Abstract
OBJECTIVES
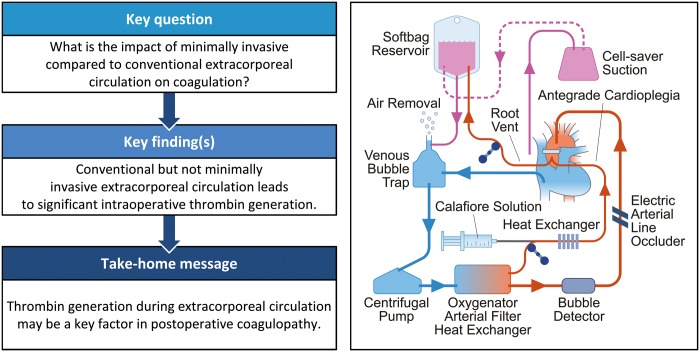
Minimally invasive extracorporeal circulation (MiECC) is suggested to have favourable impact on blood loss compared to conventional extracorporeal circulation. We aimed to compare the impact of both systems on coagulation.
METHODS
Randomized trial comparing endogenous thrombin-generating potential early after elective coronary surgery employing either MiECC group (n = 30) or conventional extracorporeal circulation group (n = 30). Secondary outcomes were in vivo thrombin generation, bleeding end points and haemodilution, as well as morbidity and mortality up to 30-day follow-up.
RESULTS
Compared to the conventional extracorporeal circulation group, the MiECC group showed (i) a trend towards a higher early postoperative endogenous thrombin-generating potential (P = 0.06), (ii) lower intraoperative levels of thrombin–antithrombin complex and prothrombin fragment 1 + 2 (P < 0.001), (iii) less haemodilution early postoperatively as measured by haematocrit and weight gain, but without correlation to coagulation factors or bleeding end points. Moreover, half as many patients required postoperative blood transfusion in the MiECC group (17% vs 37%, P = 0.14), although postoperative blood loss did not differ between groups (P = 0.84). Thrombin–antithrombin complex levels (rs = 0.36, P = 0.005) and prothrombin fragment 1 + 2 (rs = 0.45, P < 0.001), but not early postoperative endogenous thrombin-generating potential (rs = 0.05, P = 0.72), showed significant correlation to increased transfusion requirements. The MiECC group demonstrated significantly lower levels of creatine kinase-MB, lactate dehydrogenase and free haemoglobin indicating superior myocardial protection, less tissue damage and less haemolysis, respectively. Perioperative morbidity and 30-day mortality did not differ between groups.
CONCLUSIONS
Conventional but not MiECC is associated with significant intraoperative thrombin generation despite full heparinization. No correlation between coagulation factors or bleeding end points with the degree of haemodilution could be ascertained.
ClinicalTrials.gov identifier
Keywords: Minimally invasive extracorporeal circulation, Thrombin generation, Blood transfusion, Coronary artery bypass grafting
INTRODUCTION
Extracorporeal circulation (ECC) allows optimal conditions for complete myocardial revascularization during coronary artery bypass grafting (CABG). The concept of minimally invasive extracorporeal circulation (MiECC) was developed to attenuate adverse systemic effects of ECC [1]. MiECC comprises a closed circuit without venous cardiotomy reservoir or cardiotomy suction device to prevent blood–air contact, a centrifugal pump to reduce mechanical stress, a cell saver for shed blood collection, low priming and cardioplegia volumes to minimize haemodilution and bio-coated circuit to increase haemocompatibility. Meta-analyses of randomized trials have demonstrated that utilization of MiECC in CABG compared to conventional extracorporeal circulation (CECC) can reduce perioperative mortality and morbidity including reduction in transfusion requirements [2, 3]. Blood transfusions following cardiac surgery have been shown to be independently associated with increased risk of major morbidity and mortality [4, 5]. The reduced transfusion requirements following MiECC have been attributed mainly to reduced haemodilution [1]. Randomized studies comparing levels of standard coagulation markers and bleeding manifestations following CABG with MiECC and CECC yielded contradictory results [6, 7]. However, CABG with MiECC was associated with significant less intraoperative in vivo thrombin generation as measured by plasma levels of thrombin–antithrombin complex (TAT) and prothrombin fragment 1 + 2 (F1 + 2) in 2 small randomized trials [8, 9].
Endogenous thrombin-generating potential (ETP) as assessed by calibrated automated thrombography is suggested to measure the ability to generate thrombin ex vivo, thus reflecting the overall plasmatic haemostatic capacity [10]. Recent studies have shown decreased ETP early after CABG with CECC to be associated with increased postoperative bleeding [11–13]. We hypothesized that MiECC when compared to CECC would preserve haemostatic capacity as measured by the primary end point of ETP early after CABG. Secondary end points were transfusion requirements, postoperative bleeding, intraoperative thrombin generation in vivo as assessed by plasma levels of TAT and F1 + 2, perioperative haematocrit and weight as a measure of haemodilution, as well as morbidity and mortality up to 30-day follow-up.
