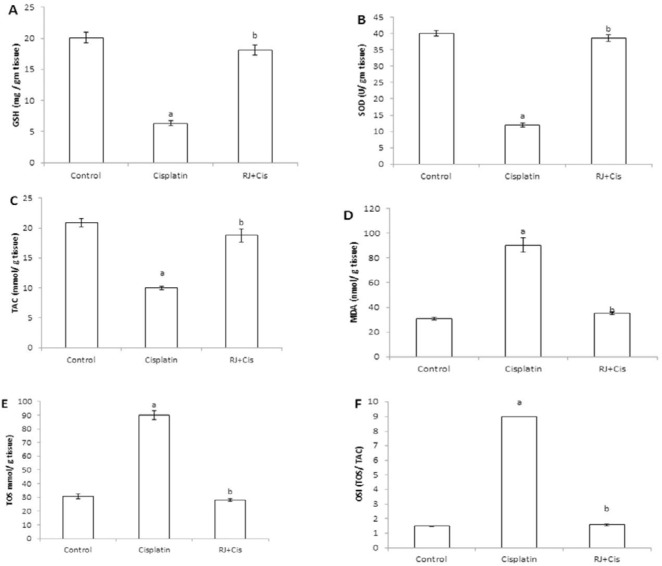
Data in Figure 2 showed that cisplatin administration significantly reduced ovarian GSH concentration (P<0.001), SOD activity (P<0.001) and TAC (P<0.001) compared to control rats. Added to that, cisplatin significantly increased MDA (P<0.001), TOS (P<0.001) and OSI (P<0.001) compared to control group. However, RJ significantly restored the ovarian antioxidant activity, which was demonstrated by the significant increase of ovarian GSH concentration (P<0.001), SOD activity (P<0.001) and TAC (P<0.001) compared to cisplatin-treated rats. In addition, RJ significantly reduced the ovarian MDA (P<0.001), TOS (P<0.001) and OSI (P<0.001) compared to cisplatin group.
Figure 2.
Antioxidant activity assays in ovarian tissues of all experimental groups
A) glutathione (GSH) levels, B) superoxidedismutase (SOD) levels, C) total antioxidant capacity (TAC), D) malondialdehyde (MDA) levels, E) total oxidative stress (TOS) and F) oxidative stress index (OSI). The data represent means±SD (n=10/group). Cisplatin administration significantly reduced ovarian GSH concentration (P<0.001), SOD activity (P<0.001) and TAC (P<0.001) compared to control rats. Added to that, cisplatin significantly increased MDA (P<0.001), TOS (P<0.001) and OSI (P<0.001) compared to control group. Royal jelly (RJ) significantly restored the ovarian antioxidant activity, which was indicated by the significant increase of ovarian GSH concentration (P<0.001), SOD activity (P<0.001) and TAC (P<0.001) compared to cisplatin-treated rats. Also, RJ significantly reduced the ovarian MDA (P<0.001), TOS (P<0.001) and OSI (P<0.001) compared to cisplatin group. a: indicates a significant difference when compared to the control group at P<0.05, b: indicates a significant difference when compared to cisplatin group at P<0.05
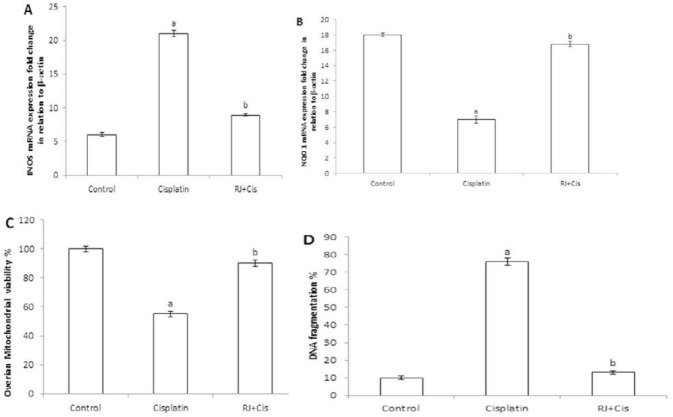
Ovarian iNOS, NQO1, mitochondrial viability and DNA fragmentation %
Results represented in Figure 3 showed that cisplatin administration significantly increased ovarian iNOS (P<0.001), and reduced NQO1 (P<0.001), mitochondrial viability (P<0.001) and DNA fragmentation % (P<0.001) as compared to control group. RJ significantly decreased iNOS (P<0.001), and increased NQO1 (P<0.001), mitochondrial viability (P<0.001) and decreased DNA fragmentation % (P<0.001) compared to cisplatin group.
Figure 3.
