Abstract
Seeing familiar faces prompts the recall of diverse kinds of person-related knowledge. How this information is encoded within the well-characterised face/person selective network is a complex and outstanding question. To address this issue, we had participants access five different kinds of person knowledge (social, episodic, semantic, physical, nominal) through ten different experimental tasks. By directly comparing different cognition domains, we are able to: 1) test the relative roles of brain regions in specific cognitive processes, such as the role of ATL in social or nominal knowledge and 2) apply a multivariate Network-level Representational Similarity Analysis (netRSA) to gain insight into underlying systems-level organisation of the person-knowledge network. NetRSA revealed a strong divide between regions involved in internalised cognition (precuneus, mPFC, ATL etc.) and other elements of the person-knowledge network. Fronto-lateral regions (IFG and OFC) coordinate closely with perceptual regions in the core system (FFA, OFA, pSTS). NetRSA also revealed a taxonomy of cognitive processes, with semantic retrieval being more similar to episodic than nominal knowledge and distinct from social and physical knowledge. Collectively these results demonstrate how coordinated activity across core-extended divisions of the person knowledge network enact the diverse cognitive capacities of this system.
Keywords: cortical network, face perception, fMRI, representational similarity analysis, semantics
Seeing a familiar person brings to awareness a variety of related attributes: biographical facts (semantic knowledge), personal experiences (episodic knowledge), perceptual attributes (physical knowledge), trustworthiness (social knowledge) and their name (nominal knowledge). We use this wealth of person specific information frequently in our day-to-day lives. The question of how this diverse information is represented in the brain is an area of active research.
Neuroimaging studies have identified an interconnected network of regions activated when we see and think about other people (Haxby, Hoffman, and Gobbini 2000; Gobbini and Haxby 2007; Fairhall and Ishai 2007). This network is composed of a perceptual ‘core system’, the occipital and fusiform face areas (OFA, FFA; Kanwisher et al. 1997; Gauthier et al. 2000) and the posterior superior temporal sulcus (pSTS). This core is complemented by an ‘extended system’ - a more loosely grouped set of regions implicated in a broad range of person-related cognition less related to perception (Haxby, Hoffman, and Gobbini 2000). The extended system includes lateral frontal regions: inferior frontal gyri (IFG), lateral orbitofrontal cortex (OFC); regions associated with internalised cognition: the anterior temporal lobes (ATL), precuneus, ventromedial and dorsmomedial prefrontal cortices (vmPFC, dmPFC); and medial temporal regions: the amygdalae and potentially the recently identified anterior temporal face patch ATFP (Moeller et al. 2008; Rajimehr et al. 2009).
Numerous investigations of the contribution of these regions have produced diverse, and at times discordant, results (Table 1). For example, while the pSTS is classically characterised as a core ‘perceptual’ region, it is frequently linked with social and other person-related cognition that is unrelated to perception (Adolphs 2003; Fairhall and Caramazza 2013a; Koster-Hale et al. 2017), potentially linked to the marked heterogeneity of the broader temporoparietal junction (TPJ)[join brackets](Gobbini and Haxby 2007; Hein and Knight 2008). Likewise, the varied functional attributions of the ATL to proper naming (Grabowski et al. 2001), semantic information (Tippett et al. 2000; Glosser et al. 2003; Olson et al. 2007) and social cognition (Simmons and Martin 2009) illustrate further the diversity and complexity of regional roles within the extended system.
Table 1.
illustrates complex cognitive landscape of the attribution of cognitive roles to various regions of the person-knowledge network. We sampled published reviews and meta-analyses reporting regional function in in the context of person perception of knowledge. Attributions have been broadly grouped into the categories such as Perception, Semantic knowledge, or Working memory.
Much insight has been gained from research addressing single cognitive function in one or a few brain regions. However, this approach has the potential to ambiguate the true regional functions of regions**. Ambiguities surrounding regional function are expounded by the tendency of research to address single cognitive functions in one or a few brain regions. Most regions of the extended system are recruited to some extent across multiple experimental contexts, including simple tasks such as repetition detection (Todorov et al. 2007) or superordinate categorization (Fairhall et al. 2014). In other words, most-all person related cognition involves most-all of the extended system. The important information may not be whether a specific region is modulated by a task but the relative change of regional activity across the entire network. Rather than attributing a function to a region or a region to a function it may be that the representation of cognition within the brain is best described in terms of the patterns of activation over distributed cortical networks.
