Abstract
The mussel-inspired properties of dopamine have attracted immense scientific interest for surface modification of nanoparticles due to the high potential of dopamine functional groups to increase the adhesion of nanoparticles to flat surfaces. Here, we report for the first time a novel type of inhibitor-loaded nanocontainer using polydopamine (PDA) as a pH-sensitive gatekeeper for mesoporous silica nanoparticles (MSNs). The encapsulated inhibitor (benzotriazole) was loaded into MSNs at neutral pH, demonstrating fast release in an acidic environment. The self-healing effect of water-borne alkyd coatings doped with nanocontainers was achieved by both on-demand release of benzotriazole during the corrosion process and formation of the complexes between the dopamine functional groups and iron oxides, thus providing dual self-healing protection for the mild steel substrate. The coatings were characterized by electrochemical impedance spectroscopy, visual observations, and confocal Raman microscopy. In all cases, the coatings with embedded benzotriazole-loaded MSNs with PDA-decorated outer surfaces demonstrated superior self-healing effects on the damaged areas. We anticipate that dopamine-based multifunctional gatekeepers can find application potential not only in intelligent self-healing anticorrosive coatings but also in drug delivery, antimicrobial protection, and other fields.
Keywords: silica, dopamine, self-healing, mild steel, corrosion protection
Introduction
Corrosion mostly affects the petroleum, cement, and concrete manufacturing industries, metal processing, water treatment, chemical processing, and power generation equipment. In recent years, there has been a considerable increase in the global anticorrosion coatings’ market. According to a 2018 report from BCC Research (https://www.bccresearch.com/), it should reach $31.0 billion by 2022, up from $23.3 billion in 2017. According to the technical classification, the market for anticorrosion coatings can be divided into solvent-borne, water-borne, powder-based, self-healing, and other paint formulations. Water-borne coatings gained a significant increase of the market share over the past few decades owing to strong regulations related to volatile organic compound emissions from solvent-borne coatings. Addition of self-healing components will endow the water-borne coatings with internal capability to repair corrosion damage by themselves (autonomic) or with the help of outside triggers such as light, heat, or mechanic pressure, which is highly desirable for novel coating products.1−3
Usually, self-healing coatings are impregnated with nanocontainers or microcapsules that encapsulate inhibitors or healing agents.4,5 Mesoporous silica nanoparticles (MSNs) are ideal nanocontainers because both their size and pore volume are easily controlled to optimize the inhibitor encapsulation process.6−9 However, the application of MSNs as delivery tools in self-healing coatings is limited by spontaneous leakage of small molecular inhibitors from mesopores.8 Bioinspired nanovalves prepared from metal precipitates and supramolecular materials have been proven to be applicable gatekeepers for nanocontainers.10−15 Besides the high cost, the major drawback of these nanovalves is their single function, serving only as pH-controlled release gatekeepers. It would be important for MSNs’ gatekeepers to have additional functionality of the pH-controlled release effect. To the best of our knowledge, multifunctional gatekeepers for controlled release have been scarcely reported up to now in the literature.16
Inspired by the adhesive nature of catechols and amines in mussel adhesive proteins, the use of polydopamine (PDA) is one of the most versatile approaches for functionalizing almost all nanomaterial surfaces.17,18 The coating with PDA can be formed in an alkaline pH solution without any external stimuli such as light or heat, and its uniformity depends on PDA diffusion and surface reactivity. Recently, PDA-coated MSNs have been shown as pH-sensitive release systems for drug delivery.19,20 It is noteworthy that the unreacted catechol groups after the oxidative polymerization of dopamine could leave abundant hydroxyl groups on the surface of MSNs, which endow the decorated nanocontainers with increased wettability. This property is crucial for nanocontainers dispersed in water-borne coatings. Moreover, catechol groups have another outstanding function for self-healing coatings. It is reported that the cracked polymer networks can be reconnected by catechol–Fe3+ coordinate bonds.21 Another paper reported that the cation−π interaction modulated by salt is a key mechanism in the mussel adhesion process.22 All these findings aroused our great interest to apply PDA as the pH-release gatekeeper for inhibitor-loaded MSNs. Although many researchers have directly applied PDA into coatings or on metal surfaces for corrosion protection,23−31 there is no evidence of using PDA as a gatekeeper in smart mesoporous nanocontainers. Besides the pH-release control property, we want to explore other PDA functionalities for anticorrosion self-healing coatings.
In this study, we designed a mussel-inspired self-healing coating by application of MCM-48 MSNs as nanocontainers for benzotriazole (BTA), a well-known inhibitor of steel corrosion.32−35 MCM-48 was chosen because of its branched internal three-dimensional mesostructure.36−38 It was used as nanoreservoirs for biocides in our previous work.39 The next step was functionalization of BTA-loaded MSNs with the PDA layer. Hence, we report here a novel design of PDA-decorated MSNs nanocontainers for self-healing coatings. We believe that our work will stimulate other researchers to explore more multifunctional gatekeepers for self-healing and other applications.
Experimental Methods
Materials
Tetraethyl orthosilicate (TEOS, 98%), hexadecyltrimethylammonium bromide (CTAB, 99%), triblock copolymer F127 (Pluronic F127), ammonium hydroxide (32%), ethanol (99.8%), 1H-benzotriazole, hydrochloride dopamine, and tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma-Aldrich, U.K. The investigated mild steel was supplied by Metal Store, U.K.
Synthesis of MSNs
MSNs were synthesized according to the method reported by Kim et al.36 CTAB (0.5 g) and Pluronic F127 (2.05 g) were dissolved in a mixture of deionized water (96 mL) and pure ethanol (43 mL), and ammonium hydroxide (11 mL of 32 wt % solution) was then added to the solution. The solution was stirred for 40 min at 600 rpm to dissolve the solid completely. In the next step, TEOS (1.8 g) was added into the mixture at once. After stirring at 1000 rpm for 24 h at room temperature, the resulting solid was recovered by filtration, washed twice with ethanol, twice with distilled water and dried in air under ambient conditions. The organic template was removed by calcination in a muffle furnace at 550 °C for 6 h after heating up with a ramp of 1 °C/min.