MATERIALS AND METHODS
Study design
Patients scheduled for primary elective isolated CABG with ECC at the Department of Cardiothoracic Surgery, Aarhus University Hospital, Denmark, were prospectively assessed for study eligibility by the multidisciplinary heart team. The study flow diagram including inclusion and exclusion criteria is depicted in Fig. 1. After written informed consent, patients were allocated to the treatment group in a 1:1 ratio by REDCap Randomization Module [14]. Allocation was inevitably known to the operating team, but participants, care providers, laboratory personal and outcome adjudicators were blinded. The study was approved by the regional ethics committee (ID 1-10-72-150-17; 1 September 2017). Data are available upon reasonable request (Supplementary Appendix SA).
Figure 1:
Study CONSORT flow diagram including inclusion and exclusion criteria. CABG: coronary artery bypass grafting; eGFR: estimated glomerular filtration rate.
Intervention
The team of surgeons, anaesthesiologists and perfusionists were identical for both groups, as were surgical procedure, anaesthesia and clinical management. Standard CABG with the employment of left internal mammary artery and no-touch saphenous vein grafts was performed. Doses of unfractionated heparin and protamine were titrated (HMS Plus®; *Medtronic International, Tolochenaz, Switzerland). We aimed for an activated clotting time of >400 s. All patients received 4 g intravenous tranexamic acid perioperatively. Normothermia and goal-directed perfusion management were applied (maintaining mixed venous oxygen saturation >65% and oxygen delivery level >270 ml/min/m2).
We employed a type III MiECC system by definition of the Minimally Invasive Extracorporeal Technologies International Society [1]: centrifugal pump (Affinity™ CP AP40*), oxygenator with integrated arterial filter (Affinity Fusion®*), automatic Venous Air Removal Device (Affinity® VARD*), polyvinylchloride/silicone tubing with biocompatible coating (Balance® Biosurface*), soft-shell reservoir bag and cell-saving device (AutoLog®*). Antegrade autologous priming and retrograde autologous priming were employed to reduce effective priming volume to ∼400 ml of Ringer’s solution.
Myocardial protection was achieved with <50 ml of antegrade intermittent cold modified Calafiore blood cardioplegia.
The CECC circuit included: roller pump (Stöckert S5®, Munich, Germany), oxygenator with integrated arterial filter (Affinity Fusion®*), hard-shell venous reservoir and silicone tubing (Costumpack M450311F*). The circuit was primed with 1400 ml of Ringer’s solution. Myocardial protection was accomplished using 300–600 ml of antegrade intermittent cold blood Harefield cardioplegia.
Clinical management
All patients were treated with 75 mg of oral acetylsalicylic acid once daily without discontinuation prior to surgery. Anaesthesia was induced and maintained with propofol, sufentanil, rocuronium and sevoflurane. All patients received postoperative paracetamol, morphine-like analgesics and ketorolac. Acetylsalicylic acid and statin therapy were resumed on the first postoperative day. Subcutaneous dalteparin 5000 IE were given once daily until full mobilization. In cases of new-onset atrial fibrillation, therapeutic doses were administered within 12–48 h. Transfusions were given according to the Danish Guidelines on blood transfusion [15]. Thrombelastometry-guided transfusion of fresh frozen plasma and platelet concentrates was triggered by continuous bleeding. Surgical re-exploration was left up to the surgeon’s discretion.
Blood sampling and laboratory analyses
We used vacuum test tubes containing following additives: 3.2% sodium citrate (thrombin generation potential, TAT, F1 + 2, standard coagulation tests), lithium heparin [lactate dehydrogenase, creatine kinase-MB (CK-MB), creatinine, lactate, intraoperative haematocrit] and ethylenediaminetetraacetic acid (haematological analyses). Study blood samples were taken at 5 time points: (i) preoperatively on induction of anaesthesia, (ii) after weaning of ECC on full heparinization, (iii) after protamine administration, (iv) after 6 h (6–10 h after completion of surgery) and (v) on the first postoperative day (16–20 h after completion of surgery). Samples were obtained through a central venous line, through which no heparin was administered, and the first 5 ml were discarded. Blood was centrifuged within 15 min of collection (3300 × g at 20°C for 25 min). Plasma was separated and stored at −80°C until subsequent batch analysis. Routine blood samples to monitor renal function were taken through antecubital venipuncture on days 2–4 (Fig. 2).
Figure 2:
Flow chart of study interventions and blood sampling. Red drops symbolize time points of blood sampling. CABG: coronary artery bypass grafting; ECC: extracorporeal circulation; preop: preoperative.