The importance of network over region is especially important considering that many elements of the extended system fall within one prominent network in the brain, the intrinsic brain network - a collection of brain regions associated with a range of internalised cognitive processes (Spreng et al. 2010). These include not only the eponymous ‘default mode’, task-deactivated states (Raichle et al. 2001) but also a broad range of internalised cognitive processes: general semantic knowledge (Binder et al. 2009; Fairhall and Caramazza 2013b; Huth et al. 2016); social cognition, (Greene et al. 2001; Van Overwalle 2009; 2011), as well as context integration episodic memory and mental time travel (Schacter and Addis 2007; Keidel, Oedekoven, Tut & Bird, 2017). As elements of the internalised cognition network are frequently activated together, establishing specific contributions of each region has proved challenging (Moran et al. 2011; Van Overwalle 2011).
In this work, we isolate the network activated when we view familiar faces during a simple stimulus repetition detection task. Then we push the network towards different aspects of person knowledge (social, semantic, episodic, nominal and physical) to understand the relative importance of these processes to each region. The goals of the current experiment are twofold. The first is to re-examine the roles of key brain regions in cognitive function by considering these regions both in context of different cognitions and other brain regions. Specifically, we investigate the role of the anterior temporal lobe in social cognition and nominal knowledge and the role of the pSTS and AG in access to person knowledge. Our second goal is to apply network level representational similarity analysis (netRSA). NetRSA entails a multivariate approach based on the regional response magnitude within network nodes, with the multivariate element coming from the changing regional biases across different tasks. We employ netRSA to address two questions: a) how are cognitive domains represented across the network and b) how do these regions work together to accomplish the diverse range of ensemble functions of the network. We hypothesise that all person-knowledge regions are involved across all cognitive dimensions related to other people and that the cognitive flexibility of this system lies within subtle differences in the pattern of activation across the network.
Methods
Participants
Twenty right-handed, native Italian participants (8 males; mean age: 23.2 years, range: 19-32 years) took part in this study. Participants had normal or corrected-to-normal vision and no history of neurological incidents. The study was approved by the University of Trento ethical committee. All participants gave informed consent and were compensated for their time.
Stimuli
Stimuli were 40 pictures of famous faces and 40 pictures of famous buildings. The stimulus set consisted of Italian and foreign politicians, actors, singers and sportsmen, as well as landmarks (Eiffel Tower, Colosseum). Stimuli were cropped with a face-shaped mask and the eyes and mouth were aligned across faces. Stimuli extended 400 pixels vertically and 300 pixels horizontally and were presented centred on the screen (1280x1024 resolution, 60hz refresh rate), with grey background. After the experiment 13/20 participants were presented with faces they saw in the experiment and asked whether they recognised the celebrity. On average, the subjects recognised M = 84% of faces.
Task
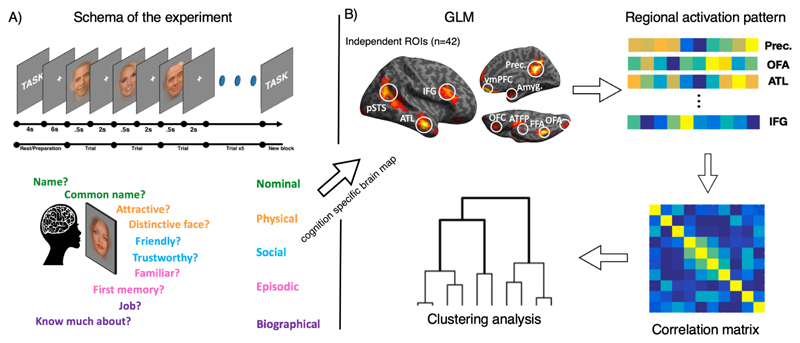
Each experimental block started with 4s instruction screen specifying the task, followed by 6s of fixation cross. After that a face was presented for .5s followed by 2s of fixation cross during which subjects provided a response via button box. Within each 8-trial block, participants were instructed to respond to questions covering five categories of person knowledge: episodic memories, semantic knowledge, social judgments, nominal knowledge and physical knowledge. For each of the categories, we chose two different probe questions that require access to each kind of knowledge (totalling ten experimental tasks; see Figure 1 and Table 2). In addition, there were two 1-back matching control tasks with either pictures of faces or famous monuments. The experiment consisted of five runs (8 min, 42s each). Sixteen blocks were presented in a randomised order (one block for each task plus three face and three monument 1-back control blocks).