BTA Loading and PDA Modification
Before loading, the MSNs were dried at 120 °C under vacuum to remove water and air from the mesoporous structure. Then, 10 mg of MSNs were directly dispersed in the solution of BTA in ethanol (10 wt %, 10 mL). The mixed solution was placed in a desiccator and evacuated using a vacuum pump at room temperature so BTA could be uploaded into the mesopores of the MSNs. The vacuum cycle was repeated thrice to obtain the maximum loading of the inhibitor (21 wt %). The as-loaded MSNs (MSNs–BTA) were separated by centrifugation and dried for 24 h at 323 K. The loading capacity of BTA is about 30 wt %, as confirmed by thermogravimetric analysis of the MSNs–BTA, which has not been shown in this paper. It is worth mentioning that any post-treatment of the MSNs–BTA in water will lead to leakage of the loaded BTA. Washing of these nanocontainers with ethanol will lead to even faster leakage of BTA due to its high solubility in organic solvents. Ultrasonic dispersion of the MSNs–BTA will cause complete release of the inhibitor within only 1 min. To deposit a PDA layer on the surface of the loaded nanocontainers, MSNs–BTA (30 mg) was dispersed in 30 mL of Tris–HCl buffer (pH 8.5) solution saturated with BTA. Then, 60 mg of hydrochloride dopamine was immediately added. The mixture was stirred for 12 h in the dark. The resulting PDA-coated MSNs–BTA (MSNs–BTA@PDA) nanocontainers were separated by centrifugation (12 000 rpm, 20 min) and washed with water to remove the unpolymerized dopamine.
Coating
The water-based alkyd paint was purchased from Crown Trade, U.K. MSNs–BTA@PDA (2 wt %) was added to the alkyd emulsion and mechanically stirred by a homogenizer. Paints without MSNs (blank coating), with 2 wt % MSNs, and 2 wt % MSNs–BTA were prepared for comparison. The coatings were deposited on mild steel plates using a paint applicator (RK Paint Applicator, U.K.) and dried at room temperature for 48 h. The wet film thickness of all studied coatings was controlled at 60 μm by the paint applicator. The dry thickness of the coating is 5 ± 0.5 μm as measured by a profilometer (AMBios XP-200).
Characterization
The morphology, size, and pore structure of the MSNs were characterized by scanning electron microscopy (SEM, JEOL 7001F), X-ray powder diffraction (XRD, Bruker D8 Venture), nitrogen adsorption isotherms (BET, Quantachrome Instruments), and transmission electron microscopy (TEM, JEOL 2100FCs). The chemical structures of MSNs, MSNs–BTA, and MSNs–BTA@PDA were determined through attenuated total reflection-Fourier transform infrared spectra (ATR-FTIR, Bruker TENSOR II, U.K.) in the wavenumber range of 4000–400 cm–1. UV–vis spectroscopy (Evolution 201 UV–visible spectrophotometer, Thermo Scientific, U.K.) was applied to characterize the release profile of BTA. The characterization method was used according to our previous research.10 To avoid the influence of PDA, the absorbance of BTA at 258 nm was plotted against time. Electrochemical impedance spectroscopy (EIS, Ivium CompactStat, the Netherlands) measurements were used to record the corrosion behavior of the self-healing coating. Mild steel substrates (3 × 6 × 0.3 cm3) with coatings were placed into special cells as shown in the Supporting Information (4 cm2 of the sample area exposed to 0.1 M NaCl electrolyte). The electrolyte was fixed in a glass tube by an O-ring. The electrolyte inside the glass tube was renewed every 24 h. The platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. Both intact and scratch samples were tested. Scratches were made with a circular-edge scalpel by a home-made machine. The scratch area is 10 mm long, 200 μm wide, and 80 μm deep. The open circuit potential was measured for 30 min following EIS measurements with a frequency ranging from 105 to 10–2 Hz using an alternating current signal amplitude of 10 mV. All electrochemical tests were performed three times to guarantee their repeatability and average values with error bars are demonstrated in the experimental results. EIS data were fitted to equivalent cell diagrams using the IviumSoft program. The corrosion products formed on mild steel substrates were characterized by Raman spectra (LabRam Xplora confocal Raman microscope, Horiba Jobin Yvon, France). After 20 days of immersion, the coating was removed by a knife. After the removal, the substrate was washed with acetone several times to completely remove the coating residues.
Results and Discussion
Characterization of MSNs, MSNs–BTA, and MSNs–BTA@PDA
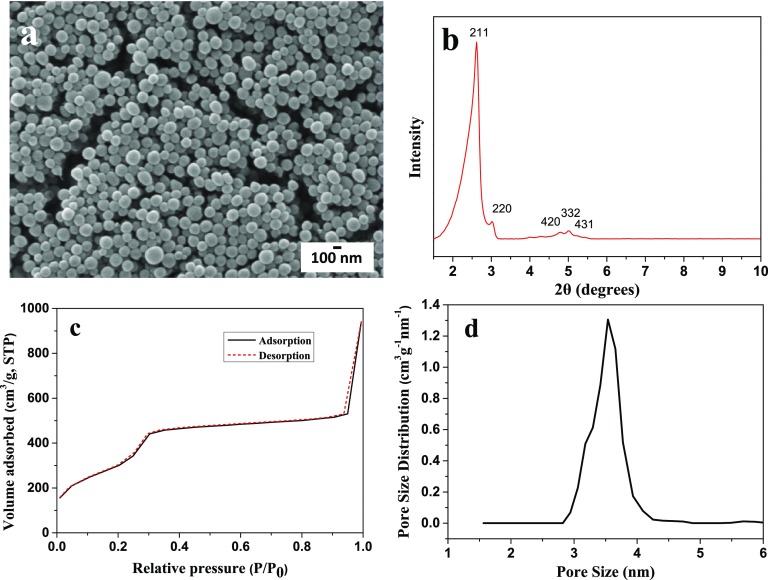
Figure 1a shows the SEM image of MSN. All particles exhibit a spherical shape with a size range between 90 and 170 nm. Figure 1b demonstrates the XRD pattern of MSNs. Five peaks were observed on the XRD pattern, which correspond to the planes (211), (220), (420), (332) and (431) of MCM-48.35 The N2 adsorption–desorption behavior and pore size distribution calculated from the nonlocal density functional theory method40 are shown in Figure 1c,d. A narrow pore size distribution with an average size of 3.5 nm can be observed in Figure 1d.
