Abstract
Background
The Royal Marsden Hospital prognostic score (RMH score) and the Gustave Roussy immune score (GRIm-score) were developed in order to select more suitable patient for phase I trials. Lactate dehydrogenase (LDH) and serum albumin concentration are common risk factors to these two systems. As the third risk factor, the RMH score and the GRIm-score adopt number of metastatic sites and neutrophil-to-lymphocyte ratio (NLR), respectively. We aimed to investigate whether these two systems are also useful for extensive disease of small cell lung cancer (ED-SCLC).
Methods
We retrospectively collected 128 patients who had initiated platinum-based chemotherapy at our hospital between September 2007 and March 2018. We divided our patients into low (score 0 - 1) and high (2 - 3) score groups, and compared overall survival (OS) and progression-free survival (PFS) between them. Multivariate Cox proportional hazard analyses found prognostic factors of survival times.
Results
Regarding GRIm-score, OS was significantly shorter in high score group than in low score group (median 6.1 vs. 11.4 months, P < 0.01), while no significant difference was observed in PFS (median 4.7 vs. 5.0 months, P = 0.12). Both OS (median 6.9 vs. 12.4 months, P < 0.01) and PFS (median 4.4 vs. 5.4 months, P = 0.01) were significantly shorter in high RMH score group than in low group. Multivariate analyses detected both high GRIm-score (hazard ratio (HR) 1.80, 95% confidence interval (CI) 1.20 - 2.72, P < 0.01) and high RMH score (HR 1.93, 95% CI 1.27 - 2.92, P < 0.01) as independent worse prognostic factors of OS, and then only high RMH score (HR 1.53, 95% CI 1.04 - 2.25, P = 0.03) as independent worse prognostic factor of PFS.
Conclusions
Both RMH score and GRIm-score are useful as independent prognostic factors of OS in ED-SCLC. However, only RMH score is an independent prognostic factor of PFS.
Keywords: Gustave Roussy immune score, Royal Marsden Hospital prognostic score, Extensive disease, Small cell lung cancer, Neutrophil-to-lymphocyte ratio, Number of metastatic sites, Lactate dehydrogenase, Serum albumin
Introduction
The Royal Marsden Hospital prognostic score (RMH score) was developed and validated in 2008 - 2009 as an objective prognostic scoring system to aid the patient selection for phase I trials of new cytotoxics and targeted therapies [1, 2]. This system is based on the three risk variables: lactate dehydrogenase (LDH) (within normal range (0) vs. higher than upper limit of normal range (ULN) (1)), serum albumin (≥ 3.5 g/dL (0) vs. < 3.5 g/dL (1)) and sites of metastasis (0 - 2 sites (0) vs. three or more sites (1)). Thereafter, in 2017, the Gustave Roussy immune score (GRIm-score) was developed on the basis of RMH scoring system in order to select better patients for phase I trials of immune-checkpoint therapies (ICTs) [3]. In the ICT phase I cohort, higher neutrophil-to-lymphocyte ratio (NLR), but not the number of metastases, was associated with a decrease in survival. Thus, the number of metastatic sites in the RMH score was replaced by NLR (≤ 6 (0) vs. > 6 (1)) in the GRIm-score. These two scoring systems were developed for phase I trials and have been validated only in phase I trials. Recently, we demonstrated these two scoring systems as useful prognostic biomarkers for practical immune-checkpoint inhibitor (ICI) monotherapy for pretreated non-small cell lung cancer (NSCLC) patients [4], and then high GRIm-score as a prognostic marker of shorter overall survival (OS) for wild-type epidermal growth factor receptor (EGFR) adenocarcinoma and as a predictive marker of poor progression-free survival (PFS) for EGFR-mutant NSCLC [5]. Thus, these two scores may be useful prognostic biomarkers not only for phase I trials but also for various types of malignancies.
Small cell lung cancer (SCLC) aggressively progresses, easily metastasizes and results in poor prognosis, despite minor histopathology and high sensitivity to chemotherapy. At the time of diagnosis, the disease is usually advanced regionally or metastatic, and is not an indication for curative-intent thoracic radiotherapy. Previous studies have indicated that high LDH [6-9], low albumin [8, 9], high NLR [10, 11] and more metastatic sites [9, 12] were associated with poor outcomes in ED-SCLC patients. However, little is known about RMH score and GRIm-score for ED-SCLC.