Figure 1.
(A) Top: Schematic representation of the experiment. Experimental blocks were preceded by 4s of instruction screen, and 6s fixation point. Each trial consisted of .5s face presentation and 2s fixation. Bottom: five domains of person knowledge and two questions per domain are noted in corresponding colours. (B) Data analysis schematic. ROI beta averages for each of the ten tasks were extracted from ROIs, correlated and subjected to RSA.
[add ‘task→’ to top right panel].
[‘correlation matrix’ might be a bit vague]
Table 2.
Experimental questions. We selected five categories of person related knowledge (nominal, perceptual, episodic, social and semantic). For each category we chose two different probe question that require accessing the specific category of knowledge. Each task was presented in a block of eight trials. On each trial, participants were instructed to relate the task question to the famous person.
| Knowledge category | Task | Participant instructions | Answer choices |
|---|---|---|---|
| Nominal | Common name? | How common is this person’s name? | Likert scale (1-4) |
| Full name? | How well can you recall the person’s full name? | Likert scale (1-4) | |
| Perceptual | Attractive? | How attractive do you find this person? | Likert scale (1-4) |
| Distinctive? | How distinctive is this person’s face? | Likert scale (1-4) | |
| Social | Friendly? | How friendly is this person? | Likert scale (1-4) |
| Trustworthy? | How trustworthy is this person? | Likert scale (1-4) | |
| Episodic | Familiar? | How familiar is this person is for you? | Likert scale (1-4) |
| First memory? | For how long have you known this person? | Likert scale (1-4) | |
| Semantic | How many facts? | How many facts could you recall about this person? | Likert scale (1-4) |
| Job? | What is this person occupation? | Predefined categories (see ‘task’) |
Participants answered 9/10 questions using a 1-4 Likert scale. Occupation question (“what is this persons’ occupation”) had predefined categories (1 = actor or TV presenter, 2 = singer or musician, 3 = politician or sportsman, 4 = none of the above). Prior to scanning, participants practiced answering experimental questions on a different set of famous people repeating each question for five trials.
Data Acquisition
Participants were scanned at the Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy. Data was collected using Bruker BioSpin MedSpec 4T, with 8-channel phased-array head coil. Five runs of 209 echo-planar volumes, consisting of 34, AC-PC aligned axial slices were acquired while participants performed the task (FOV = 64mm x 64mm, TR = 2.5s, TE = 33, FA = 73°). Voxel size was 3x3x3mm with a 1mm gap. In addition to functional data, a whole brain T1 MPRAGE anatomical image was acquired (whole brain (FOV = 256x224, 176 1mm axial slices).
Region of interest definition
Regions of interest (ROIs) were selected from an independent (N=42) experiment, conducted for high power functional localisation. In the localiser experiment participants performed a 1-back matching task with 12 second blocks of famous faces, common animals or common objects. The contrast faces > animals+tools (p < .05 FWE corrected) was used to identify face selective peaks (Table 2). 7.5mm radius spheres were drawn around the peak voxels and task evoked brain responses (beta estimates) were extracted for each subject.
To investigate differences in pSTS/Angular gyrus (see: introduction) we anatomically constrained the volume with angular and superior temporal gyri masks. Then the masks were inflated, and the overlap was removed. This allowed us to distinguish face selective anatomical activation within anatomical angular and superior temporal gyri within anatomical boundaries.
Data Analysis
Data were pre-processed with SPM12. Functional images were realigned to account for motion, grey matter segmented, warped into common space and smoothed with 8mm FWHM kernel. Subject specific response estimates (beta weights) were derived by fitting a general linear model (GLM) to the data. 12 regressors (10 tasks, 2 controls) were included as explanatory variables. Six motion parameters from re-alignment procedure were included as regressors of no interest. We drew 7.5mm spheres around chosen coordinates (Table 2) and extracted the mean beta value significantly active at p<0.001 within those ROIs (contrast faces > animals+tools). To isolate the magnitude of cognitive response, we subtracted beta value for 1-back matching face control task from each experimental task.
