Abstract
Objectives:
To investigate the effect of repeated binge drinking and moderate alcohol consumption in young adults on arterial stiffness and sympathetic activity in young adults
Methods:
We enrolled 49 healthy young adults, free of cardiovascular diseases (25 males; age: 23.5±0.4 years; body mass index: 23.4±0.4 kg/m2; mean±S.E). Subjects included were those with a history of repeated binge drinking (BDs, > 2 years duration; n=20), drank at moderate levels (MODs, > 5 years duration; n=16), and abstained from alcohol (ABs, last 2–3 years; n=13). Arterial stiffness was assessed using carotid to femoral pulse wave velocity (cfPWV) and sympathetic activity was assessed using 24-hour urinary norepinephrine (NE) levels. Also measured was aortic systolic blood pressure and augmentation index (AIx), a measure of wave reflection.
Results:
BDs and MODs had higher cfPWV compared with ABs (0.6 and 0.5 m/sec, respectively; P≤0.04). In addition, BDs had higher urinary NE levels compared with MODs and ABs (P<0.05). Higher cfPWV were correlated with higher NE levels (r=0.35. P=0.02). Aortic systolic blood pressure (P=0.2) and AIx (P=0.96) were similar among BDs, MODs, and ABs.
Conclusions:
Our findings suggest that repeated exposure to alcohol, regardless of drinking pattern, may increase aortic arterial stiffness in healthy young adults. In addition, sympathetic activation, reflected by increased 24-hour urinary NE levels may contribute to alcohol-induced arterial stiffening in young adults.
Keywords: Alcohol Drinking in College, Arterial Pressure, Binge Drinking, Vascular Function, Vascular Stiffness
INTRODUCTION
In the U.S. and worldwide, individuals aged 18 to 35 years have the highest prevalence of alcoholic beverage consumption and binge drinking [1,2]. In older and middle-aged adults, binge drinking is associated with numerous adverse cardiovascular outcomes [3–6]. Less is known about the cardiovascular effects of repeated binge drinking in young adults, but emerging data indicate binge drinking may be associated with subclinical cardiovascular disease such as elevated systolic blood pressure (SBP) [7,8]. Alcohol use has been linked to arterial stiffness, an independent risk factor of future cardiovascular disease [9] including hypertension [10]. Arterial stiffness is associated with sympathetic nerve activity [11], via the regulation on vascular smooth muscle tone. Previous studies demonstrated that arterial stiffness increases acutely following norepinephrine (NE) infusion [12], lower body negative pressure [13], and mental stress [14].
Several studies have examined the effect of alcohol consumption on sympathetic activation and there are data supporting the scientific premise that alcohol consumption in young adults may be associated with increased sympathetic activation. However, studies to date have only examined the effect of a “single, one-time” binge or moderate drinking episode [15–21]. Other studies examined the effects of “alcohol abuse” on urinary NE levels in middle-aged or older subjects [22] or in middle-aged adults with sleep apnea [23]. Data from these studies cannot be generalized to young adult healthy populations who have a history of repeated binge drinking. Repeated binge drinking as well as regular moderate drinking in young adults may alter sympathetic activity and arterial stiffness, creating a proclivity or milieu that leads to heightened vulnerability of future cardiovascular disease.
Therefore, the primary aim of our study was to investigate arterial stiffness and sympathetic activity in healthy young adults who had a history of repeated binge drinking (BDs), moderate alcohol consumption (MODs), and alcohol abstention (ABs). We also include the measurements of aortic blood pressure and pressure wave reflection, parameters associated with arterial stiffness. We hypothesized that BDs and MODs would have higher arterial stiffness and sympathetic activity compared with ABs. In addition, we hypothesized that alcohol-induced sympathetic activation would be associated with increased arterial stiffness, which may be associated with higher pressure wave reflection and SBP in young adults.
METHODS
Study design and participants
This cross-sectional study included young healthy adults (18–30 years), without cardiovascular disease and recruited from both the community and a university campus. Participants were excluded from the study if they had any known cardiovascular risk factors (as described below); history of diabetes, renal disease, seizure disorder, cancer, and inflammatory disease; current or history of cigarette smoking and illicit drug use; and active infection. Female participants were not included if they were pregnant based on a urine pregnancy test. All participants completed a detailed medical history questionnaire and an Alcohol Intake Questionnaire to determine the pattern and frequency of alcohol consumption [24]. Study procedures were performed in the morning after a 12-hour fast and abstinence of caffeine, alcohol, and medication use, and during early follicular phase for female participants. For BDs, the time from last binge drinking episode was a minimum of 48 hours. We have previously demonstrated that blood alcohol levels in a similar cohort of MODs (n=23) and BDs (n=58) are 0 at the time of study enrollment [24]. Aortic arterial stiffness, aortic blood pressure, and wave reflection were assessed in a quiet, semi-darkened, and temperature-controlled room after 10 minutes of a supine rest. Similar to others, we measured urinary NE levels rather than plasma samples since urinary levels are considered to provide a more integrated and estimated representation of sympathetic activity over an entire day [25,26]. Participants were given a urine collection container for a 24-hour urine collection to measure NE level. The study was approved by the University of Illinois at Chicago Office of Protection of Research Subjects and Institutional Review Board. All participants gave written informed consent.