Figure 1.
SEM image (a), XRD (b), N2 sorption isotherm (c), and pore size distribution (d) of MSNs.
TEM analysis was performed to observe the nanoparticle inner structure before and after encapsulation. It can be seen from Figure 2a that the well-ordered pores are extending over the whole structure of MSNs. After the encapsulation and PDA surface decoration, the diameter of MSNs–BTA@PDA is ∼10 % bigger than that of initial MSNs (Figure 2b). A rough PDA layer can be clearly seen from the surface of MSNs–BTA@PDA.
Figure 2.
TEM image of MSNs (a) and MSNs–BTA@PDA (b). The arrow indicates the PDA layer.
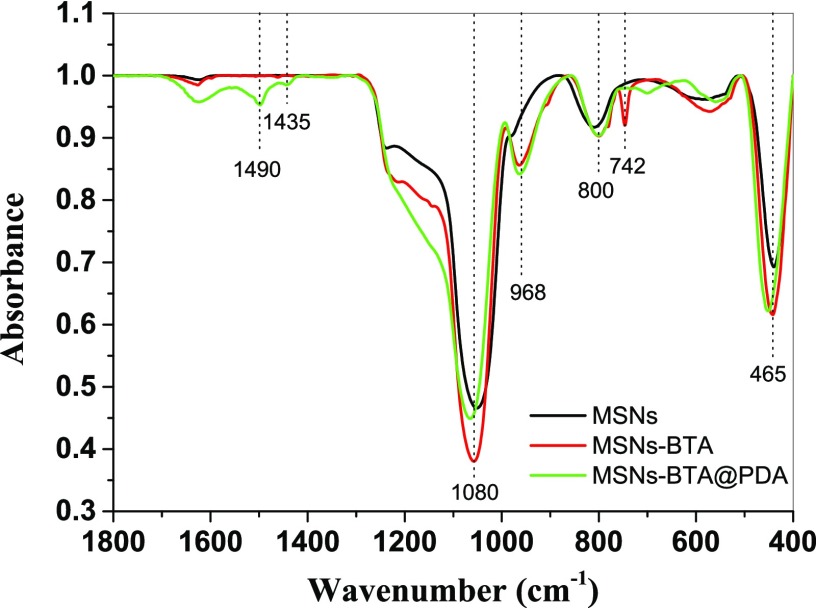
Figure 3 shows the ATR-FTIR spectra of MSNs, MSNs–BTA, and MSNs–BTA@PDA samples. The absorption peaks at 465, 800, and 1080 cm–1 correspond to Si–O–Si bending vibration, Si–O–Si symmetric stretching, and Si–O asymmetric vibration, respectively. All these peaks are characteristic of SiO2.39 There is an absorption peak at 742 cm–1 for MSNs–BTA, which represents the C–H in-plane bending vibrations of the BTA benzene ring.41 Furthermore, the peaks at 1435 and 1490 cm–1 on MSNs–BTA@PDA samples could be assigned to the skeletal vibration of aromatic double bonds, which indicates the presence of PDA.42
Figure 3.
ATR-FTIR spectra of MSNs (black), MSNs–BTA (red), and MSNs–BTA@PDA (green).
The release profiles of BTA from MSNs–BTA and MSNs–BTA@PDA nanocontainers are demonstrated in Figure 4. Fast release can be observed from the MSNs–BTA nanocontainers even at pH 7 (black line). Without the polydopamine layer, nearly 60% of the encapsulated BTA was released from the mesoporous nanocontainers within the first 100 min. When we embedded these nanocontainers into the anticorrosion coatings, the BTA would start to leak from the first minute of immersion into paint formulation. This leads to the loss of a large amount of inhibitor during the coating application and curing. In the case of MSNs–BTA@PDA, BTA release was suppressed by PDA at pH 7 (red line) as seen in Figure 4. At the same time, changing the pH value to the acidic region results in BTA release: 30% of BTA at pH 5 and 50% of BTA at pH 2 were released from the nanocontainers in the first 100 min of exposure, respectively. At neutral pH, the PDA coating anchors functional ligands on the surface of MSNs via physical bonds (hydrogen bond or van der Waals force) or chemical bonds (Michael addition or Schiff base reaction).18 At low pH, the catechol groups of PDA, the inhibitor molecules, and the silica particles have the same charge, which leads to electrostatic repulsion forces inside the nanocontainers. The PDA coatings were partially peeled off from the surface of MSNs in acidic media.19,20 Therefore, more BTA was released from the channels of the MSNs at low pH. Corrosion activity, which leads to local changes of pH, will trigger the release of BTA from MSNs–BTA@PDA nanocontainers.
Figure 4.
Release profile of BTA from the MSNs–BTA and MSNs–BTA@PDA nanocontainers at different pH values.
Characterization of Self-Healing Coatings
Ultramicrotomed SEM analysis of coatings with nanocontainers was performed to study the dispersion of the nanocontainers inside the coating. It should be noted that the samples used for the cross section observation were coated on the glass plate. So their thicknesses were different from the samples used for corrosion tests. A large amount of aggregated MSNs was observed in Figure 5a, which indicates the poor dispersity of pure MSNs in the alkyd coating matrix. In contrast, a homogeneous distribution of MSNs–BTA@PDA can be observed from Figure 5b. It could be due to the effect of −OH groups of PDA reacting with the coating matrix, thus enhancing the nanocontainer dispersion in coatings. In addition, no bubbles or cracks can be seen in Figure 5b, which indicates the intact structure of the coating.
Figure 5.
Ultramicrotomed SEM images of the coating with MSNs (a) and MSNs–BTA@PDA (b).