Multivariate Analyses
ROI responses across tasks were averaged across voxels and correlated to obtain a dissimilarity matrix (1-r), which was then subjected to Ward hierarchical agglomerative clustering. For task similarity analysis the matrix was transposed before correlating so that similarity matrix consisted of task correlation across ROIs.
Results
Behavioural Data
Mean Reaction time (RT) was M = 1203msec, SD = 111msec. Subjects reacted fastest during full name task (M=1110ms, SD=120ms), and slowest during common name task (M=1386ms, SD = 200ms). RTs differed across the ten tasks (F(9, 171) = 17.13, p < .001). Critically, this RT effect did not persist when tasks were collapsed into the five domains of knowledge used in the imaging analysis (i.e. “Nominal“, “Physical“, “Social“, “Episodic“, “Semantic“; F(4, 95) = 2.21 p = .076). RT did not significantly differ between face (M = .71, SD = .11) and place (M = .68, SD = .12) control 1-back matching tasks (t(18) = 1.96, p = .066).
Mean ratings ranged from M = 2.2, SD = 0.25 (attractiveness task) to M = 3.1, SD = .38 (full name task). Ratings differed across 10 exemplar tasks (F(4.8, 91.7) = 27.741, p < .001, Greenhouse-Geisser correction) and 5 cognitive domains (F(4,76) = 20.03, p < .001). Faces were rated highest on attractiveness (M = 2.79, SD = .025) and lowest on participants’ ability to recall full name (M = 1.82, SD = .38). To check whether task ratings influence task representation in the brain, we constructed RSA model of similarly rated tasks and compared it to task similarity across the network (see: Cognitive Taxonomy). The relationship between rating similarity and cognitive taxonomy did not approach significance (t(19) = .78. p = .442).
Role of regions in access to person knowledge
Extended but not core regions show an increased response during access to person knowledge.
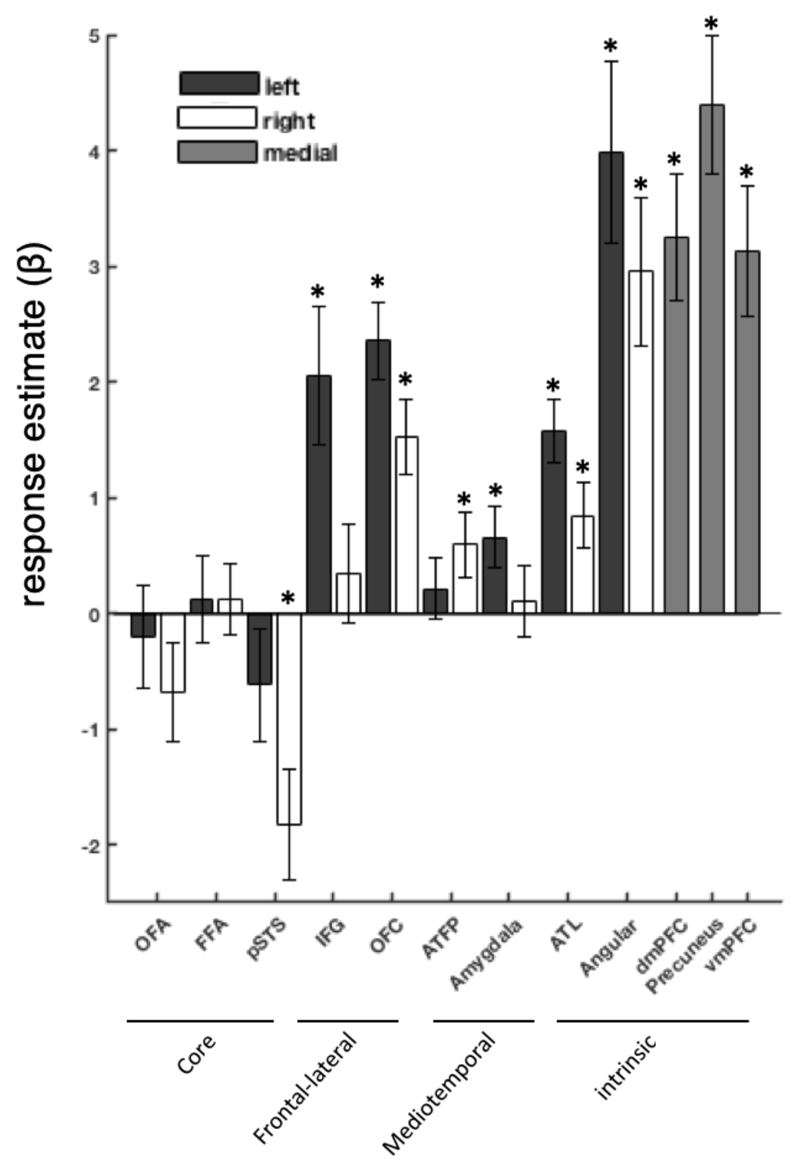
To assess the global importance of access to person knowledge in the core and extended systems for person perception/knowledge, we compared the average regional increase when participants accessed the 10 variants of person knowledge, compared to the one-back matching task on famous faces. The results are shown in Figure 2.