Measurement of alcohol use and binge drinking definition
As previously described [24,27], we used the Alcohol Intake Questionnaire to categorize alcohol use and pattern (BD, MOD, and AB) and Alcohol Use Disorders Test (AUDIT) to assess the risk of heavy alcohol drinking. Also measured, was dry blood phosphatidylethanol [24], an indicator of heavy alcohol consumption [28].
BD was defined as consuming 5 or more drinks for men and 4 or more drinks for women, either on the same occasion or within 2 hours [29], and on at least 2 days in the last month and for more than 2 years. MOD was defined as consuming no more than 3 drinks per sitting for men and no more than 2 drinks per sitting for women, and no more than 1–2 times per week in the last 5 years. AB was defined as consuming no more than 1 drink per month in the last 2–3 years (and abstention could not be due to a medical illness or prior alcohol abuse). One drink contains 14 g of pure alcohol such as 12 oz. beer, 5 oz. wine, 1.5 oz. of 80-proof spirits, 8–9 oz. of malt liquor [29].
Cardiovascular risk assessment
Body weight and height (body mass index), fasting venous blood sample analysis, and seated blood pressure measurements were preformed to screen for cardiovascular risk factors such as: obesity (body mass index ≥ 30 kg/m2), hyperlipidemia (total cholesterol > 230 mg/dL and/or low-density lipoprotein cholesterol > 160 mg/dL), or hypertension (blood pressure > 140/80 mmHg). Peripheral (seated) blood pressure was obtained on the right brachial artery after a 15-min rest using oscillometric technique (HEM-907XL, Omron Corporation, Japan), and 3 readings were obtained at 1-min intervals and averaged.
Arterial stiffness
Using the SphygmoCor XCEL system (SphygmoCor; AtCor Medical, Australia), carotid to femoral pulse wave velocity (cfPWV), was calculated from simultaneous acquisition of pressure waveforms at the carotid artery via a high-fidelity micromanometer (Millar Instruments) and at the femoral artery via a cuff [30]. The measurements were performed in duplicate and averaged, or triplicate If the difference between 2 measurements was more than 0.5 m/sec and median value was used instead [31].
Aortic blood pressure and wave reflection characteristics
Using the SphygmoCor XCEL system (SphygmoCor; AtCor Medical, Australia), aortic blood pressure and wave reflection characteristics were assessed [30]. Briefly, brachial artery pressure waveforms were obtained by using the volumetric displacement from a cuff inflated over the brachial artery. Aortic waveform was generated by applying a general transfer function to the acquired peripheral signal. Pulse wave analysis of aortic pressure waveforms provides aortic blood pressure and the following wave reflection characteristics: augmentation pressure, pressure at inflection point, forward pulse height, reflected pulse height, and end-systolic blood pressure. AIx was calculated as the ratio of augmentation pressure to the aortic pulse pressure and was adjusted for a heart rate of 75 bpm. Generally, the measurements were performed in duplicate.
24-hour urinary NE measurement
Similar to methods described by others [32], urine samples were collected into polyethylene bottles containing 25 ml of 6N hydrochloric acid. Samples were sent to Quest Diagnostics within 48 hours of collection for NE levels analysis using high performance liquid chromatography with electrochemical detection (analytic sensitivity, 3 μg/L). Also measured, were epinephrine and dopamine levels.
Statistics analyses
Statistical analyses were conducted using IBM SPSS Statistics (Essentials, Version 22) and α was set at 0.05. Data are presented as mean±SE or n (%). To examine group differences in participant characteristics, one-way ANOVA was used for continuous variables, and χ2 was used for categorical variables. To examine group differences in aortic blood pressure, arterial stiffness, wave reflection, and urinary NE levels, univariate ANOVA was used and controlled for sex. In addition, analysis with wave reflection characteristics included the covariate of body height, which is known to influence wave reflection characteristics [33]. Post hoc analysis was performed with Bonferroni correction. The correlations of interests were examined using Pearson product-moment correlation coefficients and scatter plots.