Estimation of ovarian iNOS, NQO1, mitochondrial viability % and DNA fragmentation %
A) Bar graph shows real time expression analysis of iNOS tran¬script in the ovarian tissues of rats of different experimental groups. B) Bar graph shows real time expression analysis of NQO1 transcript in the ovarian tissues of rats of different experimental groups. C) Bar graph is the representation of ovarian mitochondrial viability % in different experimental groups. D) Bar graph represents DNA fragmentation % in different experimental groups. Data of real-time PCR were used to calculate the relative expression of iNOS and NQO1 genes using the comparative threshold cycle method, and all values were normalized to the β-actin. The data represent means±SD (n=10/group). Cisplatin administration significantly increased ovarian iNOS (P<0.001), and DNA fragmentation % (P<0.001) and reduced NQO1 (P<0.001), and mitochondrial viability (P<0.001) compared to control group. RJ significantly decreased iNOS (P<0.001), and DNA fragmentation % (P<0.001) and increased NQO1 (P<0.001), and mitochondrial viability (P<0.001) compared to cisplatin group. a: indicates a significant difference when compared to the control group at P<0.05, b: indicates a significant difference when compared to cisplatin group at P<0.05. (RJ): Royal jelly, (iNOS): Inducible nitric oxide synthase, (NQO1): Quinone oxidoreductase 1
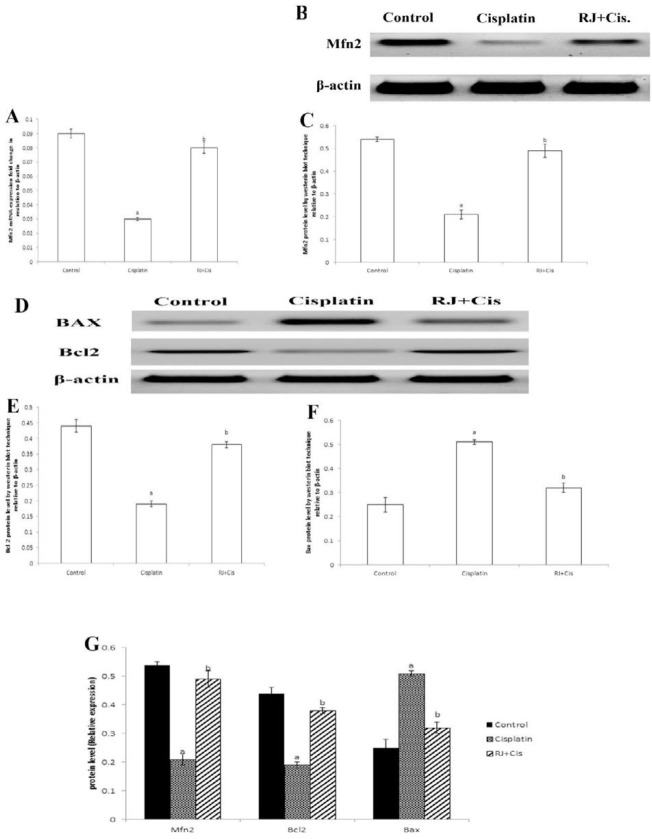
Ovarian Mfn2, Bcl2 and Bax mRNA expressions and protein concentrations
Results in Figure 4 showed that cisplatin significantly decreased mRNA expressions of Mfn2 (P<0.001), and Bcl2 and protein concentrations (P<0.001) and significantly increased Bax mRNA expression and protein concentration (P<0.001) compared to control group. RJ restored Mfn2 mRNA expression and protein and re-established ovarian Bcl2/ Bax mRNA and proteins (P<0.001) compared to cisplatin group.
Figure 4.
Ovarian Mfn2 mRNA expression, Mfn2, Bcl2 and Bax protein levels
A) Mfn2 mRNA expression of different experimental groups. B) The most representative image of western blotting of ovarian Mfn2 protein level of different experimental groups. C) Mfn2 protein level in different experimental groups. D) The most representative image of western blotting of ovarian Bcl2 and Bax protein levels of different experimental groups. E) Bcl2 protein level in different experimental groups. F) Bax protein level in different experimental groups. G) Comparison of ovarian Mfn2, Bcl-2, and Bax protein levels in different experimental groups. The data represent means±SD (n=10/group). Our data showed that cisplatin significantly decreased Mfn2 (P<0.001), and Bcl2 mRNA expressions and protein concentrations (P<0.001) and significantly increased Bax mRNA expression and protein concentration (P<0.001) compared to control group. RJ restored Mfn2 mRNA expression and protein. Added to that, RJ re-established ovarian Bcl2/ Bax mRNA and proteins (P<0.001) compared to cisplatin group. a: indicates a significant difference when compared to the control group at P˂0.05, b: indicates a significant difference when compared to cisplatin group at P<0.05. (Mfn2): Mitofusin 2, (RJ): Royal jelly
Discussion
Many previous studies highly suggested the use of protective compounds of plant source with chemotherapeutic agents to enhance their efficacy and reduce their toxic effects (63, 64). The current study intended to postulate that RJ could ameliorate cisplatin-induced ovarian oxidative stress and apoptosis.