Figure 2.
Regional response to person knowledge access (average of experimental tasks > face control task). Bars show response estimate magnitude (beta value), error bars represent standard error of the mean (SE). Retrieving person knowledge activated extended system regions to variable extent, but core system did not show an increased response.
Could we maybe space out the sub-networks (I want it visually clear that the core is donw, frontolateral is up etc, and it is not at the moment)
[change ‘estimate’ to ‘magnitude’ in the y label]
[why only single asterisks?]
The most apparent distinction is between regions of the core system, which show no increase in activity (t(19) = -1.69, p = .11; averaged across all core regions) and other elements of the network (t(19) = 6.98, p < .001; averaged across all extended regions). A second clear organisational feature of person knowledge is the left lateralisation of this process, with left hemisphere regions showing a greater relative increase when accessing person knowledge than their right hemisphere counterparts (t(19) = 4.67, p < .001).
Individually, regions of the internalised cognition part of extended system were all strongly recruited during access to person knowledge (t-values > 4.60, p-values < .001). Lateral frontal regions, the IFG and lateral OFC show an increased response most consistently in the left, with the right IFG failing to show a significant modulation. More subtle increases are seen in medial temporal lobe structures, with only the left amygdala and the right ATL exhibiting significant modulation when person knowledge was accessed.
The Role of Cognitive Domain across the Person Knowledge Network
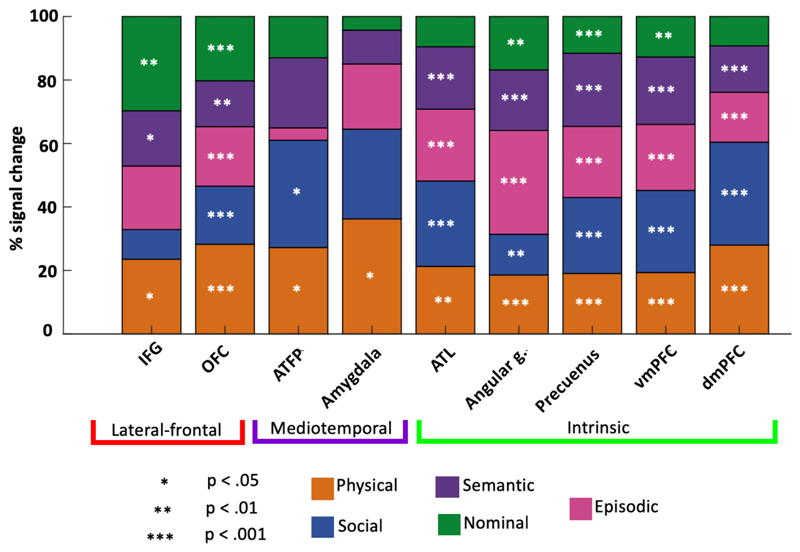
To investigate the role of different kinds of knowledge in the person knowledge network, Figure 3 shows the scaled importance of each cognitive domain in those regions that were activated during access to person knowledge. Here we focus only on regions that showed at least a significant unilateral increase when person knowledge is accessed. For simplicity, we have collapsed across hemisphere (significant interaction between domain and hemisphere were present only in IFG; F(4,76) = 2.508, p = 0.49 [.049, right?]). Regions of the intrinsic network are strongly involved in all cognitive domains with the exception of nominal knowledge, to which ATL and dmPFC were unresponsive.