This study aimed to evaluate RMH score and GRIm-score as independent prognostic markers for ED-SCLC patients treated with platinum-based chemotherapy.
Materials and Methods
Patients and study design
Our single-institutional and retrospective study included the following patients: 1) pathologically confirmed SCLC; 2) patients who had started the first-line platinum-based combination chemotherapy between September 2007 and March 2018 at our hospital; 3) clinical stage IIIB or IV in the seventh TNM classification of lung cancer by the Union for International Cancer Control (UICC) [13]; and 4) pretreatment serum albumin, LDH, differential count of leukocyte within 2 weeks before the first day of chemotherapy. We excluded the patients with clinical stage IIIB who had received curative-intent concurrent thoracic radiotherapy with chemotherapy. From our electrical medical chart, we collected the following data: sex, age, height, body weight, smoking habits and history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), metastatic sites, absolute numbers of neutrophils and lymphocytes (cells/µL), serum albumin concentration (mg/dL), first-line regimens, chemotherapeutic response according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [14], second or later line regimens, PFS and OS. The definitions of response rate (RR), disease control rate (DCR), PFS, OS, NLR, RMH score and GRIm-score followed those of our previous studies [4, 5]. According to the sum of the three factors of RMH score and GRIm-score, we divided our patients into two score groups: low (total score of 0 or 1) and high (2 or 3). The data cut-off date was December 31, 2019. The Osaka Police Hospital Ethics Committee approved this study. This study observed the Declaration of Helsinki.
Data analyses
Using median with interquartile range (IQR) and Mann-Whitney U test, frequencies and Fisher exact test, median time (months) with 95% confidential intervals (CI) and Kaplan-Meier method with log-rank test, we described and then compared continuous, categorical and survival time data, respectively. Using Spearman’s rank correlation coefficient (rs), we identified relationships between two non-parametric scores. As independent factors associated with OS and PFS, multivariate Cox proportional hazards models evaluated the following pre-defined explanatory variables: age (< 75 vs. ≥ 75 years), body mass index (BMI) (≥ 18.5 vs. < 18.5), platinum base (carboplatin vs. cisplatin), ECOG-PS (0 - 1 vs. 2 - 4), number of metastatic sites (< 3 vs. ≥ 3) with GRIm-score or NLR (≤ 6 vs. > 6) with RMH score. The cut-off age of the Japanese late-stage medical care system for the elderly is 75 years. The nutritional cut-off BMI of underweight is < 18.5. We described these results by hazard ratios (HRs) with 95% CI. We considered P-value < 0.05 as statistically significant difference. Using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [15], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria), we performed all statistical analyses.
Results
We collected 128 ED-SCLC patients treated with cisplatin (CDDP) or carboplatin (CBDCA)-based chemotherapy. Their median age and BMI were 72.0 (IQR 66.0 - 77.3) and 22.3 (19.5 - 24.9), respectively. We divided them into high and low RMH score or GRIm-score groups. RMH score and GRIm-score were significantly correlated (rs = 0.83, P < 0.01). Table 1 shows patients’ distribution of these two scores. None was in a group of low RMH and high GRIm-score, while 26 were in a group of high RMH and low GRIm-score. The patients’ numbers of NLR ≤ 6 and metastatic sites < 3, NLR ≤ 6 and metastatic sites ≥ 3, NLR > 6 and metastatic sites < 3, and NLR > 6 and metastatic sites ≥ 3 were 60, 47, 5 and 16, respectively (P < 0.01) (Tables 2 and 3). Brain and thoracic irradiations were performed in 28 and 10 patients during their cancer treatment period. Until the data cut-off, 108 patients died at our hospital (N = 81), at other hospitals (N = 18) and at home (N = 9), 16 were missing and four were still alive. Except for seven patients, 121 experienced progressive disease (PD) or death without confirmed PD. The reasons of discontinuation of the first-line chemotherapy were PD in 47 patients, completion of pre-defined courses in 45, adverse effects in five, deteriorated general conditions in 12, deteriorated other diseases in 11, patient’s refusal in five, sudden death due to unknown reason in one, suicide in one and transfer to other nursing institutions in one.