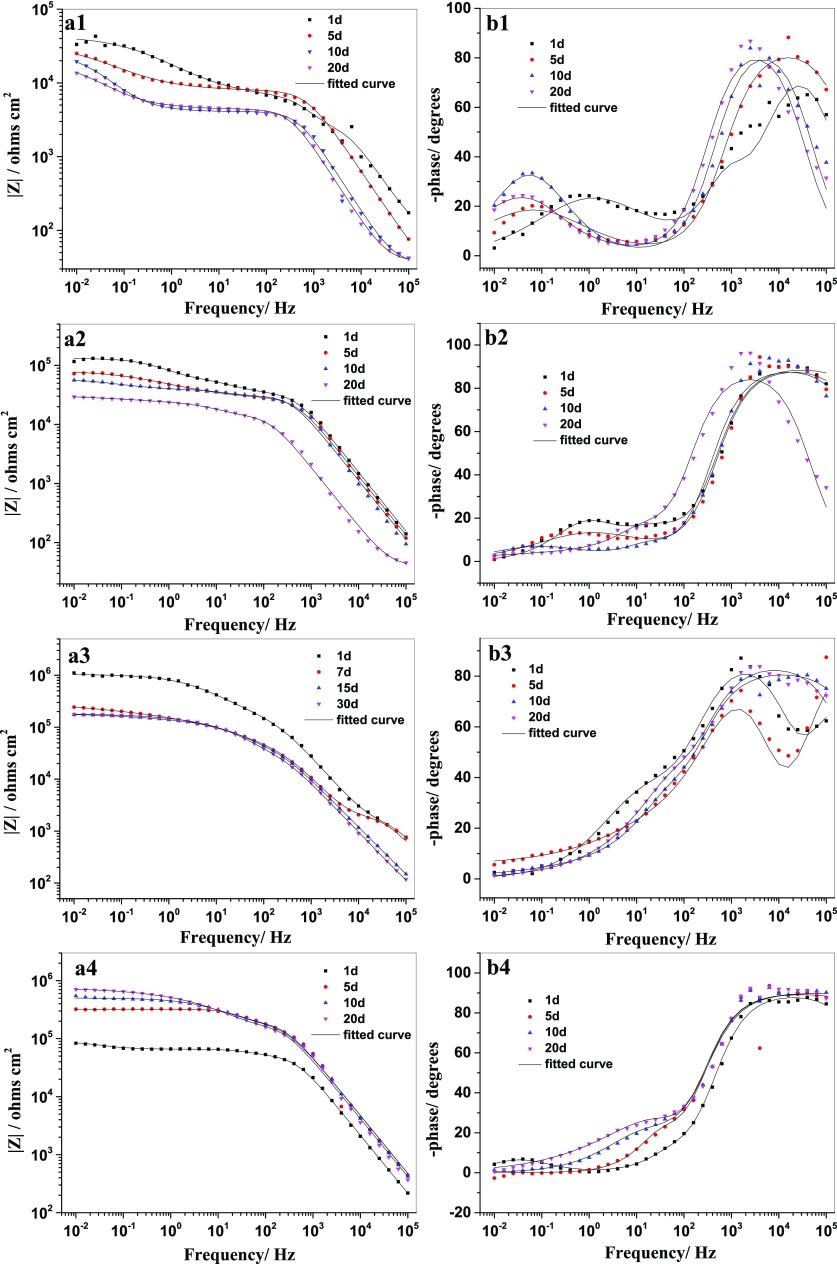
Artificial defects with 0.2 × 10 mm2 size were made on the coatings to induce the corrosion process. EIS measurements were carried out to evaluate their self-healing performance in 0.1 M NaCl. The impedance value of the coating with empty MSNs is a little higher than that of the blank coating. The Bode plots obtained from blank coating and coating with MSNs are quite similar (Figure 6a1,a2), which illustrates that the MSNs entrapped in the coating serves only as nanocontainers for the corrosion inhibitor. With the increase of immersion time, the corrosive species gradually penetrate into the scratch of the coatings, resulting in a steady decrease of the impedance modulus of the blank coating and coating with empty MSNs (Figure 6b1,b2). Figure 6a3 exhibits the highest impedance modulus (1.0 × 106 Ω cm2) after 1 day immersion for the coating doped with MSNs–BTA. The fast release of the inhibitor could suppress the development of corrosion just after immersion of the sample. However, the |Z| shows a sharp drop from 1.0 × 106 to 2.4 × 105 Ω cm2 after 5 days of immersion. The release profile (Figure 4) proves that the leakage of BTA from the MSNs–BTA could occur in neutral solution. The BTA could directly release in the whole testing area. The direct doping of the coating with the corrosion inhibitor could affect the adhesion between steel and coating or have a negative influence on the coating matrix,8 which decreases the corrosion protection of the coating. In the case of the coating with MSNs–BTA@PDA, the |Z| remains lower than the coating with MSNs and MSNs–BTA after 1 day immersion. This is probably due to the remaining hydroxyl groups on the surface of MSNs–BTA@PDA, which can promote water penetration into the coating. However, the coating with MSNs–BTA@PDA shows a stable resistance increase on a long term from 8.3 × 104 to 7.0 × 105 Ω cm2 after 20 days of immersion (Figure 6a4). Such a self-healing action was not observed for other investigated coatings. The on-demand release of BTA from the nanocontainers provides durable protection of the scratched area. Figure 7 shows the electrochemical impedance data at 0.01 Hz after 20 days of immersion, which reflects the overall corrosion resistance of the coatings.
Figure 6.
Bode plots of the scratched coatings after 20 days of immersion in 0.1 M NaCl: blank coating (a1, b1), coating with MSNs (a2, b2), with MSNs–BTA (a3, b3), and with MSNs–BTA@PDA (a4, b4).
Figure 7.
Impedance modulus |Z| measured at 0.01 Hz during immersion in 0.1 M NaCl for blank coating (1), coating with MSNs (2), with MSNs–BTA (3), and with MSNs–BTA@PDA (4).