Chemotherapeutic medicines impair fertility as they severely disturb the ovarian activities, development and hormonal balance (65). One of the most commonly used chemotherapies is cisplatin. It is used in the management of several types of tumors. However, it was reported that cisplatin caused serious unwanted effects on kidneys (66), neurons (67), stomach (68) and reproductive organs (68). Han et al. described that cisplatin increased the prevalence of premature ovarian function disturbance in humans (69).
Our generated data demonstrated that injection of cisplatin caused serious ovarian damage, which was indicated by the reduction of ovarian and uterine weight, decrease in the follicular count and increase in the number of atretic follicles. These results are in harmony with those of Ozdamar et al. (70) who reported that chemotherapies result in follicular damage followed by ovarian dysfunction. Regarding cisplatin, it was reported that its administration induced follicular and ovarian damage (70, 71). According to the results of the existing study, histopathological examination of ovaries, in cisplatin-treated animals, revealed stromal edema, severe congestion, hyalinosis and marked degeneration of follicles in cisplatin-treated rats. The findings were similar to those obtained by Altuner et al. (72).
Our results indicated that cisplatin decreased E2 and progesterone concentrations due to the extensive follicular damage, which can result in the loss of ovarian steroid hormones (73). A simultaneous increase of serum FSH and LH concentrations was noticed and harmonized with diminished ovarian negative feedback due to exhaustion of ovarian follicles (74). Rats injected by cisplatin showed a significant decrease of AMH, which is considered one of the most delicate biomarkers of ovarian damage and reserve, because serum AMH concentration decreases with low ovarian reserve and follicular destruction (71, 75). This result is in agreement with Yeh et al. who clarified that cisplatin decreases serum AMH levels in animals (76).
Many previous studies reported that free radicals overproduction and antioxidants depletion are implicated in cisplatin-induced toxicity and tissue damage (72, 77). This comes in concurrence with the results of the existing study that demonstrated low ovarian antioxidants, which were manifested by the decrease of GSH, SOD activity and TAC level coincide with cisplatin administration. A synchronized increase of lipid peroxidation was indicated by the increase of ovarian MDA concentration and OSI. These results agreed with other reports, which stated that cisplatin toxicity is closely related to increased lipid peroxidation (78, 79). Also, MDA can disturb the permeability and fluidity of the cell membrane by interrupting ionic transport and cellular enzymatic activity (80-82).
iNOS is involved in nitric oxide (NO) production and implicated in the initial step of toxicity under oxidative stress. Too much No react with superoxide anion to generate peroxynitrite radical that causes cellular injury by oxidizing cellular macromolecules as GSH, proteins, lipids and DNA to produce the peroxynitrite radical, which causes cell injury (83). In addition, excess NO depletes intracellular GSH, thereby augmenting the sensitivity to oxidative stress (84). Indeed, ovarian iNOS mRNA expression significantly increased by cisplatin confirming the induction of ovarian oxidative stress as mentioned by Krishna et al. (85). Once activated, iNOS mRNA expression increases the production of NO, which implicated in cisplatin-mediated ovarian function impairment (86). Nearby the ability of cisplatin to induce many ROS, it inhibits the ovarian antioxidant defense elements. This effect was obvious in our results and manifested by the significant reduction of ovarian NQO1 mRNA expression in cisplatin-treated rats.
Besides the function of mitochondria as the energy house of the cell (87), the mitochondrial membranes are essential sites for steroidogenesis in granulosa cells (88). Mitochondria are the target organelle for the disruption of cellular antioxidant/ oxidative dynamic equilibrium state (87). Oxidative stress-induced mitochondrial damage impairs steroidogenesis in granulosa cells and inhibits steroidogenic enzymes and a mitochondrial carrier protein (StAR protein), which possess a pivotal role in the transport of cholesterol into luteal cells mitochondria (88). In our study, cisplatin administration decreased the ovarian mitochondrial viability % in agreement with Chen et al. (14) and increased ovarian DNA fragmentation % in line with Park et al. (89) who informed that excessive ROS is associated with mitochondrial dysfunction and DNA fragmentation %.