Table 1. Distribution of GRIm-Score and RMH Score.
| GRIm-score | RMH score |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | |
| 0 | 17 | 6 | 0 | 0 | 23 |
| 1 | 0 | 30 | 26 | 0 | 56 |
| 2 | 0 | 0 | 22 | 15 | 37 |
| 3 | 0 | 0 | 4 | 8 | 12 |
| Total | 17 | 36 | 52 | 23 | 128 |
GRIm-score: Gustave Roussy immune score; RMH score: Royal Marsden Hospital prognostic score.
Table 2. Baseline Characteristics, Treatment and Laboratory Data According to GRIm-Score.
| GRIm-score |
P | ||
|---|---|---|---|
| Low | High | ||
| N | 79 | 49 | |
| Backgrounds | |||
| Sex (N) | |||
| Male/female | 65/14 | 36/13 | 0.27a |
| Age (years) | |||
| Median (IQR) | 71 (64 - 76) | 72 (66 - 81) | 0.17b |
| < 75/≥ 75 years | 49/30 | 30/19 | 1.00a |
| Smoking status (N) | |||
| NS/Ex/CS/unknown | 1/24/53/1 | 2/17/30/0 | 0.69a |
| BMI | |||
| Median (IQR) | 23.1 (20.3 - 26.2) | 21.1 (19.0 - 23.8) | < 0.01b |
| ≥ 18.5/< 18.5 (N) | 72/7 | 40/9 | 0.17a |
| ECOG-PS (N) | |||
| 0 - 1/2/3 | 57/18/4 | 20/13/16 | < 0.01a |
| Metastatic sites (N) | |||
| < 3/≥ 3 | 46/33 | 19/33 | 0.045a |
| Treatment | |||
| Regimen (N) | |||
| Platinum-based | |||
| Cisplatin/carboplatin | 20/59 | 11/38 | 0.83a |
| Partner drugs | |||
| Etoposide/irinotecan | 67/12 | 46/3 | 0.16a |
| Efficacy | |||
| CR/PR/SD/PD/NE | 2/51/11/11/4 | 0/25/6/10/8 | 0.14a |
| ORR (%) (95% CI) | 67.1 (55.6 - 77.3) | 51.0 (36.3 - 65.6) | 0.09a |
| DCR (%) (95% CI) | 81.0 (70.6 - 89.0) | 63.3 (48.3 - 76.6) | 0.04a |
| Second or later line (N) | 51 | 18 | < 0.01a |
| Amrubicin (N) | 38 | 11 | < 0.01a |
| Topotecan (N) | 10 | 3 | 0.37a |
| Irinotecan (N) | 6 | 1 | 0.25a |
| Radiotherapy | |||
| Brain | 22 | 6 | 0.048a |
| Thoracic | 6 | 4 | 1.00a |
| Laboratory data | |||
| NLR | |||
| Median (IQR) | 2.7 (2.0 - 4.1) | 4.7 (3.3 - 8.7) | < 0.01b |
| > 6 (N) | 1 | 20 | < 0.01a |
| LDH (U/L) | |||
| Median (IQR) | 233 (196.5 - 350) | 398 (280 - 493) | < 0.01b |
| > ULN (N) | 43 | 48 | < 0.01a |
| Albumin (g/dL) | |||
| Median (IQR) | 3.9 (3.6 - 4.1) | 3.1 (2.7 - 3.4) | < 0.01b |
| < 3.5 g/dL (N) | 12 | 42 | < 0.01a |
aFisher exact test. bMann-Whitney U test. BMI: body mass index; CI: confidence interval; CR: complete response; CS: current smoker; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group performance status; Ex: ex-smoker; GRIm-score: Gustave Roussy immune score; IQR: interquartile range; LDH: lactate dehydrogenase; NE: not evaluated; NLR: neutrophil-to-lymphocyte ratio; NS: non-smoker; ORR: overall response rate; PD: progressive disease; SD: stable disease; ULN: upper limit of normal.
