Abstract
Children with Down syndrome have a higher risk of stroke. Similarly, intravenous immunoglobulin (IV Ig) is also known to cause a stroke. We reported a 3-year-old boy with Down syndrome who presented with severe pneumonia and received IV Ig. He developed right hemiparesis 60 hours after the infusion. Blood investigations, echocardiography and carotid Doppler did not suggest vasculitis, thrombophilia or extracranial dissection. Brain computerised tomography (CT) showed acute left frontal and parietal infarcts. Initial magnetic resonance angiography (MRA) of cerebral vessels showed short segment attenuations of both proximal middle cerebral arteries and reduction in the calibre of bilateral supraclinoid internal carotid arteries. The boy was treated with enoxaparin and aspirin. He only had partial recovery of the hemiparesis on follow-up. A repeat MRA 13 months later showed parenchymal collateral vessels suggestive of moyamoya disease. We recommend imaging the cerebral vessels in children with a high risk of moyamoya before giving IV Ig.
Keywords: stroke, moyamoya, immunological products and vaccines, congenital disorders
Background
Children with Down syndrome have a higher risk of stroke.1 Most stroke episodes are related to thromboembolism secondary to an underlying congenital heart disease or infections causing cerebral vasculitis.1 2 Two-thirds of the cases of literature-reported angiographic abnormalities in this group of children had moyamoya syndrome and the rest were of unknown aetiology.1
Intravenous immunoglobulin (IV Ig), a pooled IgG derived from plasma of human donors, has been known to cause thrombotic complications but this is uncommon.3 4 The exact mechanism of stroke for patients receiving IV Ig is still unclear, although several studies postulated that it could be secondary to hyperviscosity, transient vasospasm, the elevation of platelet count as well as the introduction of clotting factors and vasoactive cytokines.4–8 To date, there has been no report in the literature of IV Ig-related stroke in children with Down syndrome. We describe herein a child with Down syndrome who developed stroke after IV Ig infusion given for severe bronchopneumonia, with a diagnostic challenge to ascertain the underlying cause.
Case presentation
A 3-year-old boy with Down syndrome presented with 2 days’ history of fever, cough, runny nose and rapid breathing, as well as reduced feeding and activity. He was born prematurely at 32 gestational weeks. He had chronic lung disease, bilateral moderate sensorineural hearing loss and congenital hypothyroidism. His chest X-ray showed diffuse bilateral airspace opacities suggestive of bronchopneumonia. He received 20 mL/kg of normal saline bolus and intravenous ampicillin, besides heated and humidified high flow oxygen. At the fifth hour of admission, he remained critically ill, and he was commenced on IV Ig to treat for possible adenoviral pneumonia. A total of 10 g IV Ig (1.17 g/kg) was infused over 6 hours with monitoring of close vital signs. Subsequently, his clinical condition improved and he was gradually weaned off respiratory support 2 days later.
At 60 hours after completion of IV Ig infusion, the mother noticed the boy was having right-sided body weakness and facial asymmetry. His power was 0/5 over the right upper and lower limbs, and he had a right foot sustained clonus as well as extensor plantar reflex. Cranial nerve examination showed loss of right nasolabial fold suggestive of seventh cranial nerve palsy.
Investigations
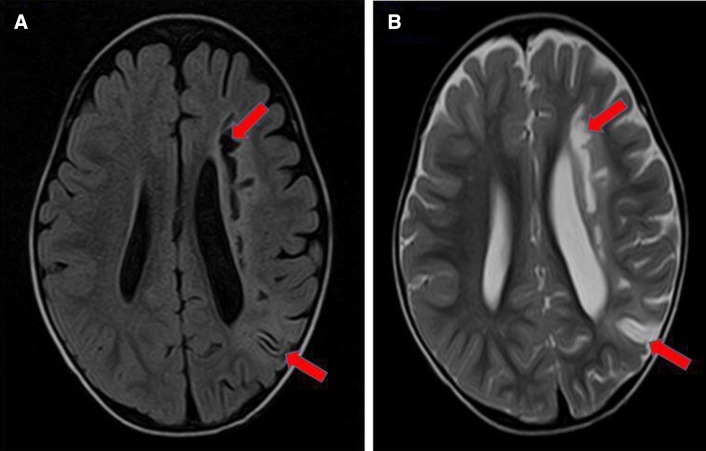
After the IV Ig infusion, the boy had higher platelet count (139×103 cells/µL) and haematocrit (29.0%) compared with 122×103 cells/µL and 26.2%, respectively, on admission. Erythrocyte sedimentation rate was high (135 mm/hour). The coagulation profile, thrombophilia and vasculitis screening, electrocardiogram, echocardiography and carotid Doppler were all normal. An urgent computerised tomography (CT)of the brain demonstrated acute left frontal and parietal infarcts (figure 1). A subsequent magnetic resonance imaging (MRI) further confirmed the infarcts involving deep white matter extending to left centrum semiovale with evidence of small vessel ischaemic changes (figure 2A and B). Magnetic resonance angiography (MRA) showed short segment attenuation of both proximal middle cerebral arteries (MCAs) involving the M1 segments (left more than right) and reduction in the calibre of both supraclinoid internal carotid arteries (ICA, figure 3). Figure 4 shows intracranial vasculopathy involving supraclinoid ICAs, M1 segments of MCAs and A1 segments of anterior cerebral arteries (ACAs). There were no collateral parenchymal vessels on either side to suggest moyamoya disease.