RESULTS
A total of 20 BDs, 16 MODs, and 13 ABs were included in this study. Phosphatidylethanol levels were greater than 50 ng/mL in BDs and 0 in abstainers. In MOD subjects, the majority of phosphatidylethanol levels were 0 ng/mL (mean: 2±1 ng/mL). The total AUDIT score was significantly greater in BDs compared to ABs and MODs (BDs: 7.7±3.9; MODs: 3.5±0.6; ABs: 0.7±0.4; P<0.0005). No differences were found in age, sex, body mass index, lipid profile, and seated blood pressure among groups (Table 1).
Table 1.
Subject characteristics
| ABs (n=13) | MODs (n=16) | BDs (n=20) | P Group | |
|---|---|---|---|---|
| Age, years | 22±1 | 24±1 | 24±1 | 0.053 |
| Male/female, n | 7/6 | 8/8 | 10/10 | 0.97 |
| Body weight, kg | 62.1±2.5 | 66.7±2.8 | 72.0±2.2* | 0.03 |
| Body height, cm | 166.2±2.2 | 166.0±2.2 | 174.8±1.9*† | 0.004 |
| Body mass index, kg/m2 | 22.5±0.8 | 24.1±0.6 | 23.5±0.6 | 0.2 |
| Waist circumference, cm | 77.4±2.4 | 82.0±7.7 | 81.2±1.6 | 0.3 |
| Systolic BP, mmHg | 110±2 | 112±2 | 112±2 | 0.6 |
| Diastolic BP, mmHg | 65±2 | 69±2 | 65±2 | 0.4 |
| Total cholesterol, mg/dL | 154±9 | 165±6 | 149±9 | 0.3 |
| Triglyceride, mg/dL | 86±10 | 90±15 | 62±5 | 0.2 |
| LDL cholesterol, mg/dL | 85±7 | 96±6 | 79±6 | 0.2 |
| HDL cholesterol, mg/dL | 52±2 | 52±3 | 58±4 | 0.3 |
| Glucose, mg/dL | 89±1 | 92±2 | 87±1 | 0.1 |
| Insulin, mIU/L | 4.5±0.6 | 5.7±0.7 | 4.3±0.4 | 0.2 |
| HOMA-IR | 1.1±0.1 | 1.3±0.2 | 0.9±0.1 | 0.1 |
| Creatinine, mg/dL | 0.85±0.03 | 0.87±0.03 | 0.86±0.03 | 0.9 |
Data are mean±SE or n. ABs, alcohol abstainers; BDs, binge drinkers; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MODs, moderate drinking.
P=0.02 vs. Abstainers
P=0.01 vs. Moderate
Aortic blood pressure, arterial stiffness and wave reflection
No differences were found in aortic SBP and diastolic blood pressure among groups (Table 2). BDs and MODs had higher cfPWV than ABs by 0.6 m/sec (P=0.006) and 0.5 m/sec (P=0.035) respectively (Figure 1). BDs had higher aortic pulse pressure than ABs and MODs, but differences were not significant in post-hoc analysis (P=0.07; Table 2). No differences were found in other aortic wave reflection characteristics among the groups (P≥0.3; Table 2). Similar results were found after adjustment for body height (P≥0.3). No differences were found in heart rate among groups (ABs: 61±3 bpm; MODs: 65±3 bpm; BDs: 59±2 bpm; P=0.3).
Table 2.
Aortic wave reflection characteristics
| ABs (n=13) | MODs (n=16) | BDs (n=20) | P Group | |
|---|---|---|---|---|
| Aortic systolic blood pressure, mmHg | 99±3 | 100±2 | 104±1 | 0.2 |
| Aortic diastolic blood pressure, mmHg | 70±2 | 71±2 | 71±1 | 0.5 |
| Aortic pulse pressure, mmHg | 29±1 | 29±1 | 32±1 | 0.04 |
| Aortic AIx, % | 4.1±3.0 | 5.0±2.8 | 4.5±1.4 | 0.96 |
| Aortic AIx@75, % | −2.5±3.9 | −0.2±3.5 | −3.4±2.1 | 0.8 |
| Augmentation pressure, mmHg | 1.5±1.0 | 1.6±0.9 | 1.5±0.4 | 0.99 |
| Pressure at inflection point, mmHg | 26±1 | 26±1 | 29±1 | 0.5 |
| Forward pulse height, mmHg | 25±1 | 25±1 | 28±1 | 0.3 |
| Reflected pulse height, mmHg | 12±1 | 12±1 | 13±1 | 0.7 |
| End-systolic blood pressure, mmHg | 89±3 | 91±2 | 92±2 | 0.4 |
Data are mean±SE. ABs, alcohol abstainers; AIx, augmentation index; AIx@75, AIx adjusted for a heart rate of 75 bpm; BDs, binge drinkers.
Figure 1.