Outcome measures
Coagulation markers
Primary end point was ETP, measured as area under the curve (nmol/min) in platelet-poor plasma using calibrated automated thrombograms (Thrombinoscope®, Maastricht, Netherlands). The following secondary end points were measured: plasma TAT and F1 + 2 determined by enzyme-linked immunosorbent assays (Enzygnost®; Siemens Healthcare Diagnostics, Marburg, Germany), reflecting in vivo thrombin generation; clot lysis measured as clot lysis area under the curve, reflecting balance between clot formation and clot lysis using an in-house dynamic turbidimetric assay [16]; fibrin d-dimer, fibrinogen, international normalized ratio and activated partial thrombin time measured by automated coagulation systems; haematological parameters by an automated haematology analyser; and free haemoglobin by Advia Chemistry XPT.
Clinical outcome measures
The number of patients receiving blood products in total, and for each component, was registered after 24 h and at 30-day follow-up. Postoperative blood loss was assessed by the measurement of chest tube output at 24 h. Postoperative end-organ dysfunction was assessed by following measures: (i) renal injury by nadir glomerular filtration rate [17], and RIFLE acute renal injury classification [18]; (ii) myocardial injury by peak CK-MB; (iii) inadequate tissue perfusion by peak blood lactate; (iv) incidence of atrial fibrillation that required medical intervention; (v) tissue damage by lactate dehydrogenase; (vi) haemolysis by free haemoglobin; and (vii) haemodilution by nadir haematocrit and body weight gain.
Major adverse cardiac and cerebrovascular events were assessed at 30-day follow-up: (i) death; (ii) cerebrovascular accident as assessed by National Institutes of Health Stroke Scale; (iii) perioperative and spontaneous myocardial infarction during follow-up [19, 20]; and (iv) repeat revascularization defined as percutaneous coronary intervention or CABG during follow-up.
Statistical analysis
A total of 30 patients in each group were required to be able to reject the null hypothesis that MiECC and CECC would not differ in ETP early after CABG based on the following assumptions: mean endogenous thrombin generation potential in platelet-poor plasma early after CABG with CECC 1508 ± 297 nmol/l × min [standard deviation (SD)] [12], estimated minimal relevant difference of 250 nmol/l × min, significance level 0.05 (2-sided) and 90% power (1 − β).
Categorical variables are presented as numbers and percentages. Continuous variables are presented as mean ± SD when distributed close to normal as assessed by quantile–quantile plots, otherwise as median (interquartile range). Intergroup comparisons were performed with the Fisher’s exact test for all binary outcomes, t-test for continuous outcomes following normal distributions, and otherwise with Wilcoxon–Mann–Whitney test. Comparisons with baseline measurements were performed with paired t-test when values followed normal distribution, and otherwise with Wilcoxon signed-rank test. P-values below 0.05 were determined as statistically significant. Spearman rank correlation was used to measure the strength of association between non-normally distributed variables with the denotation of correlation coefficient (rs). All statistical analyses were done using STATA 15® (STATA Corp., College Station, TX, USA).
RESULTS
Patients
We randomized 60 patients from 28 September 2017 to 31 October 2018 (Fig. 1). Patient demographics, procedural characteristics and laboratory findings are presented in Table 1.
Table 1:
Demographics, procedural characteristics and laboratory findings
| Variables | MiECC | CECC |
|---|---|---|
| Demographics | ||
| EuroSCORE II, median (IQR) | 0.89 (0.20) | 0.99 (1.02) |
| Age (years),a mean ± SD | 64.6 ± 9.0 | 68.2 ± 9.2 |
| Gender (male),an (%) | 28 (93) | 23 (77) |
| Moderate/severe renal impairment,an (%) | 10 (33) | 16 (53) |
| Extracardiac arteriopathy,an (%) | 2 (7) | 2 (7) |
| Poor mobility,an (%) | 0 (0) | 2 (7) |
| Chronic lung disease,an (%) | 3 (10) | 5 (17) |
| Diabetes on insulin,an (%) | 2 (7) | 3 (10) |
| Moderate/poor ejection fraction,an (%) | 7 (23) | 8 (27) |
| Recent acute myocardial infarction,an (%) | 5 (17) | 3 (10) |
| Body mass index (kg/m2), mean ± SD | 28.2 ± 3.8 | 27.6 ± 3.9 |
| Arterial hypertension, n (%) | 25 (83) | 25 (83) |
| Hypercholesterolaemia, n (%) | 30 (100) | 28 (93) |
| Smoking history, n (%) | 20 (67) | 24 (80) |
| Indication for revascularization, n (%) | ||
| Acute coronary syndrome | 10 (33) | 5 (17) |
| Stable angina pectoris | 18 (60) | 24 (80) |
| Angina equivalent (dyspnoea) | 2 (7) | 1 (3) |
| Procedural characteristics | ||
| Surgery time (min), mean ± SD | 186 ± 33 | 182 ± 39 |
| ECC time (min), median (IQR) | 87 (25) | 76 (27) |
| Aortic clamp time (min), median (IQR) | 47 (22) | 45 (26) |
| Additional pulmonary vein ablation and left atrial appendage amputation, n (%) | 2 (7) | 2 (7) |
| Number of coronary artery grafts, mean ± SD | 2.8 ± 0.9 | 2.7 ± 0.7 |
| DO2 maintained >270 ml, n (%) | 27 (90) | 24 (80) |
| SvO2 maintained >65%, n (%) | 27 (90) | 29 (97) |
| Preoperative laboratory findings | ||
| Haematocrit (%), mean ± SD | 44.9 ± 3.7 | 42.3 ± 5.6 |
| Haemoglobin (RI: 8.3–10.5 mmol/l), mean ± SD | 9.0 ± 0.7 | 8.8 ± 1.1 |
| eGFR (RI: >60 ml/min/1.73 m2), median (IQR) | 87 (10) | 82 (26) |
Definition according to the EuroSCORE II [21].