Apoptosis is defined as a programmable cellular death with certain metabolic and morphological alterations, excessive nuclear damage, chromatin condensation and the stimulation of specific markers (90, 91). Consequently, the induction of apoptosis is considered as an important pathway in ovarian toxicity and damage. Acquired data in this study showed a significant alteration of ovarian anti-apoptotic/ apoptotic markers following cisplatin treatment. This could represent the cytotoxic effect of cisplatin that is facilitated via the apoptotic pathway. Upon cisplatin treatment, the level of Bcl2 was diminished and that of Bax was augmented. These variations in protein expression changed the permeability of mitochondrial membrane and viability causing the discharge of cytochrome C into the cytosol, which results in the stimulation of the adaptor molecule apoptotic proteaseactivating factor 1 (Apaf1), and producing the apoptosome complex. Apaf1 then cleaves the preform of caspase9 to initiate the caspase cascade, resulting in apoptosis (92). Thus, mitochondrial facilitated apoptosis comprises induction of Bax, suppression of Bcl2, disturbance of mitochondrial viability, and initiation of the caspase cascade.
The reduced expression of Mfn2 can cause mitochondrial dysfunction and damage, thus provoking the cellular apoptosis (34), oocyte and follicular developmental disorders (93). In our investigation, we clarified that cisplatin administration caused a prominent suppression in ovarian Mfn2 mRNA and protein. This finding comes in line with Chen et al. (14) who revealed that expression of Mfn2 was reduced in the ovarian tissues of premature ovarian failure (POF) induced by cisplatin, and that reality might be a mechanism engaged with both ovarian mitochondrial damage and an increase in ovarian apoptosis. Also, many previous studies showed that the lower expression of Mfn2 could aggravate the stress of the ER that results in the apoptosis of granulosa cells as well as inhibiting steroids production and secretion (94, 95).
Based on the previously discussed data, oxidative stress and apoptosis are potent mechanisms of cisplatin-induced ovarian damage. So, the use of RJ that has a powerful antioxidant, and anti-apoptotic effect (96, 97) is a logic approach. Our results indicated that RJ protected the ovaries from the damaging outcome of oxidative stress mediated by cisplatin. The protective effect of RJ is manifested by increasing both ovarian and uterine weights, the follicular count, decreasing the atretic follicles and restoring normal histological structure of ovaries in RJ-pretreated rats compared to cisplatin-given rats. In addition, RJ significantly increased estrogen and progesterone level. Previous studies suggested that reduction of FSH and LH concentration in RJ-pretreated rats is a direct sequence of increasing ovarian hormones and the number of ovarian follicles (36),(98). Moreover, Kamakura stated that Royalactin, a 57-kDa protein in RJ, increases the ovarian growth (99), ovulation rate and progesterone levels in luteal phase (100) through epidermal growth factor receptor-facilitated signaling pathway.
The antioxidant effect of RJ is specified by the prominent increase of GSH, SOD, and TAC levels and the reduction of MDA, TOS and OSI that come in harmony with Karadeniz, et al. who demonstrated that RJ has a powerful antioxidant effect and reduces cisplatin-induced lipid peroxidation in kidney tissue (96,101). Proportionate to our outcomes, You et al. conveyed that RJ could improve tissue damage caused by excessive NO by reducing iNOS mRNA expression (102). Interestingly, RJ has a direct stimulatory effect on NQO1, which catalyzes two-electron reduction and reclamation of quinones and its derivatives, guarding cells from oxidative stress, and redox cycling (103). NQO1 preserves ubiquinone (co-enzyme Q) and α- tocopherol quinine in their reduced active state, which are two fundamental lipid-soluble antioxidants (104,105). Besides the antioxidant effect of RJ, it possesses a potent protective effect against mitochondria-induced ovarian apoptosis. The anti-apoptotic effect of RJ is detected by increased expression of Bcl2 and suppression of Bax in ovarian tissue. In the current study, we hypothesized that RJ targets Mfn2 to alleviate cisplatin-induced ovarian apoptosis, and this hypothesis agreed with Luo et al. (32) who concluded that Mfn2 can be used as a novel target in the treatment of ovarian stress and damage. RJ increased both Mfn2 mRNA expression and protein in ovarian tissue compared to cisplatin-treated rats. The activation of Mfn2 re-established the mitochondrial membrane permeability and integrity (106,107).
Mfn2 is widely expressed in the ovarian granular cells, follicular fluid, inner theca cells, corpus luteum and ovarian stroma, but rarely expressed in the outer theca cells. Mfn2 has the ability to inhibit the development of programmed cell death by suppressing the release of cytochrome C mediated by Bax protein, and relieving the radicals-induced cellular damage (34, 108). The re-establishment of ovarian Bcl2/ Bax levels and keeping cytochrome C inside the mitochondria prevent the activation of Apaf1, then no activation of caspase cascade and finally, no apoptotic reaction can take place.