Figure 1.
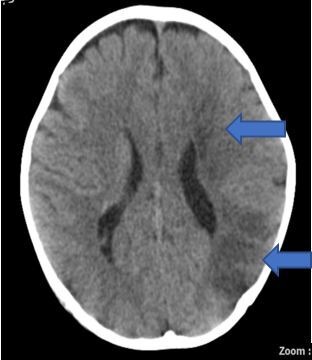
A CT of the brain demonstrated acute left frontal and parietal cerebral infarct.
Figure 2.
MRI of the brain showing non-enhancing T1 hypointensities (A) and T2 hyperintensities (B) at left periventricular deep white matter and left parietal region.
Figure 3.
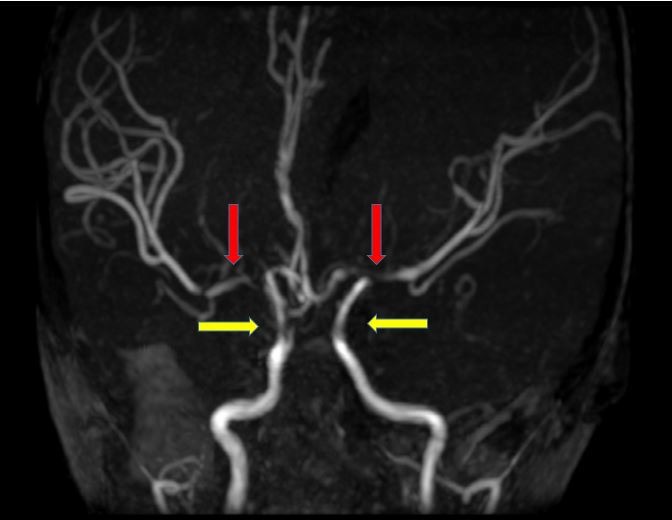
Magnetic resonance angiogram of the brain showing short segment attenuation of both proximal middle cerebral arteries (left more than right, red arrows) and reduction in calibre of both supraclinoid internal carotid arteries (yellow arrows).
Figure 4.
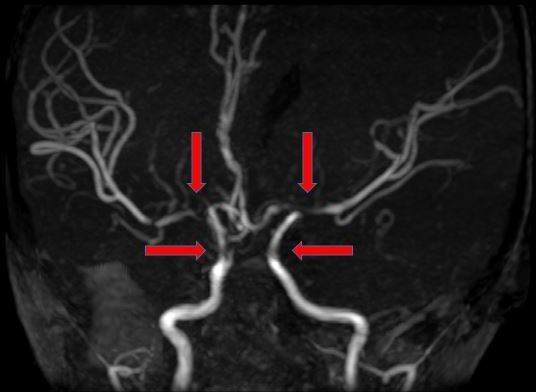
Magnetic resonance angiogram of the brain showing intracranial vasculopathy involving supraclinoid internal carotid arteries, M1 segments of middle cerebral arteries and A1 segments of anterior cerebral arteries (red arrows).
Treatment
The boy received 5 days of subcutaneous enoxaparin (twice daily, 1 mg/kg) and 5 mg/kg of aspirin (once daily). He was referred for physiotherapy.
Outcome and follow-up
The child only had partial motor recovery by 5 months with improvement in the power of the right upper limb (2/5) and right lower limb (1/5) as well as the resolution of right facial paralysis. He continued to receive physiotherapy at the community-based child developmental unit. A repeat MRA with MRI 13 months after the stroke shows slow progressive intracranial vasculopathy involving ICAs, both M1 segments of MCAs and A1 segments of the ACA with collateral vessels formation (figure 5). Although there was no ivy sign to suggest leptomeningeal collaterals, the formal finding was still suggestive of moyamoya syndrome. The chronic left MCA territory infarct remained unchanged. Three-and-half years after the event the child (6 years 3 months old) remained well and ambulating with a right hemiplegia gait. All muscle powers on the right were 4/5.