CECC: conventional extracorporeal circulation; DO2: oxygen delivery; ECC: extracorporeal circulation; eGFR: estimated glomerular filtration rate per 1.73 m2 body surface area calculated according to Chronic Kidney Disease Epidemiology Collaboration equation; EuroSCORE II: European System for Cardiac Operative Risk Evaluation II; IQR: interquartile range; MiECC: minimally invasive extracorporeal circulation; RI: reference interval; SD: standard deviation; SvO2: mixed venous oxygen saturation.
Thrombin generation and supplemental markers of coagulation
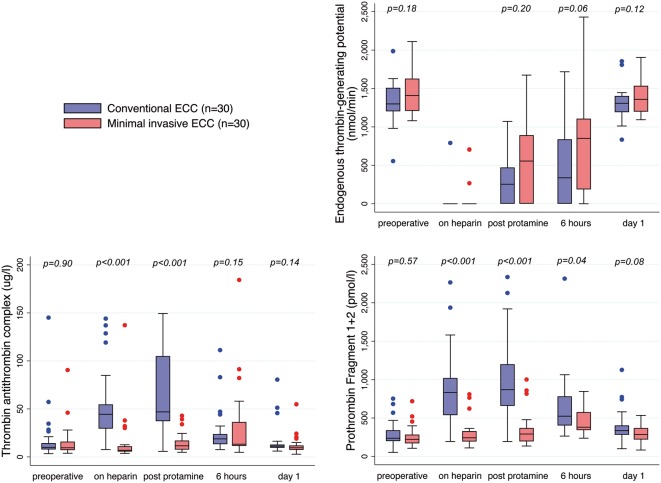
Perioperative changes in markers related to thrombin generation are shown in Fig. 3. ETP was completely suppressed in all but 4 patients during heparinization. ETP was significantly reduced up to 6 h postoperatively compared to baseline in both groups. Six hours after surgery, the median ETP in the MiECC group was twice as high as in the CECC group, but the difference did not reach statistical significance.
Figure 3:
Perioperative levels of thrombin generation markers in both groups. Centre line indicates median; box, interquartile range; error bars, upper/lower adjacent values; and dots, outside values. ECC: extracorporeal circulation. Time points: on heparin: after weaning of ECC; post-protamine: after protaminization; 6 hours: 6 h after surgery; day 1: 1 postoperative day.
In both groups, TAT and F1 + 2 levels increased significantly from baseline during surgery. Both markers were significantly lower in MiECC patients compared to CECC patients during surgery. Fibrinolysis was totally obliterated following administration of tranexamic acid in both groups as directly measured by clot lysis area under the curve. Intraoperative fibrin d-dimer levels were significantly lower in MiECC patients compared to CECC patients. Perioperative levels of other individual coagulation markers and platelets did not differ significantly between the groups (Table 2).