Conclusion
Administration of RJ potently protects against mitochondrial-mediated ovarian damage by cisplatin via increasing the ovarian antioxidants, dropping pro-apoptotic protein Bax, stimulating anti-apoptotic Bcl2 and maintaining the integrity of mitochondrial membrane by activating Mfn2. Also, RJ is a potent reproductive stimulator by increasing the follicular count and ovarian activity.
Acknowledgment
Authors appreciate all staff members of the department of pathology, faculty of veterinary medicine, Beni-Suef University, Beni-Suef, Egypt, for their great helpful efforts in the histological examinations of the ovarian sections.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 2.Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 3.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatima S, Arivarasu NA, Mahmood R. Vitamin C attenuates cisplatin-induced alterations in renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2007;26:419–426. doi: 10.1177/0960327106072389. [DOI] [PubMed] [Google Scholar]
- 5.Ciftci O, Ozdemir I, Vardi N, Gurbuz N. Novel platinum-N-heterocyclic carbene complex is more cardiotoxic than cis-platin in rats. Hum Exp Toxicol. 2011;30:1342–1349. doi: 10.1177/0960327110390064. [DOI] [PubMed] [Google Scholar]
- 6.Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet Gynecol Surv. 2007;62:58–72. doi: 10.1097/01.ogx.0000251029.93792.5d. [DOI] [PubMed] [Google Scholar]
- 7.Meraner V, Gamper EM, Grahmann A, Giesinger JM, Wiesbauer P, Sztankay M, et al. Monitoring physical and psychosocial symptom trajectories in ovarian cancer patients receiving chemotherapy. BMC Cancer. 2012;12:77. doi: 10.1186/1471-2407-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- 9.Brady HR, Kone BC, Stromski ME, Zeidel ML, Giebisch G, Gullans SR. Mitochondrial injury: an early event in cisplatin toxicity to renal proximal tubules. Am J Physiol. 1990;258:F1181–1187. doi: 10.1152/ajprenal.1990.258.5.F1181. [DOI] [PubMed] [Google Scholar]
- 10.Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995;48:761–770. doi: 10.1038/ki.1995.348. [DOI] [PubMed] [Google Scholar]
- 11.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sancho-Martinez SM, Prieto-Garcia L, Prieto M, Lopez-Novoa JM, Lopez-Hernandez FJ. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol Ther. 2012;136:35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8:e81162. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Xu X, Wang L, Bai G, Xiang W. Low expression of Mfn2 is associated with mitochondrial damage and apoptosis of ovarian tissues in the premature ovarian failure model. PLoS One. 2015;10:e0136421. doi: 10.1371/journal.pone.0136421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 16.Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251–257. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 17.Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 18.Van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 20.Ray WZ, Murphy RK, Santosa K, Johnson PJ, Mackinnon SE. Medial pectoral nerve to axillary nerve neurotization following traumatic brachial plexus injuries: indications and clinical outcomes. Hand. 2012;7:59–65. doi: 10.1007/s11552-011-9378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, et al. Blood cell mitochondrial DNA content and premature ovarian aging. PLoS One. 2012;7:e42423. doi: 10.1371/journal.pone.0042423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Archives of toxicology. 2015;89:923–939. doi: 10.1007/s00204-015-1475-z. [DOI] [PubMed] [Google Scholar]
- 23.Hirata H, Lopes GS, Jurkiewicz A, Garcez-do-Carmo L, Smaili SS. Bcl-2 modulates endoplasmic reticulum and mitochondrial calcium stores in PC12 cells. Neurochemical research. 2012;37:238–243. doi: 10.1007/s11064-011-0600-5. [DOI] [PubMed] [Google Scholar]
- 24.Stary CM, Sun X, Ouyang Y, Li L, Giffard RG. miR-29a differentially regulates cell survival in astrocytes from cornu ammonis 1 and dentate gyrus by targeting VDAC1. Mitochondrion. 2016;30:248–254. doi: 10.1016/j.mito.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Z, Gollahon L. Paclitaxel attenuates Bcl-2 resistance to apoptosis in breast cancer cells through an endoplasmic reticulum-mediated calcium release in a dosage dependent manner. Biochemical and biophysical research communications. 2013;432:431–437. doi: 10.1016/j.bbrc.2013.01.130. [DOI] [PubMed] [Google Scholar]
- 26.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta pharmaceutica sinica B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbel N, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. Journal of Biological Chemistry. 2010;285:6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Z, Syed MA, Panchal D, Joo M, Colonna M, Brantly M, et al. Triggering receptor expressed on myeloid cells 1 (TREM-1)-mediated Bcl-2 induction prolongs macrophage survival. Journal of Biological Chemistry. 2014;289:15118–15129. doi: 10.1074/jbc.M113.536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 31.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 32.Luo Q, Huo P, Wang L, Wu X. The influencing mechanism of mTOR signal pathway mediated by mitofusin-2 in development of follicle. European review for medical and pharmacological sciences. 2018;22:2212–2217. doi: 10.26355/eurrev_201804_14806. [DOI] [PubMed] [Google Scholar]