Figure 5.
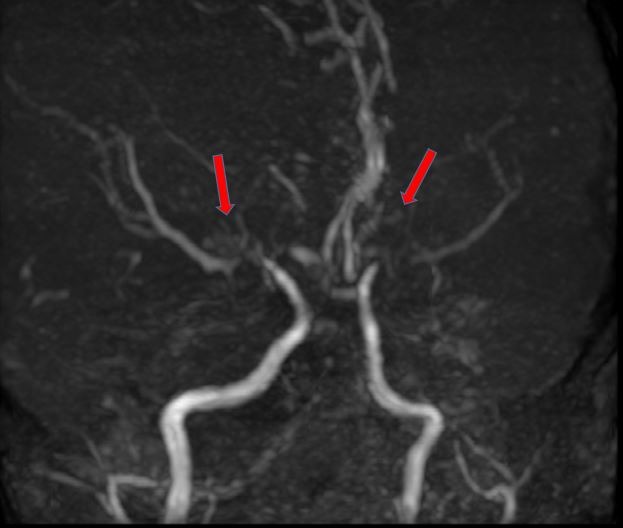
A repeat magnetic resonance angiogram 13 months after the stroke demonstrated the presence of collateral vessels (red arrows) suggestive of moyamoya syndrome.
Discussion
We presented a previously unreported case of a post IV Ig stroke event in a toddler with Down syndrome, a condition that carries a risk of developing a stroke. Despite knowing its potential risk, we had to use IV Ig as adjuvant therapy in this critically ill child to treat pneumonia, possibly due to adenovirus and improved his survival outcome. The lack of pre-existing embolic source and evidence of vasculitis suggest that IV Ig could have perhaps precipitated the thrombotic event in this child who already had a vascular risk factor. Caress et al reported that stroke episodes could occur at any time within 24 hours to 4 days of infusion, consistent with our case who presented 60 hours after completion of IV Ig.3 All of the strokes in the series also involved medium-sized cerebral arteries.3 Moreover, literature has also reported bilateral cerebral thrombotic infarction similar to that in our child.3 We treated the child with enoxaparin and searched for underlying thromboembolic causes.1 Although not conclusive, as the initial MRI showed that there was a narrowing of the ICA and MCA, features that suggested the possibility of moyamoya disease and a condition that is rather common in children with Down syndrome, we gave aspirin to the child.
Although the repeated MRA showed features of moyamoya syndrome eventually, the exact mechanism of IV Ig causing stroke in this child is unclear. It could be due to an increase in blood viscosity, but we were unable to measure his serum viscosity. The initial MRA findings suggested a risk of developing moyamoya syndrome as there was a narrowing of both supraclinoid ICA and proximal MCA on angiography.9 However, the characteristic basal collateral vessels described in moyamoya syndrome were not present in the imaging studies of this child. Differentiating moyamoya syndrome in this child who was at risk by himself remained difficult as compensatory collateral vessels may still develop later. A follow-up imaging is therefore prudent, although the duration of repeating the MRA in a year may be somewhat arbitrary. Our case also developed bilateral moyamoya, consistent with that reported in children with Down syndrome.2 We could not exclude the possibility of a coexisting condition in this child, that is developing a stroke secondary to IV Ig infusion with an asymptomatic moyamoya syndrome.10 We believe the stroke was due to a combination of the underlying vascular changes in evolution and the IV Ig, and the IV Ig itself might have aggravated the stroke scenario. We are unsure of the role of intercurrent infection in the pathogenesis of moyamoya syndrome as literature on this is scarce, although a few organisms such as measles, mycoplasma and Epstein-Barr virus have been suggested.11–13 During intercurrent infection, fever, dehydration, coagulation activation or direct involvement of organisms in the central nervous system may play a role in the development of the infarction.12
Children at risk of a stroke-like event such as Down syndrome should be given IV Ig only with clear indication (eg, life-saving situation) after judiciously weighing the risk and benefit. Parents must be counselled of the higher risk of stroke, and all patients have to be closely monitored for this adverse event during any period of IV Ig therapy till at least 4 days after completion of the infusion. Causes of post IV Ig stroke in children with risk factors are multiple and can be difficult to differentiate at times. Although it may be very effort-intensive and resource-intensive to do an emergency MRI/MRA neuroimaging (and throw up major challenges in treatment) before deciding to give IV Ig in a child with Down syndrome, the individual clinician should weigh the risk and benefit ratio holistically. A follow-up for recurrence and progression of neurology in this group of children may reveal the actual cause in cases where the initial pathology is uncertain.