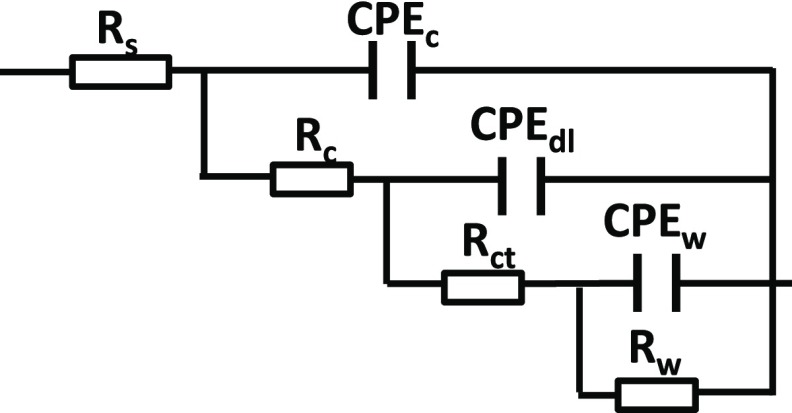
The electrical equivalent circuit shown in Figure 8 was used to analyze the impedance data. The model circuit demonstrated an excellent fitting quality as shown in Figure 6. The obtained fitting parameters for the coating response are depicted in Table 1. The value of CPEdl (double layer capacitance) for coatings with MSNs–BTA@PDA is lower than that for other coatings, which reflects a good adhesion of the coating to the metal.
Figure 8.
Electrical equivalent circuit used to fit the impedance data: Rs is the solution resistance, Rc is the coating resistance, CPEc is the constant phase element of coating capacitance, Rct is the charge transfer resistance, CPEdl stands for the constant phase element of double layer capacitance, Rw and CPEw were used to describe the mass transport.
Table 1. Values of Important Electrochemical Parameters: the Fitting Model Delivers a Good Fit Quality (χ2 < 0.01); All Fitting were Performed as a Result of Three Parallel EIS Experiments.
| coatings | time (day) | Rs (Ω cm2) | CPEc (μF cm–2) | n | Rc (kΩ cm2) | CPEdl (μF cm–2) | n | Rct (kΩ cm2) |
|---|---|---|---|---|---|---|---|---|
| blank | 1 | 23 | 0.16 | 0.90 | 7.39 | 22.90 | 0.83 | 36.59 |
| 5 | 26 | 0.35 | 0.97 | 4.36 | 150.60 | 0.83 | 20.01 | |
| 10 | 40 | 0.87 | 0.94 | 3.95 | 246.30 | 0.88 | 19.63 | |
| 20 | 38 | 1.19 | 0.96 | 3.02 | 350.90 | 0.79 | 14.63 | |
| with MSN | 1 | 8 | 0.10 | 0.97 | 34.85 | 6.88 | 0.83 | 43.82 |
| 5 | 14 | 0.12 | 0.94 | 30.82 | 26.68 | 0.88 | 31.58 | |
| 10 | 16 | 0.14 | 0.90 | 27.99 | 28.15 | 0.85 | 23.40 | |
| 20 | 23 | 0.79 | 0.90 | 12.53 | 30.71 | 0.89 | 20.61 | |
| with MSNs–BTA | 1 | 37 | 0.02 | 0.84 | 54.96 | 0.04 | 0.88 | 187.60 |
| 5 | 19 | 0.33 | 0.83 | 43.87 | 0.75 | 0.86 | 57.63 | |
| 10 | 21 | 0.93 | 0.95 | 22.21 | 2.68 | 0.79 | 11.77 | |
| 20 | 38 | 1.84 | 0.87 | 10.35 | 10.89 | 0.95 | 10.95 | |
| with MSNs–BTA@PDA | 1 | 22 | 0.07 | 0.91 | 51.97 | 5.42 | 0.91 | 23.63 |
| 5 | 11 | 0.03 | 0.98 | 170.70 | 2.08 | 0.89 | 31.46 | |
| 10 | 24 | 0.04 | 0.94 | 182.50 | 0.04 | 0.96 | 250.80 | |
| 20 | 18 | 0.04 | 0.99 | 196.80 | 0.03 | 0.98 | 374.10 |
Figure 9 shows the optical images of coatings after 20 days of immersion in 0.1 M NaCl solution. Massive corrosion products are observed in the scratched area of the coatings in Figure 9a,b for blank coating and coating with MSNs, which indicates their poor corrosion resistance. A lower amount of rust is found in the image of the coating with MSNs–BTA (Figure 9c). However, a large area of coating was delaminated from the metal substrate, showing weak adhesion between the coating and substrate. This is in accordance with the impedance results, which suggests that the fast release of BTA decreases the adhesion between the coating and steel substrate. In the case of the coating with MSNs–BTA@PDA (Figure 9d), no corrosion products and no delamination were found in the whole immersion area. Salt spray tests were taken to achieve a better knowledge of the failure process. Images of mild steel and mild steel coated with all kinds of coatings before and after 288 h of salt spray tests are shown in Figure 10. More detailed salt spray test images at different time periods are shown in Figure S2. Better performances were obtained for the mild steel coated with alkyd paint with MSNs–BTA@PDA, which proves their self-healing functionality and better barrier properties. Although all of the coatings failed after the 288 h salt spray test, the mild steel coated with alkyd paint with MSNs–BTA@PDA shows less rust on the surface than other samples. The coating with MSNs started to blister and bubble after 24 h, whereas the coating with MSNs–BTA@PDA started to blister and bubble after 72 h (see Figure S2). These results confirm the improved anticorrosion properties of the coating with MSNs–BTA@PDA nanocontainers.
Figure 9.
Images of the blank coating (a), coatings with MSNs (b), MSNs–BTA (c), and MSNs–BTA@PDA (d) after 20 days of immersion in 0.1 M NaCl solution.
Figure 10.
Images of mild steel (a), blank coating (b), coatings with MSNs (c), MSNs–BTA (d), and MSNs–BTA@PDA (e) before and after 288 h of salt spray test.