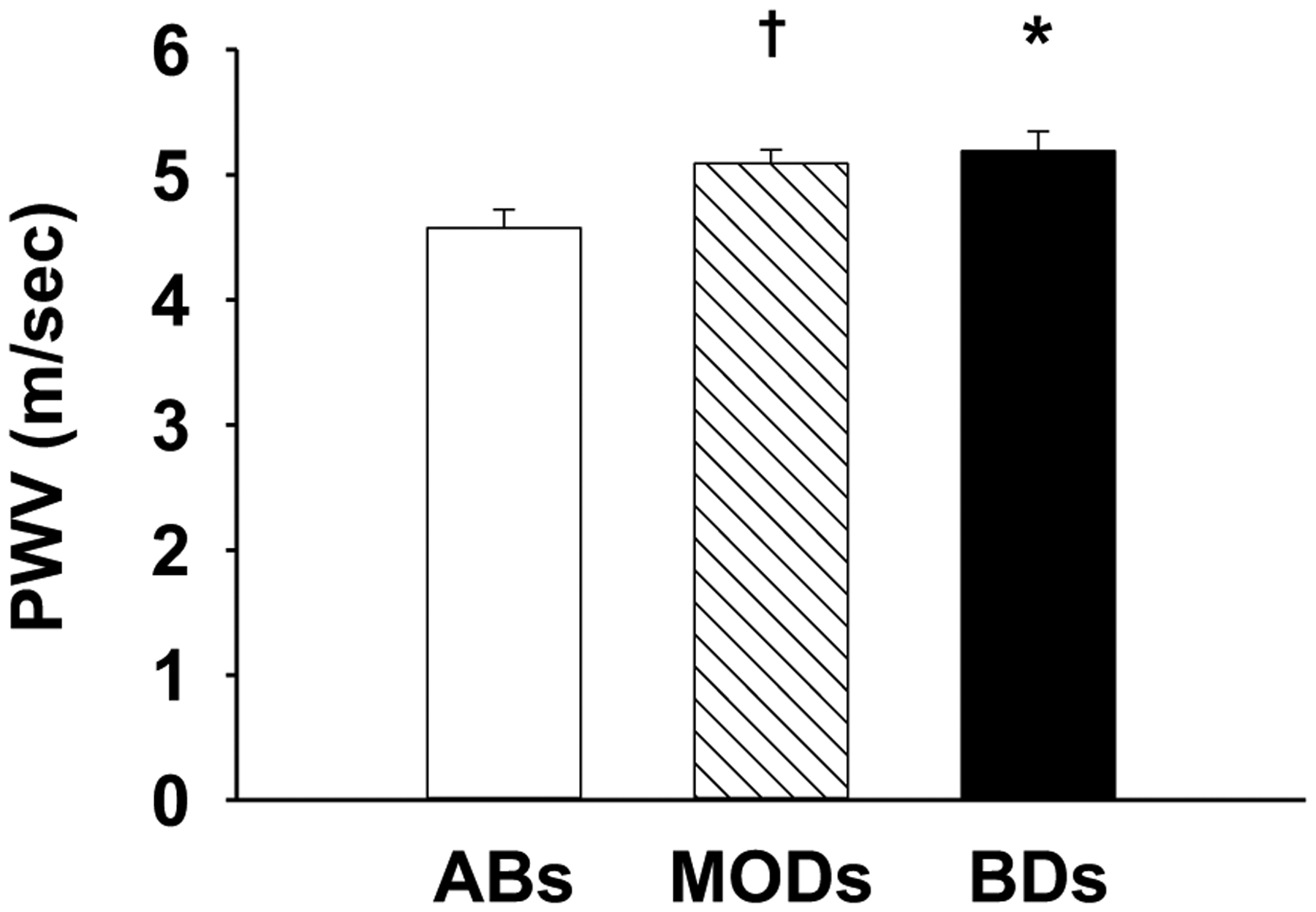
Aortic arterial stiffness measured as carotid to femoral pulse wave velocity (cfPWV) in young healthy adults who had a history of repeated binge drinking (BDs), moderate alcohol consumption (MODs), and alcohol abstention (ABs). *P=0.006 vs. ABs; †P=0.035 vs. ABs.
Urinary NE, epinephrine, and dopamine levels
Urinary samples were obtained in 46 participants. NE levels were significantly greater in BDs compared to MODs (P=0.048) and ABs (P=0.003; Figure 2). Compared to ABs, BDs had higher dopamine levels, while no difference was found in epinephrine among groups (Table 3).
Figure 2.

Urinary norepinephrine (NE) levels in young healthy adults who had a history of repeated binge drinking (BDs), moderate alcohol consumption (MODs), and alcohol abstention (ABs). *P=0.003 vs. ABs; †P=0.048 vs. MODs.
Table 3.
Urinary catecholamine levels
| ABs (n=13) | MODs (n=14) | BDs (n=19) | P Group | |
|---|---|---|---|---|
| Epinephrine, μg/24 hours | 7.5±1.4 | 5.4±0.6 | 9.1±1.4 | 0.1 |
| Dopamine, μg/24 hours | 195±22 | 265±43 | 324±28* | 0.03 |
Data are mean±SE.
