Abstract
This is a case report of a 67-year-old patient with castration resistant metastatic prostate cancer who developed an immune-mediated large vessel vasculitis following treatment with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1).
Keywords: prostate cancer, vasculitis, unwanted effects / adverse reactions, immunology, cancer intervention
Background
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies which are being increasingly used as systemic anticancer therapies. They function by blockade of inhibitory pathways, which limit T-cell activity, therefore, allowing increased T-cell activity and antitumour immune responses.
Although ICIs have revolutionised the management of many patients with advanced malignancies, they are associated with a broad spectrum of immune-related adverse events (IRAEs), which can occur in any tissue or organ, during or after treatment. The most common IRAEs involve the skin, colon, endocrine organs and lungs.1
The incidence and prevalence of rheumatic IRAEs are not well reported.2 There are a small number of cases of ICI-mediated large vessel vasculitides described in the published literature, including one case of ICI-induced periaortitis and only one other reported case of ICI-induced aortitis.3–5
Case presentation
In April 2014, a 62-year-old man with a medical history of chronic lower back pain was diagnosed with metastatic prostate cancer with a presenting prostate specific antigen (PSA) of 1280 and retroperitoneal and paraaortic lymphadenopathy. He was commenced on androgen deprivation therapy with a good PSA response. After 20 months, he developed castration-resistant prostate cancer with a rising PSA and a bone scan which demonstrated small volume osteoblastic metastases. He was, therefore, commenced on the androgen receptor antagonist enzalutamide. Ten months later, in October 2018, CT imaging showed evidence of disease progression in conjunction with a rise in PSA, and he was commenced on ipilimumab and nivolumab within the NEPTUNES clinical trial (ClinicalTrials.gov Identifier: NCT03061539).
He became unwell with recurrent fevers, hypotension, breathlessness and a C reactive protein (CRP) > 320 mg/L on day 12 following the first cycle of combination treatment and was treated empirically with broad-spectrum antibiotics, although no source of infection was detected. On day 20, he developed lethargy, Common Terminology Criteria for Adverse Events (CTCAE) grade 3 diarrhoea and grade 2 immunotherapy-associated thyroiditis. He was admitted to hospital for treatment of these IRAEs with intravenous methylprednisolone and had a flexible sigmoidoscopy, the biopsies of which confirmed mild inflammation consistent with immunotherapy-associated colitis. He was subsequently discharged from hospital with a reducing regimen of prednisolone starting at 60 mg/day. On resolution of his symptoms, he received one further cycle of single agent nivolumab but did not restart the ipilimumab as it was thought to be the most likely cause of the grade 3 colitis.
The prednisolone was tapered and he initially remained well. However, 4 weeks after stopping steroids (4 months following the first cycle of combination treatment), he complained of right-sided chest and shoulder pain, and breathlessness.
Investigations
Blood tests revealed a raised CRP and erythrocyte sedimentation rate (ESR). A CT pulmonary angiogram was performed, which did not show evidence of a pulmonary embolus. It did, however, show new thickening in the wall of the thoracic aorta, which had not been present on the baseline CT, and was suspicious for vasculitis. Subsequent 18F-fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography (PET/CT) confirmed extensive large vessel vasculitis and enthesopathy in the spine (figure 1). FDG uptake was observed to involve the entire aorta, but was most marked at the proximal/mid aorta. FDG uptake was also demonstrated in the major branches of the aorta, including the subclavian arteries, axillary arteries, and proximal carotid and proximal vertebral arteries bilaterally. There was also some uptake at the common iliac arteries bilaterally, with sparing of the internal and external iliac arteries. Reassuringly, ECG and echocardiogram were normal and troponin was not raised. Immunological tests, including antineutrophil cytoplasmic antibody, antinuclear antibodies and complement, were negative.
Figure 1.
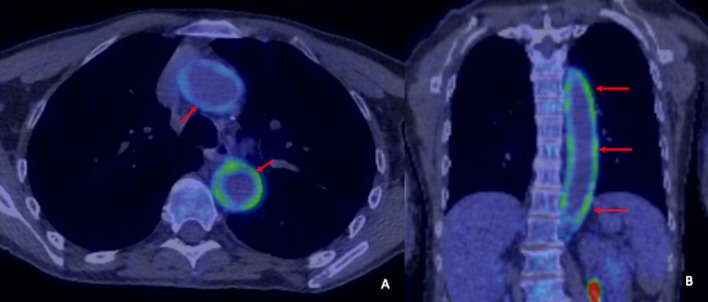
18F-FDG-PET-CT. Axial (A) and coronal (B) reformats at diagnosis demonstrate murally based 18F-FDG uptake throughout the aorta (red arrows), in keeping with large vessel vasculitis.
Treatment
The patient was given 3 days of intravenous methylprednisolone and had symptomatically improved after one dose. This was then converted to oral prednisolone at a dose of 60 mg/day. ESR, which had been raised at 130, decreased to 5. Similarly, CRP decreased from 200 to 4.
Outcome and follow-up
After discussion with the rheumatology team, the patient was discharged with a reducing regimen of prednisolone starting at 60 mg daily. After 2 weeks, this was tapered to 50 mg for 4 weeks, then 40 mg for 3 weeks, then 30 mg for 3 weeks, followed by 20 mg for 4 weeks. At this point, the tapering slowed to 17.5 mg for 4 weeks, then 15 mg for 4 weeks, then 12.5 mg for 4 weeks, followed by a maintenance dose of 10 mg. 18F-FDG-PET/CT performed after 6 months demonstrated resolution of murally based FDG uptake of the large vessels (figure 2).