Figure 11a,b shows the confocal Raman images of the coatings after immersion for 20 days in 0.1 M NaCl. These images were taken by a Raman microscope to observe the rust and complex more clearly. Rust (yellow color) could be found in the unscratched area of the blank coating (Figure 11a). Figure 11b shows some dark complexes inside the micropores of the coating with MSNs–BTA@PDA nanocontainers. To eliminate the signal interference of the coating matrix, the Raman spectra were performed directly on the mild steel substrate after careful removal of the coatings. No obvious peak can be seen from the blank sample (line 1 in Figure 11c), which indicates that no rust or oxide were formed on the surface of mild steel before immersion. Three peaks are observed at 532, 654, and 1300 cm–1 for the steel coated with a blank coating after immersion (line 2 in Figure 11c), which are assigned to γ-FeOOH and α-Fe2O3.43 The Raman spectrum of mild steel (line 3 in Figure 11c) beneath the self-healing coatings with MSNs–BTA@PDA is different from the blank sample. Raman peaks between 470 and 670 cm–1 are chelate peaks between Fe3+ and the PDA catechol group.44,45 The other peaks at 812, 1272, 1330, 1483, and 1566 cm–1 are characteristic Raman vibrations of dopamine.46 Raman spectra confirm the existence of complexes between the steel substrate and PDA.
Figure 11.
Images of the blank coating (a) and coating with MSNs–BTA@PDA nanocontainers (b) taken by a confocal Raman microscope after 20 days of immersion in 0.1 M NaCl; (c) Raman spectra of (1) mild steel before immersion, (2) mild steel after removal of the blank coating or coating with MSNs–BTA@PDA (3) after 20 days of immersion.
Conclusions
In this paper, we designed a novel pH-sensitive inhibitor release system by using polydopamine (PDA) as the gatekeeper for mesoporous silica nanocontainers (MSNs) of 90–170 nm size loaded with a corrosion inhibitor benzotriazole (BTA). TEM and ATR-FTIR results confirmed the presence of a PDA layer on the surface of nanoparticles. Release profiles at different pH values confirm the pH-responsive kinetics of MSNs–BTA@PDA nanocontainers. The encapsulated inhibitor was trapped inside the MSNs at neutral pH while being rapidly released in an acidic environment. We embedded MSNs–BTA@PDA nanocontainers into a water-based alkyd coating and painted it on the surface of mild steel (Figure 12). A stable increase of the impedance modulus at 0.01 Hz for coatings with 2 wt % of incorporated MSNs–BTA@PDA after 20 days of immersion in 0.1 M NaCl solution demonstrated high corrosion resistance and self-healing effects. Raman spectra confirmed the existence of complexes between the steel substrate and PDA component of MSNs–BTA@PDA nanocontainers. PDA makes the complex with corrosion products in the micropores of water-borne coatings as shown in Figure 12. The PDA layer not only controls the release of the inhibitor but also serves as a chelating agent to form protective complexes with corrosion products. Furthermore, the outer surface of MSNs–BTA@PDA is functionalized with −OH groups from PDA for better dispersion in water-borne coatings. We hope that our work will inspire other researchers working in the field of corrosion protection to explore the multifunctional properties of dopamine as a gatekeeper for other types of core–shell nanocontainers.
Figure 12.
Self-healing mechanism of mussel-inspired coatings. In the scratched area, the released BTA creates a protective film on the surface of mild steel. The detached dopamine also forms coordination complexes, which provide an additional protective effect for mild steel.
Acknowledgments
Dr B.Q. was supported by the Shandong Provincial Government Scholarship (China) to visit the University of Liverpool. The work was financially supported by the Research Foundation for Distinguished Scholars of Qingdao Agricultural University (663-1115017), Shandong Province Natural Science Foundation, China (ZR2017BD038, ZR2016DM21), and Natural Science Foundation of China (51801110), ERC Projects Enercapsule (647969) and Enerpaint (767173).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.8b21197.
Schematic diagram of the cell for electrochemical impedance spectroscopy and images of salt spray tests (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hua Z.; Hongxia W.; Haitao N.; Yan Z.; Zhiguang X.; Tong L. A Waterborne Coating System for Preparing Robust, Self-healing, Superamphiphobic Surfaces. Adv. Funct. Mater. 2017, 27, 1604261 10.1002/adfm.201604261. [DOI] [Google Scholar]
- Li J.; Feng Q.; Cui J.; Yuan Q.; Qiu H.; Gao S.; Yang J. Self-assembled Graphene Oxide Microcapsules in Pickering Emulsions for Self-healing Waterborne Polyurethane Coatings. Compos. Sci. Technol. 2017, 151, 282–290. 10.1016/j.compscitech.2017.07.031. [DOI] [Google Scholar]
- Wan T.; Chen D. Synthesis and Properties of Self-healing Waterborne Polyurethanes Containing Disulfide Bonds in the Main Chain. J. Mater. Sci. 2017, 52, 197–207. 10.1007/s10853-016-0321-x. [DOI] [Google Scholar]
- Shchukin D. G.; Zheludkevich M.; Yasakau K.; Lamaka S.; Ferreira M. G. S.; Möhwald H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. 10.1002/adma.200502053. [DOI] [Google Scholar]