Table 2:
Supplemental laboratory coagulation findings
| Variables | Reference interval | Treatment group | Preoperativea |
Full heparinization |
Post-protamine |
6 h |
Day 1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levels | P-value | Levels | P-value | Levels | P-value | Levels | P-value | Levels | P-value | |||
| Clot lysis (AUC) | 219–1051 | CECC | 2888 ± 133 | 0.25 | No analysis | |||||||
| MiECC | 3075 ± 94 | |||||||||||
| Fibrin d-dimer (mg/l FEU) | <0.60 | CECC | 0.3 (0.3) | 0.4 | 0.4 (0.3) | 0.01 | 0.5 (0.4) | 0.01 | 0.7 (0.3) | 0.07 | 0.5 (0.3) | 0.33 |
| MiECC | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.2) | 0.6 (0.3) | 0.5 (0.3) | |||||||
| Fibrinogen (μmol/l) | 5.5–12 | CECC | 8.1 ± 1.9 | 0.21 | 6.3 ± 1.3 | 0.16 | 6.4 ± 1.3 | 0.19 | 7.7 ± 1.6 | 0.63 | 10.3 ± 1.7 | 0.08 |
| MiECC | 8.8 ± 2.3 | 6.8 ± 1.7 | 6.9 ± 1.7 | 7.9 ± 1.7 | 11.2 ± 10.5 | |||||||
| INR | <1.2 | CECC | 1.1 (0.1) | 0.98 | 2.2 (0.7) | 0.89 | 1.2 (0.1) | 0.15 | 1.1 (0.1) | 0.06 | 1.2 (0.1) | 0.14 |
| MiECC | 1.1 (0.1) | 2.2 (0.7) | 1.3 (0.2) | 1.2 (0.1) | 1.2 (0.1) | |||||||
| APTT (s) | 20–29 | CECC | 24 (3) | 0.71 | 150 (0) | 0.31 | 36 (18) | 0.14 | 24 (6) | 0.45 | 24 (2) | 0.88 |
| MiECC | 24 (3) | 150 (0) | 33 (16) | 23 (3) | 24 (3) | |||||||
| Platelets | 145–350 | CECC | 251 (109) | 0.92 | No analysis | 221 (63) | 0.73 | 194 (65) | 0.99 | |||
| MiECC | 245 (67) | 211 (45) | 188 (65) | |||||||||
Data are presented as mean ± standard deviation and median with interquartile range as indicated.
Measurement following administration of tranexamic acid.
AUC: area under the curve; APTT: activated partial thromboplastin time; CECC: conventional extracorporeal circulation; FEU: fibrinogen equivalent unit; INR: international normalized ratio; MiECC: minimally invasive extracorporeal circulation.
Bleeding end points
MiECC patients required half as many transfusions after 24 h and after 30 days compared to CECC patients (Table 3). No difference in 24-h blood loss was observed between groups. One patient in the CECC group required surgical re-exploration due to haemorrhage. No correlation was found between early postoperative ETP and 30-day transfusion requirements (rs = 0.05, P = 0.72). However, intraoperative levels of TAT and F1 + 2 showed significant correlations with increased transfusion requirement at 30-day follow-up (TAT: rs = 0.36, P = 0.005; F1 + 2: rs = 0.45, P = 0.0003).
Table 3:
Clinical outcome
| Variables | MiECC | CECC | P-value |
|---|---|---|---|
| Bleeding-related outcome | |||
| Drain loss after 24 h (ml), median (IQR) | 448 (290) | 468 (270) | 0.84 |
| Transfusions after 24 h, n (%) | 3 (10, 2–27) | 6 (20, 8–39) | 0.47 |
| Packed red blood cells | 1 (3, 0–17) | 4 (13, 4–31) | 0.35 |
| Pooled platelets concentrate | 2 (7, 1–22) | 4 (13, 4–31) | 0.67 |
| Fresh frozen plasma | 1 (3, 0–17) | 2 (7, 1–22) | 1.00 |
| Total blood transfusion after 30 days, n (%) | 5 (17, 6–35) | 11 (37, 20–56) | 0.14 |
| Re-exploration due to haemorrhage, n (%) | 0 (0–12) | 1 (3, 0–17) | 0.50 |
| In-hospital outcome | |||
| Postoperative requirement noradrenaline, n (%) | 5 (17, 6–35) | 6 (20, 8–39) | >0.99 |
| Postoperative requirement other inotropes, n (%) | 3 (10, 2–27) | 2 (7, 1–22) | >0.99 |
| Maximal SVRI (dynes/s/cm−5), mean ± SD | 1783 ± 374 | 2063 ± 638 | 0.04 |
| Nadir cardiac index (l/min/m2), mean ± SD | 2.0 ± 0.3 | 1.8 ± 0.3 | 0.10 |
| Intensive care stay >24 h, n (%) | 1 (3, 0–17) | 1 (3, 0–17) | >0.99 |
| Mechanical ventilation >12 h, n (%) | 0 (0–12) | 1 (3, 0–17) | >0.99 |
| Length of hospital stay (days), median (IQR) | 7 (2) | 7 (2) | 0.62 |
| Atrial fibrillation/flutter, n (%) | 12 (40, 23–59) | 13 (43, 26–63) | >0.99 |
| Fluid balance, median (IQR) | |||
| Weight gain day 1 (kg) | 1.0 (1.7) | 1.5 (2.1) | 0.05 |
| Weight gain day 2 (kg) | 2.4 (4.2) | 2.1 (1.7) | 0.77 |
| Nadir eGFR in-hospital, median (IQR) | 80 (25) | 66 (36) | 0.07 |
| Acute kidney injury,an (%) | 0.28 | ||
| No acute kidney injury | 26 (87) | 23 (77) | |
| Risk: >25% reduced | 2 (7) | 6 (20) | |
| Injury: eGFR >50% reduced | 2 (7) | 1 (3) | |
| Failure: eGFR >75% reduced | 0 | 0 | |
| Loss: eGFR <15 ml/min, >27 days | 0 | 0 | |
| End-stage kidney disease | 0 | 0 | |
| Peak postoperative CK-MB (μg/l), median (IQR) | 19 (7) | 24 (17) | 0.03 |
| Peak postoperative lactate (mmol/l), median (IQR) | 1.45 (0.9) | 1.45 (0.8) | 0.75 |