P=0.02 vs. Abstainers
Relationship of arterial stiffness to urinary NE levels, aortic blood pressure, and wave reflection
Higher cfPWV was correlated with higher NE levels (Figure 3), higher aortic SBP (r=0.59, p<0.0005), and higher AIx (r=0.34, p=0.02). No association between NE levels and AIx was found (r=−0.07, p=0.6). No significant association was found with peripheral SBP to cfPWV, AIx, and NE levels (P≥0.3).
Figure 3.

The correlation between urinary norepinephrine (NE) levels and carotid to femoral pulse wave velocity (cfPWV) in healthy young adults who had a history of repeated binge drinking (●), moderate alcohol consumption (▲), and alcohol abstention (○).
DISCUSSION
This is the first study to investigate the effect of repeated binge drinking and moderate drinking on arterial stiffness and sympathetic activity in healthy young adults. This study also examined the relationship of arterial stiffness to sympathetic activity as well as wave reflection and aortic SBP. The major findings of this study are as follows: 1) cfPWV, a measure of aortic arterial stiffness, was higher in BDs and MODs compared with ABs; 2) urinary NE levels, a measure of sympathetic activity, was higher in BDs than MODs and ABs, and 3) higher NE level was correlated with higher cfPWV, which was positively correlated with AIx and aortic SBP.
To our knowledge no studies have investigated the effect of repeated binge drinking on arterial stiffness in young adults. We have found that BDs had higher cfPWV than ABs, suggesting that repeated binge drinking is associated with increased arterial stiffness. In agreement with our findings, Kweon and Lee demonstrated that among community dwelling adults (average age 50 years), binge drinking (7 or more drinks for men and 5 or more for women on a single occasion) ≥1 day/week was associated with increased risk for high brachial to ankle pulse wave velocity in men (OR=1.61, 95% CI=1.04–2.50) and in women(OR=3.12, 95% CI=1.16–8.34) [34]. In this study, we have also found that MODs had increased cfPWV than ABs. Clinical experimental studies have demonstrated that a one-time low-to-moderate level of drinking (~14g to 20 g of ethanol) in young men [35,36] and binge-like drinking (~56 g of ethanol within 10 min) in young and middle-age adults transiently decreases cfPWV [37]. The transient decrease in cfPWV was accompanied by a drop in SBP, which may in part explain the corresponding decreases cfPWV [12]. It has been demonstrated that the consumption of 2 glasses of wine (12% of ethanol) reduces overall arterial compliance (indicating increases in arterial stiffness) throughout a day in young and middle-aged adults (age range 25–53 years) [38]. Collectively, our findings suggest that repeated exposure to alcohol is associated with arterial stiffening in young adults.
This study, for the first time, demonstrated that BDs had higher urinary NE levels compared with MODs and ABs. Experimental studies with young adults demonstrated that one-time binge drinking (0.8–1.0 g of ethanol/kg of body weight; mean blood alcohol level≥0.08 g/dL) increases sympathetic activity [15,16]. Our findings further support that repeated exposure to binge drinking is associated with sympathetic activation. In addition, we have found that urinary NE levels were positively associated with cfPWV. Enhanced NE production plays a role in tension and stiffness of muscular arteries [39], collagen production in vascular smooth muscle cells [40], and proliferation of vascular smooth muscle and fibroblasts in aorta [41]. These factors may contribute to increased arterial stiffness associated with alcohol use. The underlying mechanisms by which repeated binge drinking contributes to sympathetic activation remains unknown. Studies suggested that sympathetic activation may be mediated by enhanced endothelin-1 pathway [42] and/or reduced nitric oxide bioavailability [43], both of which are subclinical features of young adults who have a history of repeated binge drinking [27,44]. Whether binge drinking induced endothelial dysfunction is mechanistically linked to sympathetic activation remains to be determined.
In contrast to findings of the effect of one-time moderate alcohol consumption (0.5–0.75 g of ethanol/kg or 2 drinks) on sympathetic activity in young adults [17–21], we found that MODs did not have increased urinary NE levels compared with ABs. It has been suggested that alcohol-induced sympathetic activity may be dose dependent, with no effect of alcohol consumption at low level (1 drink) [21]. In this current study, our MODs included those consuming alcohol at low to moderate levels, which may contribute to the no difference in NE between MODs and ABs. Increased vasoreactivity to NE induced by alcohol [45] may be a possible contributing factor to increased cfPWV in MODs. Besides NE, vascular tone and elastic properties (i.e., elastin and collagen) are regulated by other neurohormones such as angiotensin [46]. Study investigating the repeated exposure of alcohol consumption and its dose-response on sympathetic nerve activity, neurohormones, and arterial stiffness is warranted.