Table 3. Baseline Characteristics, Treatment and Laboratory Data According to RMH Score.
| RMH score |
P | ||
|---|---|---|---|
| Low | High | ||
| N | 54 | 74 | |
| Backgrounds | |||
| Sex (N) | |||
| Male/female | 45/9 | 56/18 | 0.38a |
| Age (years) | |||
| Median (IQR) | 72 (66 - 76) | 71.5 (65.3 - 80) | 0.83b |
| < 75/≥ 75 years | 33/21 | 46/28 | 1.00a |
| Smoking status (N) | |||
| NS/Ex/CS/unknown | 1/19/34/0 | 2/22/49/1 | 0.93a |
| BMI | |||
| Median (IQR) | 22.8 (19.8 - 26.1) | 21.3 (19.4 - 24.3) | 0.22b |
| ≥ 18.5/< 18.5 (N) | 49/5 | 63/11 | 0.42a |
| ECOG-PS (N) | |||
| 0 - 1/2/3 | 39/13/2 | 38/18/18 | < 0.01a |
| Metastatic sites (N) | |||
| < 3/≥ 3 | 47/7 | 18/56 | < 0.01a |
| Treatment | |||
| Regimen (N) | |||
| Platinum-based | |||
| Cisplatin/carboplatin | 12/42 | 19/55 | 0.68a |
| Partner drugs | |||
| Etoposide/irinotecan | 46/8 | 67/7 | 0.41a |
| Efficacy | |||
| CR/PR/SD/PD/NE | 1/35/10/7/1 | 1/41/7/14/11 | 0.04a |
| ORR (%) (95% CI) | 66.7 (52.5 - 78.9) | 56.8 (44.7 - 68.2) | 0.27a |
| DCR (%) (95% CI) | 85.2 (72.9 - 93.4) | 66.2 (54.3 - 76.8) | 0.02a |
| Second or later line (N) | 39 | 30 | < 0.01a |
| Amrubicin (N) | 28 | 21 | < 0.01a |
| Topotecan (N) | 4 | 9 | 0.56a |
| Irinotecan (N) | 4 | 3 | 0.45a |
| Radiotherapy | |||
| Brain | 15 | 13 | 0.20a |
| Thoracic | 5 | 5 | 0.74a |
| Laboratory data | |||
| NLR | |||
| Median (IQR) | 2.6 (2.0 - 4.0) | 4.1 (2.7 - 5.8) | < 0.01b |
| > 6 (N) | 2 | 19 | < 0.01a |
| LDH (U/L) | |||
| Median (IQR) | 212 (186 - 314) | 332 (266 - 490) | < 0.01b |
| > ULN (N) | 23 | 68 | < 0.01a |
| Albumin (g/dL) | |||
| Median (IQR) | 3.9 (3.7 - 4.1) | 3.3 (2.8 - 3.8) | <0.01b |
| < 3.5 g/dL (N) | 7 | 47 | <0.01a |
aFisher exact test. bMann-Whitney U test. BMI: body mass index; CI: confidence interval; CR: complete response; CS: current smoker; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group performance status; Ex: ex-smoker; IQR: interquartile range; LDH: lactate dehydrogenase; NE: not evaluated; NLR: neutrophil-to-lymphocyte ratio; NS: non-smoker; ORR: overall response rate; PD: progressive disease; RMH score: Royal Marsden Hospital prognostic score; SD: stable disease; ULN: upper limit of normal.
Poorer ECOG-PS, more frequent metastatic sites ≥ 3, lower DCR, lower rate of second or later line and amrubicin regimen, higher NLR, higher LDH and lower serum albumin concentration were common to high GRIm-score and high RMH score groups (Tables 2 and 3). Lower proportion of brain irradiation and lower BMI were observed in high GRIm-score group than in low group (Table 2).