| Peak LDH (U/l), median (IQR) | 229 (80) | 380 (114) | <0.001 |
| Infectious complications, n (%) | |||
| Harvest site infection | 5 (17, 6–35) | 3 (10, 2–27) | 0.71 |
| Superficial sternal wound infection | 0 (0–12) | 2 (7, 1–22) | 0.49 |
| Deep sternal wound infection | 0 (0–12) | 1 (3, 0–17) | >0.99 |
| Pneumonia | 5 (17, 6–35) | 6 (20, 8–39) | >0.99 |
| Other infection requiring antibiotics | 4 (13, 4–31) | 4 (13, 4–31) | >0.99 |
Classification of acute kidney injury according to RIFLE criteria [18].
CECC: conventional extracorporeal circulation; CK-MB: creatine kinase-MB; eGFR: estimated glomerular filtration rate per 1.73 m2 body surface area calculated according to Chronic Kidney Disease Epidemiology Collaboration equation; IQR: interquartile range; LDH: lactate dehydrogenase; MiECC: minimally invasive extracorporeal circulation; SD: standard deviation; SVRI: systemic vascular resistance index.
Haemodilution
Haematocrit levels were significantly higher during and early after CABG with MiECC compared to CECC (Fig. 4). Likewise, we observed significant lower weight gain at day 1. No correlation between decrease in haematocrit and individual coagulation analyses could be detected. Consequently, we omitted adjustment for haemodilution.
Figure 4:

Perioperative haematocrit levels in both groups. Centre line indicates median; box, interquartile range; error bars, upper/lower adjacent values; and dots, outside values. ECC: extracorporeal circulation; nadir intraoperative: lowest intraoperative value; 6 hours: 6 h after surgery; day 1: 1 postoperative day.
End-organ dysfunction
Postoperative peak CK-MB, lactate dehydrogenase and free haemoglobin levels were significantly lower in the MiECC group than in the CECC group indicative of superior myocardial protection, less tissue damage and less haemolysis. Other indices of in-hospital clinical outcome did not differ between groups (Table 3).
Safety and feasibility
All patients received the allocated intervention. Quality indices of perfusion and surgery were comparable between groups (Table 1). We encountered 3 cases of VARD alarm during MiECC (1 accidental disconnection of central venous catheter, 1 displacement of venous cannula and 1 without obvious finding). Air was effectively removed in all cases by the VARD with no resulting air lock or subsequent neurological dysfunction. Two patients in the CECC group required graft revision after the removal of cross-clamp.
Requirement of prolonged intensive care stay, mechanical ventilation or inotropic support did not differ between groups (Table 3). We registered no death, no stroke and 1 perioperative myocardial infarction in the CECC group (CK-MB elevation without clinical signs of ischaemia) and 1 incomplete surgical revascularization in each group, which necessitated completion percutaneous coronary intervention prior to discharge.
DISCUSSION
This is the first randomized study to investigate the impact of MiECC compared to CECC on thrombin generation, and concomitantly on bleeding end points and haemodilution. Reduced ETP has been suggested to be associated with increased postoperative bleeding after CABG with CECC [12, 13]. Reduced ETP is thought to be more indicative of postoperative bleeding than reduced levels of individual plasma coagulation factors as it reflects overall suppression of plasma haemostatic capacity.
We found a significant decrease in early postoperative ETP in both groups with a non-significant trend of better preservation of ETP in the MiECC group. In the MiECC group, we found very low intraoperative levels of TAT and F1 + 2, indicating the absence of significant in vivo thrombin generation during surgery. In contrast, we confirmed previous findings of CABG with CECC to be associated with significant intraoperative thrombin generation as measured by levels of TAT and F1 + 2 [22, 23]. Correspondingly, we observed significantly lower intraoperative levels of fibrin d-dimer in the MiECC group, which following tranexamic acid-induced suppression of fibrinolysis in both groups indirectly reflects the extent of thrombin generation. Thus, our results substantiate prior findings that ‘open-circuit’ ECC in contrast to ‘closed-circuit’ ECC triggers significant in vivo thrombin generation despite full heparinization [8, 9].