We demonstrated that AIx and aortic SBP was not different among BDs, MODs, and ABs, despite of higher cfPWV in BDs and MODs. As arterial stiffening, reflected pressure waves from peripheral sites to the heart may return earlier and thus augments aortic SBP [47]. However, the results of cfPWV (aortic arterial stiffness) and AIx may not always align. For example, in young men form the ARYA study, investigators found a positive association of alcohol consumption with AIx [48] but not with cfPWV [49]. In addition, AIx, a simplified index to assess complex wave reflection, is derived from augmentation pressure and pulse pressure, determined by the height and travel time of both forward and reflected pressure waves. AIx itself may not be sensitive to detect early alcohol-induced changes in wave reflection characteristics.
Findings from this study have contributed to a growing body of literature which supports that repeated binge drinking in young adults may be a trigger that leads to premature cardiovascular disease. We have previously reported that binge drinking in young adults is associated with microvascular dysfunction, characterized by alterations in flow induced vasodilation [27] and increased responsiveness of the microcirculation vasoconstrictors such as endothelin 1 [44]. Other indicators of subclinical disease, such as increased coronary calcification [50], and elevated blood pressure (SBP >120 mm Hg) [7,8,51] have been associated with binge drinking in young adults. Even though our participants had normal blood pressure (seated peripheral SBP <120 mmHg), BDs had higher, although not significant, aortic pulse pressure and SBP (~5 mmHg) than ABs and MODs. We found that higher cfPWV was associated with higher AIx and aortic SBP, suggesting that increased cfPWV may be present at an initial stage of alcohol-induced vascular changes, later leading to changes in wave reflection and increases in aortic SBP.
Our study had several limitations. To isolate the effect of alcohol on arterial stiffness, we included healthy young adults without presence of traditional cardiovascular risk factors which may limit the study generalizability. Demographic characteristics, cardiovascular metrics as well as metabolic parameters were not different among the groups (Table 1). The only exception is body weight and height which were greater in the BDs compared to ABs and MODs. We did not find differences in body mass index. Findings related to alcohol consumption and body mass index have been equivocal among adult studies [52]. Alcohol contains approximately 7 kilocalories per gram, and the increased energy intake resulting from alcohol consumption can contribute to a positive energy balance and weight gain [52]. Our research design was cross sectional, not allowing for the determination of the cause and effect. And, our study may not be powered to detect sex differences in the cardiovascular effect of alcohol. As a measure of sympathetic activation, we only measured 24-hour NE levels. Future studies should include the measurement of other variables such as 24-hour ambulatory blood pressure, heart rate variability, cardiovascular parameters influenced by the sympathetic nervous system and can be used to predict increased risk for cardiovascular disease. For example, in experimental studies, investigators have found transient increases in SBP [53,54], as well as dampening of the circadian night time fall (dipping) of SBP [55] in young adults following a binge-like drinking episode. Finally, although we did not measure indices of liver function in this study, we have previously reported in a similar cohort of young adult BDs (n=58) and MODs (n=23) drinkers that mean corpuscular volume and gamma glutamyl transpepetidase values are not increased and are within normal limits [24].
In conclusion, young healthy adults who binge drank and who drank at moderate levels on a regular basis had higher aortic arterial stiffness than those who abstained from alcohol. Furthermore, repeated binged drinking was associated with sympathetic activation, reflected by increased 24-hour urinary NE levels which may contribute the arterial stiffening in young adults.
ACKNOWLEDGEMNTS
This study was supported by the National Institutes of Health grants R21AA024535 (SAP and MRP). We would like to thank all study participants for their time and participation. We would like to thank Maryann Holtcamp MS, APN and the rest of the staff at the Center for Clinical and Translational Science (CCTS) for their support with the study. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Source of funding: This study was supported by the National Institutes of Health grants R21AA024535 (SAP and MRP) and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
None was declared.