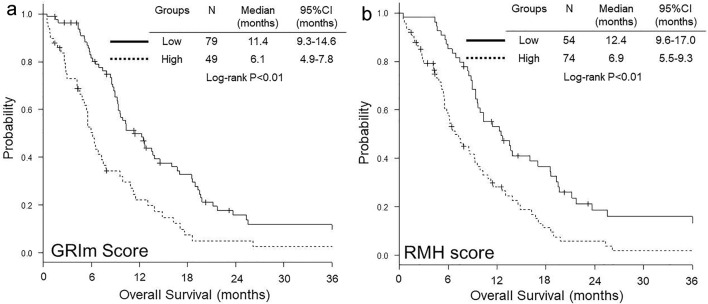
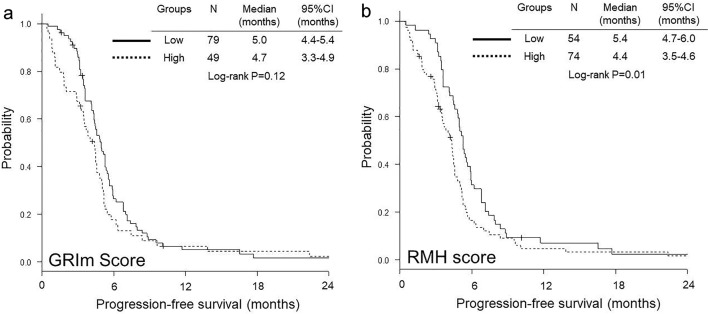
OS was significantly shorter in high GRIm-score group than in low group (median 6.1 vs. 11.4 months, P < 0.01) (Fig. 1a), while no significant difference was observed in PFS between low and high GRIm-score groups (median 4.7 vs. 5.0 months, P = 0.12) (Fig. 2a). In contrast, both OS (median 6.9 vs. 12.4 months, P < 0.01) (Fig. 1b) and PFS (median 4.4 vs. 5.4 months, P = 0.01) (Fig. 2b) were significantly shorter in high RMH score group than in low group.
Figure 1.
Kaplan-Meier curves of overall survival according to GRIm-score (a) and RMH score (b). GRIm-score: Gustave Roussy immune score; RMH score: Royal Marsden Hospital prognostic score.
Figure 2.
Kaplan-Meier curves of progression-free survival according to GRIm-score (a) and RMH score (b). GRIm-score: Gustave Roussy immune score; RMH score: Royal Marsden Hospital prognostic score.
In addition to ECOG-PS, multivariate Cox hazard proportional analyses detected number of metastases ≥ 3 (hazard ratio (HR) 1.97, 95% CI 1.29 - 3.02, P < 0.01), high GRIm-score (HR 1.80, 95% CI 1.20 - 2.72, P < 0.01) and high RMH score (HR 1.93, 95% CI 1.27 - 2.92, P < 0.01) as independent prognostic factors of OS (Table 4). Multivariate analyses found number of metastases (HR 1.60, 95% CI 1.09 - 2.34, P = 0.02) and high RMH score (HR 1.53, 95% CI 1.04 - 2.25, P = 0.03) as independent prognostic factors of PFS (Table 5).
Table 4. Multivariate Cox Hazard Proportional Analyses of Overall Survival of All Patients.
| Variable | GRIm-score |
RMH score |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||
| < 75 | 1 (Reference) | 1 (Reference) | ||
| ≥ 75 | 1.34 (0.87 - 2.06) | 0.19 | 1.29 (0.82 - 2.02) | 0.27 |
| BMI | ||||
| ≥ 18.5 | 1 (Reference) | 1 (Reference) | ||
| < 18.5 | 1.39 (0.79 - 2.45) | 0.25 | 1.57 (0.89 - 2.77) | 0.12 |
| Platinum-based | ||||
| Cisplatin | 1 (Reference) | 1 (Reference) | ||
| Carboplatin | 0.94 (0.54 - 1.62) | 0.82 | 0.82 (0.48 - 1.41) | 0.48 |
| ECOG-PS | ||||
| 0 - 1 | 1 (Reference) | 1 (Reference) | ||
| 2 - 4 | 2.16 (1.41 - 3.31) | < 0.01 | 2.12 (1.38 - 3.24) | < 0.01 |
| No. of metastases | ||||
| < 3 | 1 (Reference) | |||
| ≥ 3 | 1.97 (1.29 - 3.02) | < 0.01 | ||
| GRIm-score | ||||
| Low (0 - 1) | 1 (Reference) | |||
| High (2 - 3) | 1.80 (1.20 - 2.72) | < 0.01 | ||
| NLR | ||||
| < 6 | 1 (Reference) | |||
| ≥ 6 | 1.17 (0.65 - 2.09) | 0.60 | ||
| RMH score | ||||
| Low (0 - 1) | 1 (Reference) | |||
| High (2 - 3) | 1.93 (1.27 - 2.92) | < 0.01 | ||
BMI: body mass index; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group performance status; GRIm-score: Gustave Roussy immune score; HR: hazard ratio; NLR: neutrophil-to-lymphocyte ratio; RMH score: Royal Marsden Hospital prognostic score.