- 33.Zorzano A, Hernandez-Alvarez MI, Sebastian D, Munoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22:1020–1031. doi: 10.1089/ars.2014.6208. [DOI] [PubMed] [Google Scholar]
- 34.Zhao N, Zhang Y, Liu Q, Xiang W. Mfn2 affects embryo development via mitochondrial dysfunction and apoptosis. PloS one. 2015;10:e0125680. doi: 10.1371/journal.pone.0125680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid Med Cell Longev. 2017;2017:1259510. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghanbari E, Khazaei MR, Khazaei M, Nejati V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. International journal of fertility & sterility. 2018;11:263. doi: 10.22074/ijfs.2018.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto M, Kanda M, Ikeno K, Hayashi Y, Nakamura T, Ogawa Y, et al. Oral administration of royal jelly facilitates mRNA expression of glial cell line-derived neurotrophic factor and neurofilament H in the hippocampus of the adult mouse brain. Biosci Biotechnol Biochem. 2005;69:800–805. doi: 10.1271/bbb.69.800. [DOI] [PubMed] [Google Scholar]
- 38.Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chemistry. 2004;84:181–186. [Google Scholar]
- 39.Ramadan MF, Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: A review. Journal of Functional Foods. 2012;4:39–52. [Google Scholar]
- 40.Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. Journal of nutritional science and vitaminology. 2008;54:191–195. doi: 10.3177/jnsv.54.191. [DOI] [PubMed] [Google Scholar]
- 41.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez J. Functional properties of honey, propolis, and royal jelly. Journal of food science. 2008;73:R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 42.Tamura S, Kono T, Harada C, Yamaguchi K, Moriyama T. Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chemistry. 2009;114:1491–1497. [Google Scholar]
- 43.Sugiyama T, Takahashi K, Mori H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr Metab Immune Disord Drug Targets. 2012;12:368–376. doi: 10.2174/187153012803832530. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim A, Eldaim MAA, Abdel-Daim MM. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology. 2016;68:1039–1048. doi: 10.1007/s10616-015-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsunaga Y, Sakata Y, Yago T, Nakamura H, Shimizu T, Takeda Y. Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice. Nutrients. 2018;10:753. doi: 10.3390/nu10060753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. Journal of nutrition and metabolism. 2014:2014. doi: 10.1155/2014/239750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida M, Hayashi K, Watadani R, Okano Y, Tanimura K, Kotoh J, et al. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. Journal of Veterinary Medical Science . 2016:16–0458. doi: 10.1292/jvms.16-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi Y, Hijikata K, Seike K, Nakano S, Banjo M, Sato Y, et al. Effects of Royal Jelly Administration on Endurance Training-Induced Mitochondrial Adaptations in Skeletal Muscle. Nutrients. 2018;10:1735. doi: 10.3390/nu10111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chtourou Y, Aouey B, Kebieche M, Fetoui H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-kappaB and P53 signaling pathways. Chem Biol Interact. 2015;239:76–86. doi: 10.1016/j.cbi.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed MM, El-Shazly SA, Alkafafy ME, Mohamed AA, Mousa AA. Protective potential of royal jelly against cadmium-induced infertility in male rats. Andrologia. 2018;50:e12996. doi: 10.1111/and.12996. [DOI] [PubMed] [Google Scholar]
- 51.Warren B. Theory and Practice of Histological Techniques. Pathology. 1996;28:381. [Google Scholar]
- 52.Patil SR, Ravindra S, Patil R, Londonkar R, Patil SB. Nicotine induced ovarian and uterine changes in albino mice. Indian journal of physiology and pharmacology. 1998;42:503–508. [PubMed] [Google Scholar]
- 53.Li X, Kang X, Deng Q, Cai J, Wang Z. Combination of a GnRH agonist with an antagonist prevents flare-up effects and protects primordial ovarian follicles in the rat ovary from cisplatin-induced toxicity: a controlled experimental animal study. Reproductive Biology and Endocrinology. 2013;11:16. doi: 10.1186/1477-7827-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 55.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 56.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 57.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19:2101–2113. [PubMed] [Google Scholar]
- 60.Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, et al. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chemical research in toxicology. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- 61.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 63.Gasic S, Vucevic D, Vasilijic S, Antunovic M, Chinou I, Colic M. Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacology and Immunotoxicology. 2007;29:521–536. doi: 10.1080/08923970701690977. [DOI] [PubMed] [Google Scholar]