- Shchukin D. G.; Grigoriev D. O.; Mohwald H. Application of Smart Organic Nanocontainers in Feedback Active Coatings. Soft Matter 2010, 6, 720–725. 10.1039/B918437F. [DOI] [Google Scholar]
- Hollamby M. J.; Fix D.; Dönch I.; Borisova D.; Möhwald H.; Shchukin D. Hybrid Polyester Coating Incorporating Functionalized Mesoporous Carriers for the Holistic Protection of Steel Surfaces. Adv. Mater. 2011, 23, 1361–1365. 10.1002/adma.201003035. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Schenderlein M.; Huang X.; Brownbill N. J.; Blanc F.; Shchukin D. Influence of Functionalization of Nanocontainers on Self-Healing Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2015, 7, 22756–22766. 10.1021/acsami.5b08028. [DOI] [PubMed] [Google Scholar]
- Borisova D.; Möhwald H.; Shchukin D. G. Mesoporous Silica Nanoparticles for Active Corrosion Protection. ACS Nano 2011, 5, 1939–1946. 10.1021/nn102871v. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Huang X.; Schenderlein M.; Borisova D.; Cao R.; Möhwald H.; Shchukin D. Self-Healing and Antifouling Multifunctional Coatings Based on pH and Sulfide Ion Sensitive Nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. 10.1002/adfm.201203180. [DOI] [Google Scholar]
- Zheng Z.; Huang X.; Schenderlein M.; Moehwald H.; Xu G. K.; Shchukin D. G. Bioinspired Nanovalves With Selective Permeability and pH Sensitivity. Nanoscale 2015, 7, 2409–2416. 10.1039/C4NR06378C. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Huang X.; Shchukin D. A Cost-effective pH-sensitive Release System for Water Source pH Detection. Chem. Commun. 2014, 50, 13936–13939. 10.1039/C4CC05597G. [DOI] [PubMed] [Google Scholar]
- Chen T.; Fu J. J. An Intelligent Anticorrosion Coating Based on pH-responsive Supramolecular Nanocontainers. Nanotechnology 2012, 23, 505705 10.1088/0957-4484/23/50/505705. [DOI] [PubMed] [Google Scholar]
- Chen T.; Fu J. J. pH-responsive Nanovalves Based on Hollow Mesoporous Silica Spheres for Controlled Release of Corrosion Inhibitor. Nanotechnology 2012, 23, 235605 10.1088/0957-4484/23/23/235605. [DOI] [PubMed] [Google Scholar]
- Ding C.; Xu J.; Tong L.; Gong G.; Jiang W.; Fu J. Design and Fabrication of a Novel Stimulus-Feedback Anticorrosion Coating Featured by Rapid Self-Healing Functionality for the Protection of Magnesium Alloy. ACS Appl. Mater. Interfaces 2017, 9, 21034–21047. 10.1021/acsami.7b06347. [DOI] [PubMed] [Google Scholar]
- Fu J.; Chen T.; Wang M.; Yang N.; Li S.; Wang Y.; Liu X. Acid and Alkaline Dual Stimuli-Responsive Mechanized Hollow Mesoporous Silica Nanoparticles as Smart Nanocontainers for Intelligent Anticorrosion Coatings. ACS Nano 2013, 7, 11397–11408. 10.1021/nn4053233. [DOI] [PubMed] [Google Scholar]
- Ding C.; Tong L.; Fu J. Quadruple Stimuli-Responsive Mechanized Silica Nanoparticles: A Promising Multifunctional Nanomaterial for Diverse Applications. Chem. - Eur. J. 2017, 23, 15041–15045. 10.1002/chem.201704245. [DOI] [PubMed] [Google Scholar]
- Ryu J. H.; Messersmith P. B.; Lee H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.; Gao Y.; Wang L.; Liu G.; Chen Y.; Wang T.; Tao W.; Mei L.; Huang L.; Zeng X. Polydopamine-based Surface Modification of Mesoporous Silica Nanoparticles as pH-sensitive Drug Delivery Vehicles for Cancer Therapy. J. Colloid Interface Sci. 2016, 463, 279–287. 10.1016/j.jcis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Zeng X.; Tao W.; Liu G.; Mei L. Polydopamine-based Surface Modification of Copolymeric Nanoparticles as a Targeted Drug Delivery System for Cancer Therapy. J. Controlled Release 2017, 259, e150–e151. 10.1016/j.jconrel.2017.03.303. [DOI] [Google Scholar]
- Xia N. N.; Xiong X. M.; Wang J.; Rong M. Z.; Zhang M. Q. A Seawater Triggered Dynamic Coordinate Bond and its Application for Underwater Self-healing and Reclaiming of Lipophilic Polymer. Chem. Sci. 2016, 7, 2736–2742. 10.1039/C5SC03483C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Yoo H. Y.; Huang J.; Lee Y.; Park S.; Park Y.; Jin S.; Jung Y. M.; Zeng H.; Hwang D. S.; Jho Y. Salt Triggers the Simple Coacervation of an Underwater Adhesive When Cations Meet Aromatic π Electrons in Seawater. ACS Nano 2017, 11, 6764–6772. 10.1021/acsnano.7b01370. [DOI] [PubMed] [Google Scholar]
- Chen S.; Chen Y.; Lei Y.; Yin Y. Novel Strategy in Enhancing Stability and Corrosion Resistance for Hydrophobic Functional Films on Copper Surfaces. Electrochem. Commun. 2009, 11, 1675–1679. 10.1016/j.elecom.2009.06.021. [DOI] [Google Scholar]
- Chen Y.; Zhao S.; Chen M.; Zhang W.; Mao J.; Zhao Y.; Maitz M. F.; Huang N.; Wan G. Sandwiched Polydopamine (PDA) Layer for Titanium Dioxide (TiO2) Coating on Magnesium to Enhance Corrosion Protection. Corros. Sci. 2015, 96, 67–73. 10.1016/j.corsci.2015.03.020. [DOI] [Google Scholar]
- Ghelichkhah Z.; Sharifi-Asl S.; Farhadi K.; Banisaied S.; Ahmadi S.; Macdonald D. D. L-cysteine/polydopamine Nanoparticle-coatings for Copper Corrosion Protection. Corros. Sci. 2015, 91, 129–139. 10.1016/j.corsci.2014.11.011. [DOI] [Google Scholar]