Table 5. Multivariate Cox Hazard Proportional Analyses of Progression-Free Survival of First-Line Chemotherapy.
| Variables | GRIm-score |
RMH score |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||
| < 75 | 1 (Reference) | 1 (Reference) | ||
| ≥ 75 | 0.98 (0.65 - 1.47) | 0.91 | 1.00 (0.66 - 1.51) | 1.00 |
| BMI | ||||
| ≥ 18.5 | 1 (Reference) | 1 (Reference) | ||
| < 18.5 | 1.30 (0.76 - 2.22) | 0.34 | 1.21 (0.71 - 2.06) | 0.49 |
| Platinum-based | ||||
| Cisplatin | 1 (Reference) | 1 (Reference) | ||
| Carboplatin | 1.22 (0.73 - 2.01) | 0.45 | 1.08 (0.66 - 1.78) | 0.76 |
| ECOG-PS | ||||
| 0 - 1 | 1 (Reference) | 1 (Reference) | ||
| 2 - 4 | 1.27 (0.84 - 1.92) | 0.26 | 1.33 (0.88 - 2.02) | 0.18 |
| No. of metastases | ||||
| < 3 | 1 (Reference) | |||
| ≥ 3 | 1.60 (1.09 - 2.34) | 0.02 | ||
| GRIm-Score | ||||
| Low (0 - 1) | 1 (Reference) | |||
| High (2 - 3) | 1.13 (0.76 - 1.67) | 0.55 | ||
| NLR | ||||
| < 6 | 1 (Reference) | |||
| ≥ 6 | 0.87 (0.49 - 1.55) | 0.65 | ||
| RMH score | ||||
| Low (0 - 1) | 1 (Reference) | |||
| High (2 - 3) | 1.53 (1.04 - 2.25) | 0.03 | ||
BMI: body mass index; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group performance status; GRIm-score: Gustave Roussy immune score; HR: hazard ratio; NLR: neutrophil-to-lymphocyte ratio; RMH score: Royal Marsden Hospital prognostic score.
Discussion
This was the first study that evaluated RMH score and GRIM-score for ED-SCLC. We demonstrated that, based on our comparisons of survival curves and multivariate analyses, both pretreatment RMH score and GRIm-score are significant prognostic markers of OS of ED-SCLC patients. Thus, these two scores are useful not only for experimental phase I trials [1-3] and some subsets of NSCLC practically treated with chemotherapy or ICT [4, 5], but also for practical setting of ED-SCLC treated with standard regimen, platinum-based chemotherapy. We may use these two scoring systems in various practical settings and for various malignancies.
Interestingly, a significant prognostic biomarker of PFS of first-line platinum-based chemotherapy was not GRIm-score, but RMH score. Serum albumin and LDH levels are common to these two systems. The difference between them is only NLR or number of metastases. Furthermore, in our multivariate analyses, a significant factor associated with PFS and OS was not NLR, but number of metastases. Our two hypotheses on the discrepancy between these two variables are as follows. 1) The cut-off point, 6, of NLR in GRIm-score is much higher than those, 3 - 4, of the previous studies that had detected NLR as a significant prognostic factor for SCLC [16-18]. 2) The number of metastases, i.e. extent of cancer spread, may be more important in contribution to survival than NLR, i.e. a marker of patient’s inflammatory response.
We have to be careful to some limitations in our study. First, a selection bias might exist in such a retrospective, single-institutional and small sample-sized study. Second, our study accrued patients who had initiated chemotherapy before August 2019, when atezolizumab was approved as a combination partner of carboplatin plus etoposide by Japanese medical insurance. Thus, our study is unable to respond to a new era of combination immunotherapy for ED-SCLC. It is interesting whether these biomarkers are also useful for this new treatment option.