Consumption of coagulation factors through in vivo thrombin generation may explain the positive correlation of intraoperatively increased TAT and F1 + 2 levels with increased transfusion requirements. However, the results of our study question the ability of early postoperative thrombin-generating potential to reflect transfusion requirements due to the spread of values over a large range.
In addition, the difference in early postoperative ETP between groups may have been mitigated by the fact that we used heparin concentration-based anticoagulation management during ECC in both groups. Heparin titration has been shown to significantly reduce thrombin generation as compared to conventional management based on activated clotting time [24].
In accordance with other studies, we observed reduced haemodilution until first postoperative day in the MiECC group as measured by significantly lower haematocrit levels and significantly less weight gain [7, 25, 26]. Our results disprove the theory that haemodilution is a main factor contributing to impaired haemostasis as neither coagulation factors nor bleeding end points were correlated to the degree of haemodilution.
In contrast to others, our results did not confirm any correlation between markers of intraoperative thrombin generation and blood loss [11, 13]. A possible explanation for this discrepancy could be the selection of low-risk CABG patients and treatment by only 2 experienced cardiac surgeons resulting in low volumes of blood loss.
Limitations
The major limitation of our study is not adequately powered to evaluate clinical outcomes such as bleeding end points. Thus, these findings must be considered strictly observational and interpreted with caution. Larger-scale studies or meta-analyses are necessary to provide adequate statistical power to assess the assumed benefits of MiECC in terms of clinical outcome.
With the perspective of future improvement of ECC, our data suggest the absence of significant in vivo thrombin generation during MiECC as compared to CECC and not minimized haemodilution as a possible explanation for preserved haemostatic capacity.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all MiECC group members at Aarhus University Hospital for enthusiastic support: Karsten L. Søberg and Peter F. Nielsen (Perfusion), Pia K. Ryhammer and Sandeep Prataprao Tambe (Department of Anaesthesiology and Intensive Care). Statistical input was provided by Erik T. Parner (Department of Biostatistics, Aarhus University) and Jakob Hjort (Department of Clinical Medicine, Aarhus University).
Funding
This study was supported by Medtronic External Research Program [ERP-2018-11272] and NIH/NCRR Colorado CTSI [UL1 RR025780] (REDCap).
Conflict of interest: none declared.
ABBREVIATIONS
- CABG
Coronary artery bypass grafting
- CECC
Conventional extracorporeal circulation
- CK-MB
Creatine kinase-MB
- ECC
Extracorporeal circulation
- ETP
Endogenous thrombin-generating potential
- F1 + 2
Fragment 1 + 2
- MiECC
Minimally invasive extracorporeal circulation
- TAT
Thrombin–antithrombin complex
Presented at the 33rd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 3–5 October 2019.
Author contributions
Ivy Susanne Modrau: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Debbie Richards Halle: Conceptualization; Data curation; Investigation; Methodology; Project administration; Validation; Writing—original draft; Writing—review & editing. Per Hostrup Nielsen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing—original draft; Writing—review & editing. Hans Henrik Kimose: Conceptualization; Investigation; Methodology; Project administration; Writing—original draft; Writing—review & editing. Jacob Raben Greisen: Conceptualization; Methodology; Project administration; Writing—original draft; Writing—review & editing. Michael Kremke: Conceptualization; Investigation; Project administration; Writing—original draft; Writing—review & editing. Anne-Mette Hvas: Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
REFERENCES
- 1. Anastasiadis K, Murkin J, Antonitsis P, Bauer A, Ranucci M, Gygax E. et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact CardioVasc Thorac Surg 2016;22:647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun Y, Gong B, Yuan X, Zheng Z, Wang G, Chen G. et al. What we have learned about minimized extracorporeal circulation versus conventional extracorporeal circulation: an updated meta-analysis. Int J Artif Organs 2015;38:444–53. [DOI] [PubMed] [Google Scholar]
- 3. Anastasiadis K, Antonitsis P, Haidich A-B, Argiriadou H, Deliopoulos A, Papakonstantinou C.. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2013;164:158–69. [DOI] [PubMed] [Google Scholar]