- 64.Cayir K, Karadeniz A, Yildirim A, Kalkan Y, Karakoc A, Keles M, et al. Protective effect of L-carnitine against cisplatin-induced liver and kidney oxidant injury in rats. Open Medicine. 2009;4:184–191. [Google Scholar]
- 65.Smith J. Erlotinib: Small-molecule targeted therapy in the treatmentof non-small-cell lung cancer. Clinical therapeutics. 2005;27:1513–1534. doi: 10.1016/j.clinthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. The oncologist. 2017;22:609–619. doi: 10.1634/theoncologist.2016-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binnetoglu D, Hacimuftuoglu A, Aricioglu F. Neuroprotective effects of agmatine in antineoplastic drugs induced neurotoxicity: In vitro study. Life sciences. 2019;221:311–318. doi: 10.1016/j.lfs.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Adefisayo MA, Adeyemi WJ, Alabi QK. Combined but not single administration of vitamin C and l-carnitine ameliorates cisplatin-induced gastric mucosa damage in male rats. Canadian journal of physiology and pharmacology. 2018;96:830–838. doi: 10.1139/cjpp-2017-0751. [DOI] [PubMed] [Google Scholar]
- 69.Han SE, Park MJ, Kim HJ, Kim HG, Kim CW, Joo BS, et al. Establishment of Effective Mouse Model of Premature Ovarian Failure Considering Treatment Duration of Anticancer Drugs and Natural Recovery Time. Journal of menopausal medicine. 2018;24:196–203. doi: 10.6118/jmm.2018.24.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ozdamar S, Taskin MI, Onder GO, Kaymak E, Baran M, Yay A. Progesterone decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. 2019;28:25–33. doi: 10.17219/acem/76858. [DOI] [PubMed] [Google Scholar]
- 71.Said RS, Mantawy EM, El-Demerdash E. Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn-Schmiedeberg’s archives of pharmacology . 2019:1–14. doi: 10.1007/s00210-019-01662-x. [DOI] [PubMed] [Google Scholar]
- 72.Altuner D, Gulaboglu M, Yapca OE, Cetin N. The effect of mirtazapine on cisplatin-induced oxidative damage and infertility in rat ovaries. The Scientific World Journal. 2013:2013. doi: 10.1155/2013/327240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoyer PB. Damage to ovarian development and function. Cell and tissue research. 2005;322:99–106. doi: 10.1007/s00441-005-1083-y. [DOI] [PubMed] [Google Scholar]
- 74.Mishra B, Ortiz L, Luderer U. Charged iron particles, components of space radiation, destroy ovarian follicles. Human Reproduction. 2016;31:1816–1826. doi: 10.1093/humrep/dew126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertility and sterility. 2010;93:855–864. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 76.Yeh J, Kim B, Liang YJ, Peresie J. Müllerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochemical and biophysical research communications. 2006;348:337–344. doi: 10.1016/j.bbrc.2006.06.195. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, et al. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. Journal of gynecologic oncology. 2013;24:177–185. doi: 10.3802/jgo.2013.24.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ANTUNES LMG, DARIN JDAC, BIANCHI MDLP. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacological Research. 2000;41:405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 79.Wozniak K, Czechowska A, Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chemico-biological interactions. 2004;147:309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. Journal of toxicology. 2012:2012. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rashed LA, Hashem RM, Soliman HM. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomedicine & Pharmacotherapy. 2011;65:474–480. doi: 10.1016/j.biopha.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Jacob KD, Hooten NN, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mechanisms of ageing and development. 2013;134:139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Omar HA, Mohamed WR, Arafa E-SA, Shehata BA, El Sherbiny GA, Arab HH, et al. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacological Reports. 2016;68:349–356. doi: 10.1016/j.pharep.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Chtourou Y, Aouey B, Kebieche M, Fetoui H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chemico-biological interactions. 2015;239:76–86. doi: 10.1016/j.cbi.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 85.Krishna MB, Joseph A, Thomas PL, Dsilva B, Pillai SM, Laloraya M. Impaired arginine metabolism coupled to a defective redox conduit contributes to low plasma nitric oxide in polycystic ovary syndrome. Cellular Physiology and Biochemistry. 2017;43:1880–1892. doi: 10.1159/000484107. [DOI] [PubMed] [Google Scholar]
- 86.Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, Saglam YS. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomedicine & Pharmacotherapy. 2018;102:517–530. doi: 10.1016/j.biopha.2018.03.119. [DOI] [PubMed] [Google Scholar]