Figure 3.
[asterisk are still not centred.] [lateral-frontal axis label seems uncentered] [labels for cog domain are un-aligned] Figure 3. Regional preference patterns. Percentage of total activation elicited by each cognitive domain. Stars denote significance threshold. Most regions are involved in most cognitive domains. Although patterns vary across components of the network, it can also be seen that regions that respond to episodic knowledge tend to respond to semantic access as well.
The ATL is weighted towards Social not Nominal knowledge
The involvement of ATL in social or nominal (proper names) knowledge is a matter of current contention (Grabowski et al. 2001; Olson et al. 2013). We exploited the presence of all these cognitive aspects within one single study to address their relative importance in bilateral ATL. ANOVA revealed significant differences across cognitive domains (F(4, 95) = 8.13, p = < .001). A planned comparison specifically testing the role of social and nominal tasks revealed that access to social knowledge recruited this region to a greater extent than access to nominal knowledge (t(19) = 5.79, p < .001), with nominal knowledge showing no significant increase compared to control (t(19) = 2.08, p = .052. Follow up analyses revealed that, while the largest response was evident during access to social knowledge, this was not significantly greater than episodic, semantic or physical cognitive domains, each of which showed a greater response than the nominal tasks (post hoc: all t-values > 2.49, all p-values < .02, uncorrected). The response during the nominal tasks were not diminished in all regions, with IFG responding more strongly to access to nominal than social knowledge (t(19) = 3.1, p = .006). These results show a distinction between the way social and nominal knowledge is processed, social tasks engage ATL more than nominal ones, while the IFG exhibits the opposite pattern, being more responsive to nominal than social knowledge.
Perceptual and non-perceptual processing in the pSTS/Angular gyrus
Here we sought to test whether the pSTS and AG, have diverse functional roles. While these regions sometimes form a contiguous activation cluster, pSTS has been implicated in perceptual processes, while AG is involved in cognitive processes such as knowledge retrieval.** The functional division between these two regions was apparent even at a level of global access to person-knowledge, (c.f. figure 2). Specifically, while the pSTS showed a suppressed response during access to stored person-knowledge (pSTS left: t(19) = -1.26, ns.; right: t(19) = -3.81, p = .001), the angular gyrus conversely showed a robust response (AG left: t(19) = 5.81, p < .001.; right: t(19) = 2.99, p = .008). This difference was also apparent in the global laterality pattern. pSTS showed a stronger inhibition in response on the right (t(19) = 3.47, p = .001), consistent with the pattern of right laterality in core regions. Conversely, there was no laterality effect in global response of the angular gyrus (t(19) = 1.57, p = .134). Collectively, these patterns underscore the pronofunctional subdivision of these regions of the temporo-parietal junction.
At the level of the single cognitive domain (see Figure 3) an increased response in AG was evident across each cognitive domain (t > 3.12, p < .006) while the pSTS did not show and increase for any cognitive domain. Finally, while cognitive domain did not have a variable effect in pSTS at the univariate level, the angular gyrus responded more during episodic memory retrieval than any other task (t > 2.8, p < .01). Together, these results demonstrate a pattern of response in the pSTS consistent with core perceptual processing and a pattern in the AG consistent with access to knowledge. Differences in inter-regional coordination patterns will be discussed in the next section.