- 4. Paone G, Brewer R, Theurer PF, Bell GF, Cogan CM, Prager RL.. Preoperative predicted risk does not fully explain the association between red blood cell transfusion and mortality in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2012;143:178–85. [DOI] [PubMed] [Google Scholar]
- 5. Vivacqua A, Koch CG, Yousuf AM, Nowicki ER, Houghtaling PL, Blackstone EH. et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg 2011;91:1780–90. [DOI] [PubMed] [Google Scholar]
- 6. Anastasiadis K, Asteriou C, Deliopoulos A, Argiriadou H, Karapanagiotidis G, Antonitsis P. et al. Haematological effects of minimized compared to conventional extracorporeal circulation after coronary revascularization procedures. Perfusion 2010;25:197–203. [DOI] [PubMed] [Google Scholar]
- 7. Nollert G, Schwabenland I, Maktav D, Kur F, Christ F, Fraunberger P. et al. Miniaturized cardiopulmonary bypass in coronary artery bypass surgery: marginal impact on inflammation and coagulation but loss of safety margins. Ann Thorac Surg 2005;80:2326–32. [DOI] [PubMed] [Google Scholar]
- 8. Farneti P, Sbrana S, Spiller D, Cerillo A, Santarelli F, Di Dario D. et al. Reduction of blood coagulation and monocyte-platelet interaction following the use of a minimal extracorporeal circulation system (Synergy®) in coronary artery bypass grafting (CABG). Perfusion 2008;23:49–56. [DOI] [PubMed] [Google Scholar]
- 9. Wippermann J, Albes JM, Hartrumpf M, Kaluza M, Vollandt R, Bruhin R. et al. Comparison of minimally invasive closed circuit extracorporeal circulation with conventional cardiopulmonary bypass and with off-pump technique in CABG patients: selected parameters of coagulation and inflammatory system. Eur J Cardiothorac Surg 2005;28:127–32. [DOI] [PubMed] [Google Scholar]
- 10. Hemker HC, Al Dieri R, De Smedt E, Béguin S.. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 2006;96:553–61. [PubMed] [Google Scholar]
- 11. Moorlag M, Schurgers E, Krishnamoorthy G, Bouwhuis A, Lindhout T, Kelchtermans H. et al. Near patient thrombin generation in patients undergoing elective cardiac surgery. J Appl Lab Med 2017;1:613–25. [DOI] [PubMed] [Google Scholar]
- 12. Bosch Y, Al Dieri R, ten Cate H, Nelemans P, Bloemen S, Hemker C. et al. Preoperative thrombin generation is predictive for the risk of blood loss after cardiac surgery: a research article. J Cardiothorac Surg 2013;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coakley M, Hall JE, Evans C, Duff E, Billing V, Yang L. et al. Assessment of thrombin generation measured before and after cardiopulmonary bypass surgery and its association with postoperative bleeding. J Thromb Haemost 2011;4:1523–9. [DOI] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danish National Board of Health [Sundhedsstyrelse]. Guidelines on Blood Transfusion [Vejledning om blodtransfusion]. Sundhedsstyrelsen [Internet] 2015. http://www.dasaim.dk/wp-content/uploads/2017/01/Vejledning-om-blodtransfusion-SST.pdf (15 January 2020, date last accessed).
- 16. Neergaard-Petersen S, Grove EL, Mogensen VB, Kristensen SD, Hvas A-M, Veirup MS.. Fibrin clot lysis assay: establishment of a reference interval. Thromb Res 2018;167:9–11. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P.. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES. et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the society for cardiovascular angiography and interventions (SCAI). J Am Coll Cardiol 2013;62:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. et al. ; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Hear J 2012;33:2551–67. [Google Scholar]
- 21. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR. et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–44; discussion 744–5. [DOI] [PubMed] [Google Scholar]
- 22. Raivio P, Kuitunen A, Suojaranta-Ylinen R, Lassila R, Petäjä J.. Thrombin generation during reperfusion after coronary artery bypass surgery associates with postoperative myocardial damage. J Thromb Haemost 2006;4:1523–9. [DOI] [PubMed] [Google Scholar]
- 23. Chandler W, Velan T.. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003;101:4355–20. [DOI] [PubMed] [Google Scholar]
- 24. Koster A, Fischer T, Praus M, Haberzettl H, Kuebler WM, Hetzer R. et al. Hemostatic activation and inflammatory response during impact of heparin management. Anesthesiology 2002;97:837–41. [DOI] [PubMed] [Google Scholar]
- 25. Aal M. A, ElNahal N, Bakir BM, Fouda M.. Mini-cardiopulmonary bypass impact on blood conservation strategy in coronary artery bypass grafting. Int Cardiovasc Thorac Surg 2011;12:600–4. [DOI] [PubMed] [Google Scholar]
- 26. Zeitani J, Buccisano F, Nardella S, Flaminio M, Prati P, Chiariello G. et al. Mini-extracorporeal circulation minimizes coagulation abnormalities and ameliorates pulmonary outcome in coronary artery bypass grafting surgery. Perfusion 2013;28:298–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.