Learning points.
Children with Down syndrome and immunoglobulin infusion are known as risk factors for stroke development.
The role of intravenous immunoglobulin (IV Ig) in a child with Down syndrome who develops stroke is unknown.
Moyamoya disease is a known and common cause of stroke in children with Down syndrome. Still, the diagnosis is difficult and often requires repeat imaging as well as follow-up to ascertain the actual cause.
Children at risk of a stroke-like event such as Down syndrome should be given IV Ig with caution after weighing the risk-benefit ratio, and requires monitoring for a possible adverse event.
It is advisable to image the cerebral vessels in children with a high risk of moyamoya disease before giving IV Ig.
Acknowledgments
The authors thank Director of Health Malaysia for his permission to present and publish this case report. The authors also thank Dr Shabhana Sivandan for reporting all the initial brain imaging and Dr Menaga A/P Sellamuthoo for reporting the repeat MRA.
Footnotes
Contributors: T-HT conceptualised and designed the manuscript, critically revised the manuscript as submitted and approved the final manuscript. ECS drafted the first draft of the manuscript and approved the final manuscipt. C-HC conceptualised and designed the manuscript, critically revised the manuscript as submitted and approved the final manuscript. HIMI critically revised the manuscript as submitted and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pearson E, Lenn NJ, Cail WS. Moyamoya and other causes of stroke in patients with Down syndrome. Pediatr Neurol 1985;1:174–9. 10.1016/0887-8994(85)90060-8 [DOI] [PubMed] [Google Scholar]
- 2.See AP, Ropper AE, Underberg DL, et al. . Down syndrome and moyamoya: clinical presentation and surgical management. J Neurosurg Pediatr 2015;16:58–63. 10.3171/2014.12.PEDS14563 [DOI] [PubMed] [Google Scholar]
- 3.Caress JB, Cartwright MS, Donofrio PD, et al. . The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology 2003;60:1822–4. 10.1212/01.WNL.0000068335.01620.9D [DOI] [PubMed] [Google Scholar]
- 4.Okuda D, Flaster M, Frey J, et al. . Arterial thrombosis induced by IVIg and its treatment with tPA. Neurology 2003;60:1825–6. 10.1212/01.WNL.0000068334.04500.08 [DOI] [PubMed] [Google Scholar]
- 5.Dalakas MC. High-Dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994;44:223–6. 10.1212/WNL.44.2.223 [DOI] [PubMed] [Google Scholar]
- 6.Reinhart WH, Berchtold PE. Effect of high-dose intravenous immunoglobulin therapy on blood rheology. Lancet 1992;339:662–4. 10.1016/0140-6736(92)90806-E [DOI] [PubMed] [Google Scholar]
- 7.Sztajzel R, Le Floch-Rohr J, Eggimann P. High-Dose intravenous immunoglobulin treatment and cerebral vasospasm: a possible mechanism of ischemic encephalopathy? Eur Neurol 1999;41:153–8. 10.1159/000008040 [DOI] [PubMed] [Google Scholar]
- 8.Woodruff RK, Grigg AP, Firkin FC, et al. . Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986;2:217–8. 10.1016/S0140-6736(86)92511-0 [DOI] [PubMed] [Google Scholar]
- 9.Bagdasarian A, Tonetta S, Harel W, et al. . Ivig adverse reactions: potential role of cytokines and vasoactive substances. Vox Sang 1998;74:74–82. 10.1046/j.1423-0410.1998.7420074.x [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Lee K-S, Weon YC. Asymptomatic moyamoya syndrome, atlantoaxial subluxation and basal ganglia calcification in a child with Down syndrome. Korean J Pediatr 2013;56:540–3. 10.3345/kjp.2013.56.12.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco F, Castellano Chiodo D, Sorge A, et al. . [Multiple arterial ischemic strokes in a child with moyamoya disease and Mycoplasma pneumoniae infection]. Minerva Pediatr 2006;58:63–8. [PubMed] [Google Scholar]
- 12.Takasugi H, Maemoto T, Kitazawa K, et al. . [A case of Down syndrome with moyamoya syndrome presenting extensive multiple cerebral infarction during measles infection]. No To Hattatsu 2000;32:39–43. [PubMed] [Google Scholar]
- 13.Tanigawara T, Yamada H, Sakai N, et al. . Studies on cytomegalovirus and Epstein-Barr virus infection in moyamoya disease. Clin Neurol Neurosurg 1997;99 Suppl 2:S225–8. 10.1016/S0303-8467(97)00049-8 [DOI] [PubMed] [Google Scholar]