- 87.Cortés-Rojo C, R Rodriguez-Orozco A. Importance of oxidative damage on the electron transport chain for the rational use of mitochondria-targeted antioxidants. Mini reviews in medicinal chemistry. 2011;11:625–632. doi: 10.2174/138955711795906879. [DOI] [PubMed] [Google Scholar]
- 88.Tanabe M, Tamura H, Taketani T, Okada M, Lee L, Tamura I, et al. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. Journal of Reproduction and Development. 2014 doi: 10.1262/jrd.2014-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park C, Choi EO, Kim GY, Hwang HJ, Kim BW, Yoo YH, et al. Protective Effect of Baicalein on Oxidative Stress-induced DNA Damage and Apoptosis in RT4-D6P2T Schwann Cells. Int J Med Sci. 2019;16:8–16. doi: 10.7150/ijms.29692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peitsch M, Polzar B, Stephan H, Crompton T, MacDonald HR, Mannherz H, et al. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death) The EMBO journal. 1993;12:371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong Y, Zhao Q, Gu L, Liu C, Wang C. Shenqi Fuzheng Injection Reverses Cisplatin Resistance through Mitofusin-2-Mediated Cell Cycle Arrest and Apoptosis in A549/DDP Cells. Evidence-Based Complementary and Alternative Medicine. 2018:2018. doi: 10.1155/2018/8258246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desagher S, Martinou J-C. Mitochondria as the central control point of apoptosis. Trends in cell biology. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 93.Jiang G-J, Pan L, Huang X-Y, Han M, Wen J-K, Sun F-Z. Expression of HSG is essential for mouse blastocyst formation. Biochemical and biophysical research communications. 2005;335:351–355. doi: 10.1016/j.bbrc.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 94.Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. Journal of Biological Chemistry. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duarte A, Poderoso C, Cooke M, Soria G, Maciel FC, Gottifredi V, et al. Mitochondrial fusion is essential for steroid biosynthesis. PLoS One. 2012;7:e45829. doi: 10.1371/journal.pone.0045829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karadeniz A, Simsek N, Karakus E, Yildirim S, Kara A, Can I, et al. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative medicine and cellular longevity. 2011:2011. doi: 10.1155/2011/981793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pavel CI, Mărghitaş LA, Bobiş O, Dezmirean DS, Şapcaliu A, Radoi I, et al. Biological activities of royal jelly-review. Scientific Papers Animal Science and Biotechnologies. 2011;44:108–118. [Google Scholar]
- 98.Imai M, Qin J, Yamakawa N, Miyado K, Umezawa A, Takahashi Y. Molecular Alterations during Female Reproductive Aging: Can Aged Oocytes Remind Youth? Embryology-Updates and Highlights on Classic Topics: IntechOpen. 2012 [Google Scholar]
- 99.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473 doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 100.Husein M, Kridli R. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Animal reproduction science. 2002;74:45–53. doi: 10.1016/s0378-4320(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 101.El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon. 2007;50:256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 102.You M-M, Chen Y-F, Pan Y-M, Liu Y-C, Tu J, Wang K, et al. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediators of inflammation. 2018:2018. doi: 10.1155/2018/7834381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radical Biology and Medicine. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 104.Landi L, Fiorentini D, Galli MC, Segura-Aguilar J, Beyer RE. DT-Diaphorase maintains the reduced state of ubiquinones in lipid vesicles thereby promoting their antioxidant function. Free Radical Biology and Medicine. 1997;22:329–335. doi: 10.1016/s0891-5849(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 105.Beyer RE, Segura-Aguilar J, Di Bernardo S, Cavazzoni M, Fato R, Fiorentini D, et al. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proceedings of the National Academy of Sciences. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vidoni S, Zanna C, Rugolo M, Sarzi E, Lenaers G. Why mitochondria must fuse to maintain their genome integrity. Antioxidants & redox signaling. 2013;19:379–388. doi: 10.1089/ars.2012.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wakai T, Harada Y, Miyado K, Kono T. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Molecular human reproduction. 2014;20:1090–1100. doi: 10.1093/molehr/gau064. [DOI] [PubMed] [Google Scholar]
- 108.Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, et al. Mff-Dependent Mitochondrial Fission Contributes to the Pathogenesis of Cardiac Microvasculature Ischemia/Reperfusion Injury via Induction of mROS-Mediated Cardiolipin Oxidation and HK 2/VDAC 1 Disassociation-Involved mPTP Opening. Journal of the American Heart Association. 2017;6:e005328. doi: 10.1161/JAHA.116.005328. [DOI] [PMC free article] [PubMed] [Google Scholar]