References:
- 1.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med 2018; 54 (4):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global status report on alcohol and health 2018. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Mukamal KJ, Maclure M, Muller JE, Mittleman MA. Binge drinking and mortality after acute myocardial infarction. Circulation 2005; 112 (25):3839–3845. [DOI] [PubMed] [Google Scholar]
- 4.Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation 2014; 130 (5):390–398. [DOI] [PubMed] [Google Scholar]
- 5.Ruidavets JB, Ducimetiere P, Evans A, Montaye M, Haas B, Bingham A, et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 2010; 341:c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J Am Coll Cardiol 2017; 70 (8):913–922. [DOI] [PubMed] [Google Scholar]
- 7.Wellman RJ, Vaughn JA, Sylvestre MP, O’Loughlin EK, Dugas EN, O’Loughlin JL. Relationships Between Current and Past Binge Drinking and Systolic Blood Pressure in Young Adults. J Adolesc Health 2016; 58 (3):352–357. [DOI] [PubMed] [Google Scholar]
- 8.Piano MR, Burke L, Kang M, Phillips SA. Effects of Repeated Binge Drinking on Blood Pressure Levels and Other Cardiovascular Health Metrics in Young Adults: National Health and Nutrition Examination Survey, 2011–2014. J Am Heart Assoc 2018; 7 (13):e008733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55 (13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 10.Koivistoinen T, Lyytikainen LP, Aatola H, Luukkaala T, Juonala M, Viikari J, et al. Pulse Wave Velocity Predicts the Progression of Blood Pressure and Development of Hypertension in Young Adults. Hypertension 2018; 71 (3):451–456. [DOI] [PubMed] [Google Scholar]
- 11.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, et al. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens 2010; 28 (5):979–984. [DOI] [PubMed] [Google Scholar]
- 12.Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension 2003; 42 (5):915–918. [DOI] [PubMed] [Google Scholar]
- 13.Holwerda SW, Luehrs RE, Collins MT, Wooldridge NA, Stroud AK, Fadel PJ, et al. Elevated Muscle Sympathetic Nerve Activity Contributes to Central Artery Stiffness in Young and Middle-Age/Older Adults. Hypertension 2019:HYPERTENSIONAHA11812462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol 2017; 243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter JR, Stream SF, Durocher JJ, Larson RA. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am J Physiol Endocrinol Metab 2011; 300 (5):E771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Borne P, Mark AL, Montano N, Mion D, Somers VK. Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension 1997; 29 (6):1278–1283. [DOI] [PubMed] [Google Scholar]
- 17.Randin D, Vollenweider P, Tappy L, Jequier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. N Engl J Med 1995; 332 (26):1733–1737. [DOI] [PubMed] [Google Scholar]
- 18.Ireland M, Vandongen R, Davidson L, Beilin L, Rouse I. Pressor effect of moderate alcohol consumption in man: a proposed mechanism. Clin Exp Pharmacol Physiol 1983; 10 (3):375–379. [DOI] [PubMed] [Google Scholar]
- 19.Iwase S, Matsukawa T, Ishihara S, Tanaka A, Tanabe K, Danbara A, et al. Effect of oral ethanol intake on muscle sympathetic nerve activity and cardiovascular functions in humans. J Auton Nerv Syst 1995; 54 (3):206–214. [DOI] [PubMed] [Google Scholar]
- 20.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. J Hypertens Suppl 1989; 7 (6):S20–21. [DOI] [PubMed] [Google Scholar]
- 21.Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, et al. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol 2008; 294 (2):H605–612. [DOI] [PubMed] [Google Scholar]
- 22.Ransome Y, Slopen N, Karlsson O, Williams DR. The association between alcohol abuse and neuroendocrine system dysregulation: Race differences in a National sample. Brain Behav Immun 2017; 66:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J 2000; 16 (5):909–913. [DOI] [PubMed] [Google Scholar]
- 24.Piano MR, Tiwari S, Nevoral L, Phillips SA. Phosphatidylethanol Levels Are Elevated and Correlate Strongly with AUDIT Scores in Young Adult Binge Drinkers. Alcohol Alcohol 2015; 50 (5):519–525. [DOI] [PubMed] [Google Scholar]
- 25.Steptoe A The assessment of sympathetic nervous function in human stress research. J Psychosom Res 1987; 31 (2):141–152. [DOI] [PubMed] [Google Scholar]
- 26.Weinkove C ACP Broadsheet No 127: April 1991. Measurement of catecholamines and their metabolites in urine. J Clin Pathol 1991; 44 (4):269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bian JT, Piano MR, Kotlo KU, Mahmoud AM, Phillips SA. MicroRNA-21 Contributes to Reduced Microvascular Function in Binge Drinking Young Adults. Alcohol Clin Exp Res 2018; 42 (2):278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci 2012; 13 (11):14788–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015. [Google Scholar]