Inter-Regional Coordination and Network organisation
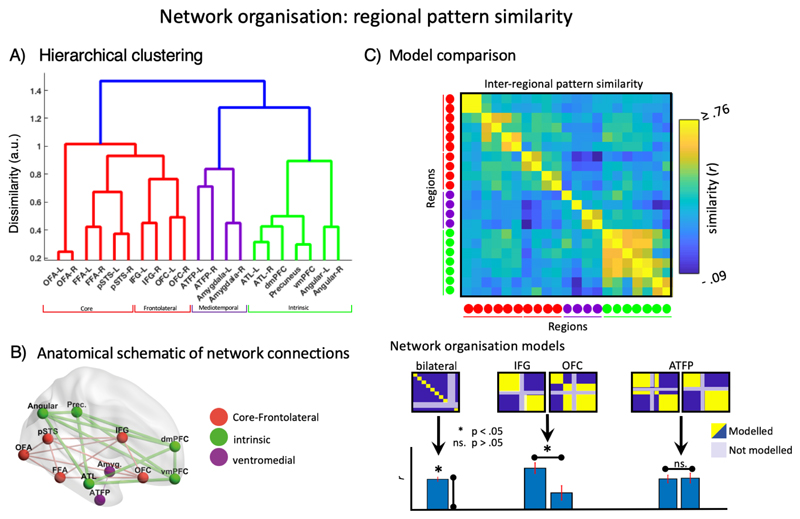
Which brain regions work together to accomplish the person-knowledge network’s varied functions? The functional coordination between ROIs was examined through a network level Representational Similarity Analysis (netRSA). The ten task-induced beta patterns were correlated between each pair of ROIs and subjected to hierarchical clustering analysis (see figure 1 and methods). The validity of this netRSA approach is confirmed by the close proximity of left and right regional homologues (figure 4): In all cases, despite the anatomical distance, a given ROI’s cognitive profile matched most closely to that of its contralateral counterpart. An RSA template model (figure 4), confirmed the high reliability of this effect across subjects (t(19) = 13.89, p < 0.001). This result highlights the commonality of function between hemispheric homologues despite hemispheric asymmetries in the overall response.
Figure 4.
[straighten colourbar labels] [use more asterisks to indicate robustness of significance] [make blue line in A) black] [the IFG anf OFC labels on the network organisation models confused me]. [Y-label on model evidence] Figure 4. A) ROI clustering. First major division separates core together with IFG & OFC from the rest of the extended system (red vs (green + purple)). Within the extended system, a further division is evident between medial temporal regions and regions involved in the intrinsic system (purple, green). B) Schematic representation of clusters projected onto the brain. C) model comparison schematic. Competing models of network organisation were constructed, fitted to observed data for each participant, and compared in a paired samples t-test.
At a descriptive level, netRSA revealed the expected cognitive clustering of core regions (OFA, FFA, pSTS). Interestingly, the lateral frontal regions the orbito-frontal and inferior frontal gyri cluster with the core system, rather than the other components of the extended system (figure 4 – red cluster). To test whether lateral frontal regions coordinate more closely with core or extended systems, we built competing models of regional coordination (see figure 4). Post hoc comparisons of the goodness of fit between the two competing models confirmed that lateral frontal regions co-ordinate more closely with core than other extended system regions (t(19) = 3.09, p = .006). Considering regions separately, this effect persisted for IFG (t(19) = 4.35, p < 0.001), while no preference was evident for OFC (t(19) = 1.59, p = 0.13).
Within the extended system, regions overlapping with those associated with internalised cognition form a distinct cluster (Cluster 2 - green) with respect to the amygdala and anterior face patch of the medial temporal lobe (Cluster 3 – purple). However, planned comparison of whether the ATFP groups more closely with the core or extended system resulted in no evidence for either hypothesis (t(19) = -0.21, p = 0.84).