Figure 2.
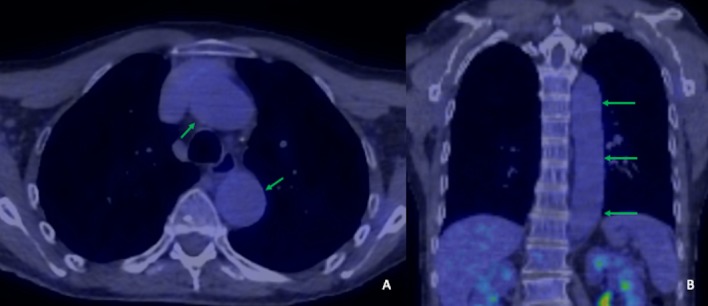
Axial (A) and coronal (B) reformats 6 months following treatment show resolution of vasculitis with no residual 18F-FDG uptake (green arrows).
Treatment within the study was stopped 2 months after commencement, at which point his PSA was stable, compared with a pre-treatment PSA. Two months later, however, his PSA started to rise with a PSA doubling time of 1 month. Since he had bone only metastatic disease he was commenced on radium-223 6 months after commencing treatment within the clinical trial.
Discussion
Despite the significant benefits of ICIs, the non-specific activation of T cells and augmented immune response can lead to a range of toxicities, often experienced simultaneously. Arthralgia and myalgia have been widely reported; however, the prevalence of other rheumatic IRAEs is less well described.3 Vasculitis is rarely documented and has mainly been reported in single organs (retina and uterus) and in the form of giant cell arteritis and polymyalgia rheumatica.6–8 We have found one published case of aortitis secondary to ICIs in a patient treated with ipilimumab for malignant melanoma and another case of periaortitis in a patient treated with nivolumab for non-small cell lung cancer.3 5 Interestingly, in the development of spontaneous autoimmune inflammation of the aorta (giant cell arteritis), inefficiency of the immunoprotective PD-1/PD-L1 immune checkpoint has been shown to be a contributory factor, with in vivo PD-1 blockade exacerbating vascular inflammation and promoting effector T cell infiltration.9
There are currently no standardised diagnostic criteria for rheumatic IRAEs and no defined treatment algorithms. Given the increasing use of ICIs as systemic anticancer management, rheumatic IRAEs are an important area for future research.
Learning points.
Checkpoint inhibitors are now used widely to treat a growing number of malignancies. In prostate cancer, their use is currently experimental (other than the use of pembrolizumab in the setting of a tumour with microsatellite instability-high), and under investigation in multiple clinical trials.
Non-specific activation of T cells may result in a broad spectrum of immune-related adverse events (IRAEs), often experienced simultaneously.
Increased awareness of the non-specific symptoms of rheumatic IRAEs is important for early diagnosis and initiation of management.
18F-fluorodeoxyglucose-positron emission tomography/computed tomography may be useful in the diagnosis of immunotherapy-related aortitis in patients that present with chest pain.
Collaboration between oncologists and rheumatologists is critical for optimal management of potential rheumatological IRAEs.
Footnotes
Twitter: @RadDSP
Contributors: DH, GE, DP and DJ: conception and design of case report; acquisition, analysis and interpretation of data and drafting and revising the article. SC, RJ, PS, BK and ML: analysis and interpretation of data and drafting and revising the article. All authors gave final approval of the article and have agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Haanen J, Carbonnel F, Robert C, et al. . On behalf of the ESMO guidelines Committee, management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:119–42. [DOI] [PubMed] [Google Scholar]
- 2.Cappelli LC, Gutierrez AK, Bingham CO, et al. . Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017;69:1751–63. 10.1002/acr.23177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy AK, Tathireddy HR, Roy M. Aftermath of induced inflammation: acute periaortitis due to nivolumab therapy. BMJ Case Rep 2017;2017:bcr-2017-221852. 10.1136/bcr-2017-221852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 2018;37:2579–84. 10.1007/s10067-018-4177-0 [DOI] [PubMed] [Google Scholar]
- 5.Ban B, Crowe J, Graham R. Rheumatology case report: immune-related aortitis associated with ipilimumab. The rheumatologist. Available: https://www.the-rheumatologist.org/article/rheumatology-case-report-immune-related-aortitis-associated-ipilimumab/ [Accessed 24th May 2019].
- 6.Minor DR, Bunker SR, Doyle J. Lymphocytic vasculitis of the uterus in a patient with melanoma receiving ipilimumab. JCO 2013;31:e356 10.1200/JCO.2012.47.5095 [DOI] [PubMed] [Google Scholar]
- 7.Manusow JS, Khoja L, Pesin N, et al. . Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. J Immunother Cancer 2014;2:41. 10.1186/s40425-014-0041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein BL, Gedmintas L, Todd DJ. Drug-Associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol 2014;66:768–9. 10.1002/art.38282 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Watanabe R, Berry GJ, et al. . Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci U S A 2017;114:E970–9. 10.1073/pnas.1616848114 [DOI] [PMC free article] [PubMed] [Google Scholar]