- Ou J.; Wang J.; Zhou J.; Liu S.; Yu Y.; Pang X.; Yang S. Construction and Study on Corrosion Protective Property of Polydopamine-based 3-layer Organic Coatings on Aluminum Substrate. Prog. Org. Coat. 2010, 68, 244–247. 10.1016/j.porgcoat.2010.01.004. [DOI] [Google Scholar]
- Wang C.; Shen J.; Xie F.; Duan B.; Xie X. A Versatile Dopamine-induced Intermediate Layer for Polyether Imides (PEI) Deposition on Magnesium to Render Robust and High Inhibition Performance. Corros. Sci. 2017, 122, 32–40. 10.1016/j.corsci.2017.03.021. [DOI] [Google Scholar]
- Wang N.; Zhang Y.; Chen J.; Zhang J.; Fang Q. Dopamine Modified Metal-organic Frameworks on Anti-corrosion Properties of Waterborne Epoxy Coatings. Prog. Org. Coat. 2017, 109, 126–134. 10.1016/j.porgcoat.2017.04.024. [DOI] [Google Scholar]
- Wei N.; Jiang Y.; Ying Y.; Guo X.; Wu Y.; Wen Y.; Yang H. Facile Construction of a Polydopamine-based Hydrophobic Surface for Protection of Metals Against Corrosion. RSC Adv. 2017, 7, 11528–11536. 10.1039/C7RA00267J. [DOI] [Google Scholar]
- Singer F.; Schlesak M.; Mebert C.; Höhn S.; Virtanen S. Corrosion Properties of Polydopamine Coatings Formed in One-Step Immersion Process on Magnesium. ACS Appl. Mater. Interfaces 2015, 7, 26758–26766. 10.1021/acsami.5b08760. [DOI] [PubMed] [Google Scholar]
- Zain N. M.; Hussain R.; Abdul Kadir M. R. Quinone-rich Polydopamine Functionalization of Yttria Stabilized Zirconia for Apatite Biomineralization: The Effects of Coating Temperature. Appl. Surf. Sci. 2015, 346, 317–328. 10.1016/j.apsusc.2015.04.007. [DOI] [Google Scholar]
- Liang L. G.; Matthias S.; Yongjun M.; Helmuth M.; Shchukin D. G. Monodisperse Polymeric Core–Shell Nanocontainers for Organic Self-Healing Anticorrosion Coatings. Adv. Mater. Interfaces 2014, 1, 1300019 10.1002/admi.201300019. [DOI] [Google Scholar]
- Cao P. G.; Yao J. L.; Zheng J. W.; Gu R. A.; Tian Z. Q. Comparative Study of Inhibition Effects of Benzotriazole for Metals in Neutral Solutions As Observed with Surface-Enhanced Raman Spectroscopy. Langmuir 2002, 18, 100–104. 10.1021/la010575p. [DOI] [Google Scholar]
- Mennucci M. M.; Banczek E. P.; Rodrigues P. R. P.; Costa I. Evaluation of Benzotriazole as Corrosion Inhibitor for Carbon Steel in Simulated Pore Solution. Cem. Concr. Compos. 2009, 31, 418–424. 10.1016/j.cemconcomp.2009.04.005. [DOI] [Google Scholar]
- Gomma G. K. Corrosion Inhibition of Steel by Benzotriazole in Sulphuric Acid. Mater. Chem. Phys. 1998, 55, 235–240. 10.1016/S0254-0584(98)00043-1. [DOI] [Google Scholar]
- Kim T.-W.; Chung P.-W.; Lin V. S. Y. Facile Synthesis of Monodisperse Spherical MCM-48 Mesoporous Silica Nanoparticles with Controlled Particle Size. Chem. Mater. 2010, 22, 5093–5104. 10.1021/cm1017344. [DOI] [Google Scholar]
- Schumacher K.; Ravikovitch P. I.; Du Chesne A.; Neimark A. V.; Unger K. K. Characterization of MCM-48 Materials. Langmuir 2000, 16, 4648–4654. 10.1021/la991595i. [DOI] [Google Scholar]
- Schumacher K.; Grün M.; Unger K. K. Novel Synthesis of Spherical MCM-48. Microporous Mesoporous Mater. 1999, 27, 201–206. 10.1016/S1387-1811(98)00254-6. [DOI] [Google Scholar]
- Michailidis M.; Sorzabal-Bellido I.; Adamidou E. A.; Diaz-Fernandez Y. A.; Aveyard J.; Wengier R.; Grigoriev D.; Raval R.; Benayahu Y.; D’Sa R. A.; Shchukin D. Modified Mesoporous Silica Nanoparticles with a Dual Synergetic Antibacterial Effect. ACS Appl. Mater. Interfaces 2017, 9, 38364–38372. 10.1021/acsami.7b14642. [DOI] [PubMed] [Google Scholar]
- Landers J.; Gor G. Y.; Neimark A. V. Density Functional Theory Methods for Characterization of Porous Materials. Colloids Surf., A 2013, 437, 3–32. 10.1016/j.colsurfa.2013.01.007. [DOI] [Google Scholar]
- Barreto L. S.; Tokumoto M. S.; Guedes I. C.; Melo H. G. d; Amado F. D. R.; Capelossi V. R.. Evaluation of the Anticorrosion Performance of Peel Garlic Extract as Corrosion Inhibitor for ASTM 1020 Carbon Steel in Acidic Solution Matéria 2017, 22, e11852. 10.1590/s1517-707620170003.0186. [DOI]
- Müller M.; Keßler B. Deposition from Dopamine Solutions at Ge Substrates: An in Situ ATR-FTIR Study. Langmuir 2011, 27, 12499–12505. 10.1021/la202908b. [DOI] [PubMed] [Google Scholar]
- de la Fuente D.; Alcántara J.; Chico B.; Díaz I.; Jiménez J. A.; Morcillo M. Characterisation of Rust Surfaces Formed on Mild Steel Exposed to Marine Atmospheres Using XRD and SEM/Micro-Raman Techniques. Corros. Sci. 2016, 110, 253–264. 10.1016/j.corsci.2016.04.034. [DOI] [Google Scholar]
- Holten-Andersen N.; Harrington M. J.; Birkedal H.; Lee B. P.; Messersmith P. B.; Lee K. Y. C.; Waite J. H. pH-induced Metal-ligand Cross-links Inspired by Mussel Yield Self-healing Polymer Networks with Near-covalent Elastic Moduli. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 2651–2655. 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalchyk W. K.; Davis K. L.; Morris M. D. Surface-enhanced Resonance Raman Spectroscopy of Iron-dopamine Complexes. Spectrochim. Acta, Part A 1995, 51, 145–151. 10.1016/0584-8539(94)00153-3. [DOI] [Google Scholar]
- Ciubuc J. D.; Bennet K.; Qiu C.; Alonzo M.; Durrer W.; Manciu F. Raman Computational and Experimental Studies of Dopamine Detection. Biosensors 2017, 7, 43. 10.3390/bios7040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.