- 30.Hwang MH, Yoo JK, Kim HK, Hwang CL, Mackay K, Hemstreet O, et al. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens 2014; 28 (8):475–481. [DOI] [PubMed] [Google Scholar]
- 31.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015; 66 (3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Missouris CG, Markandu ND, He FJ, Papavasileiou MV, Sever P, MacGregor GA. Urinary catecholamines and the relationship with blood pressure and pharmacological therapy. J Hypertens 2016; 34 (4):704–709. [DOI] [PubMed] [Google Scholar]
- 33.Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol 1998; 31 (5):1103–1109. [DOI] [PubMed] [Google Scholar]
- 34.Kweon SS, Lee YH. Relationship of average volume of alcohol consumption and binge drinking to arterial stiffness in community-dwelling healthy adults. J Agric Med Community Health 2012; 37 (1):23–35. [Google Scholar]
- 35.Karatzi K, Rontoyanni VG, Protogerou AD, Georgoulia A, Xenos K, Chrysou J, et al. Acute effects of beer on endothelial function and hemodynamics: a single-blind, crossover study in healthy volunteers. Nutrition 2013; 29 (9):1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishiwaki M, Kora N, Matsumoto N. Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiol Rep 2017; 5 (15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmud A, Feely J. Divergent effect of acute and chronic alcohol on arterial stiffness. Am J Hypertens 2002; 15 (3):240–243. [DOI] [PubMed] [Google Scholar]
- 38.Fantin F, Bulpitt CJ, Zamboni M, Cheek E, Rajkumar C. Arterial compliance may be reduced by ingestion of red wine. J Hum Hypertens 2016; 30 (1):68–72. [DOI] [PubMed] [Google Scholar]
- 39.Girerd X, Chamiot-Clerc P, Copie X, Renaud JF, Laurent S, Safar ME. Effects of norepinephrine on the mechanical properties of the human radial artery in vitro. Am Heart J 1998; 136 (4 Pt 1):624–631. [DOI] [PubMed] [Google Scholar]
- 40.O’Callaghan CJ, Williams B. The regulation of human vascular smooth muscle extracellular matrix protein production by alpha- and beta-adrenoceptor stimulation. J Hypertens 2002; 20 (2):287–294. [DOI] [PubMed] [Google Scholar]
- 41.Faber JE, Yang N, Xin X. Expression of alpha-adrenoceptor subtypes by smooth muscle cells and adventitial fibroblasts in rat aorta and in cell culture. J Pharmacol Exp Ther 2001; 298 (2):441–452. [PubMed] [Google Scholar]
- 42.Bruno RM, Sudano I, Ghiadoni L, Masi L, Taddei S. Interactions between sympathetic nervous system and endogenous endothelin in patients with essential hypertension. Hypertension 2011; 57 (1):79–84. [DOI] [PubMed] [Google Scholar]
- 43.Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 2015; 308 (3):R208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goslawski M, Piano MR, Bian JT, Church EC, Szczurek M, Phillips SA. Binge drinking impairs vascular function in young adults. J Am Coll Cardiol 2013; 62 (3):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eby JM, Majetschak M. Effects of ethanol and ethanol metabolites on intrinsic function of mesenteric resistance arteries. PLoS One 2019; 14 (3):e0214336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dab H, Kacem K, Hachani R, Dhaouadi N, Hodroj W, Sakly M, et al. Physiological regulation of extracellular matrix collagen and elastin in the arterial wall of rats by noradrenergic tone and angiotensin II. J Renin Angiotensin Aldosterone Syst 2012; 13 (1):19–28. [DOI] [PubMed] [Google Scholar]
- 47.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 2005; 18 (1 Pt 2):3S–10S. [DOI] [PubMed] [Google Scholar]
- 48.van Trijp MJ, Beulens JW, Bos WJ, Uiterwaal CS, Grobbee DE, Hendriks HF, et al. Alcohol consumption and augmentation index in healthy young men: the ARYA study. Am J Hypertens 2005; 18 (6):792–796. [DOI] [PubMed] [Google Scholar]
- 49.van den Elzen AP, Sierksma A, Oren A, Vos LE, Witteman JC, Grobbee DE, et al. Alcohol intake and aortic stiffness in young men and women. J Hypertens 2005; 23 (4):731–735. [DOI] [PubMed] [Google Scholar]
- 50.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 2005; 161 (5):423–433. [DOI] [PubMed] [Google Scholar]
- 51.Hayibor LA, Zhang J, Duncan A. Association of binge drinking in adolescence and early adulthood with high blood pressure: findings from the National Longitudinal Study of Adolescent to Adult Health (1994–2008). J Epidemiol Community Health 2019; 73 (7):652–659. [DOI] [PubMed] [Google Scholar]
- 52.Traversy G, Chaput JP. Alcohol Consumption and Obesity: An Update. Curr Obes Rep 2015; 4 (1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bau PF, Bau CH, Naujorks AA, Rosito GA. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol 2005; 37 (1):53–58. [DOI] [PubMed] [Google Scholar]
- 54.Seppa K, Sillanaukee P. Binge drinking and ambulatory blood pressure. Hypertension 1999; 33 (1):79–82. [DOI] [PubMed] [Google Scholar]
- 55.Rosito GA, Fuchs FD, Duncan BB. Dose-dependent biphasic effect of ethanol on 24-h blood pressure in normotensive subjects. Am J Hypertens 1999; 12 (2 Pt 1):236–240. [DOI] [PubMed] [Google Scholar]