Conclusion
Both RMH score and GRIm-score are useful as independent prognostic factors of OS in ED-SCLC. However, only RMH score is an independent prognostic factor of PFS.
Acknowledgments
We are grateful to Kazunori Moriizumi, Kanako Nishimatsu, Saori Ikebe, Hideyasu Okada and Kensuke Kanaoka at the Department of Respiratory Medicine, Osaka Police Hospital, and Tsunehiro Tanaka and Kazuki Hashimoto at the Department of Respiratory Medicine, Daini Osaka Police Hospital for their medical records, diagnosis, treatment and care of their patients.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The Osaka Police Hospital Ethics Committee approved waiver of the written informed consents because of the retrospective and anonymous design.
Author Contributions
Seigo Minami designed, performed the statistical analysis of the data, and drafted the manuscript. All authors were involved in the conceptual design, review of the draft, and approved the final manuscript. Komuta Kiyoshi supervised all aspects of the study.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27(16):2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 2.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98(6):1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, Angevin E. et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score) Eur J Cancer. 2017;84:212–218. doi: 10.1016/j.ejca.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Minami S, Ihara S, Ikuta S, Komuta K. Gustave roussy immune score and Royal Marsden hospital prognostic score are biomarkers of immune-checkpoint inhibitor for non-small cell lung cancer. World J Oncol. 2019;10(2):90–100. doi: 10.14740/wjon1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minami S, Ihara S, Komuta K. Gustave Roussy Immune Score Is a Prognostic Factor for Chemotherapy-Naive Pulmonary Adenocarcinoma With Wild-Type Epidermal Growth Factor Receptor. World J Oncol. 2019;10(1):55–61. doi: 10.14740/wjon1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer - a retrospective single institution analysis. Respir Med. 2010;104(12):1937–1942. doi: 10.1016/j.rmed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Quoix E, Purohit A, Faller-Beau M, Moreau L, Oster JP, Pauli G. Comparative prognostic value of lactate dehydrogenase and neuron-specific enolase in small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer. 2000;30(2):127–134. doi: 10.1016/S0169-5002(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 8.Shirasawa M, Fukui T, Kusuhara S, Hiyoshi Y, Ishihara M, Kasajima M, Nakahara Y. et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag Res. 2018;10:6039–6047. doi: 10.2147/CMAR.S181789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura M, Ueoka H, Kiura K, Tabata M, Shibayama T, Miyatake K, Gemba K. et al. Prognostic factors of small-cell lung cancer in Okayama Lung Cancer Study Group Trials. Acta Med Okayama. 1998;52(2):105–111. doi: 10.18926/AMO/31310. [DOI] [PubMed] [Google Scholar]
- 10.Kang MH, Go SI, Song HN, Lee A, Kim SH, Kang JH, Jeong BK. et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111(3):452–460. doi: 10.1038/bjc.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7(3):498–506. doi: 10.3892/mco.2017.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirasawa M, Fukui T, Kusuhara S, Harada S, Nishinarita N, Hiyoshi Y, Ishihara M. et al. Prognostic differences between oligometastatic and polymetastatic extensive disease-small cell lung cancer. PLoS One. 2019;14(4):e0214599. doi: 10.1371/journal.pone.0214599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15(1):4–9. [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakin A, Sahin S, Yasar N, Demir C, Arici S, Geredeli C, Cihan S. The Relation between Hemogram Parameters and Survival in Extensive-Stage Small Cell Lung Cancer. Oncol Res Treat. 2019;42(10):506–515. doi: 10.1159/000501595. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R, Lin SH, Wei X, Allen PK, Welsh JW, Byers LA, Komaki R. Prognostic significance of pretreatment total lymphocyte count and neutrophil-to-lymphocyte ratio in extensive-stage small-cell lung cancer. Radiother Oncol. 2018;126(3):499–505. doi: 10.1016/j.radonc.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Teng F, Kong L, Yu J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther. 2016;9:5761–5770. doi: 10.2147/OTT.S106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.