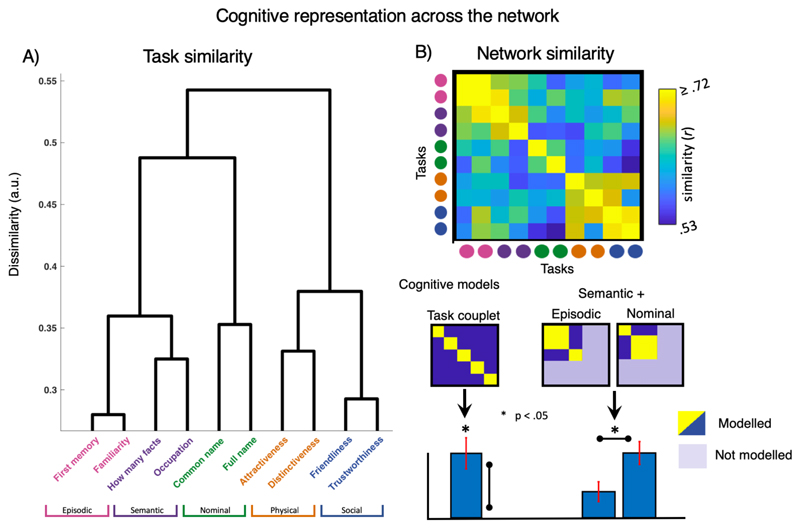
Cognitive Domain Similarity in the Brain Cortical Similarity between Cognitive Domains
Congnivite Domain Similarity in Cortical Profile cognitive taxonomy grounded in brain representation
To address the fundamental question of how different forms of cognition relate to one another, netRSA was performed across ROIs to investigate how similar the neural representations of cognitive domains are in the brain (see Figure 5 and methods). Despite variance across tasks, reactions times, and in one case response scales, the task pairs for each cognitive domain (e.g. ‘common name’ and ‘full name’ for nominal knowledge) are grouped together. This illustrates the efficacy of netRSA in this context, providing another internal validation. To test our a priori selection of task couplets, we built a model to test their similarity. Results show that tasks from the same cognitive domain have highly similar representation across the person knowledge network (t(19) = 4.14, p < 0.001).
Figure 5.
A) [random bar for ‘task couplet bar plot- either shift the asterisk of kill the bar]] Task similarity in core and extended system ROIs. Tasks are grouped according to the domain they were sampled from. Episodic and semantic knowledge retrieval tasks elicit differentiable patters from nominal, physical or social ones (dendrogram, left). B) fMRI pattern similarity matrix and models tested. [change range for b to .5 – .75.]
[could add ***s to indicate greater significance]
At the next level of hierarchical clustering, results reveal three distinct cognitive clusters. Network activity is more similar for physical (yellow) and social (blue) knowledge than to other forms of knowledge. Likewise, episodic (pink) and semantic (purple) knowledge form a cluster, that is distinct from nominal knowledge. We compared competing models (see Figure 5) to make inference about whether semantic knowledge more closely relates to the episodic or nominal domain. Results confirm that semantic knowledge is more similar to episodic memories, than nominal knowledge (t(19) = 3.97, p < .001).
Whole Brain Analysis - Beyond the person-selective network
The motivation of this project was to gain insight into the normal function of the well-characterised network for person knowledge by strongly pushing the system towards access to different domains of knowledge. While we focus on the network for perceiving and knowing about others, cognitive processes are not enacted solely by the person knowledge system. It is important to consider that these systems presumably pair and couple with brain regions outside the person-selective network, with the network’s periphery potentially driving transient specialisation within the network itself.
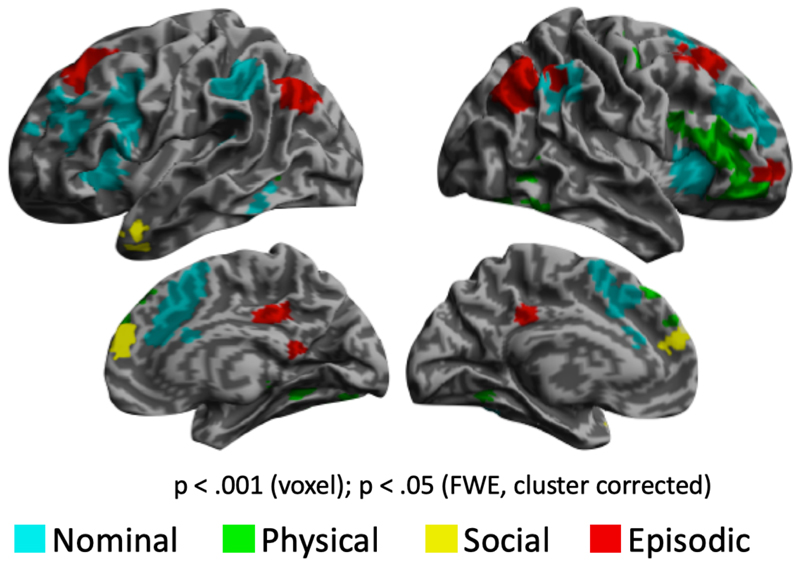
A whole brain analysis comparing each cognitive domain to the average of the other domains is presented in figure 6. Notably, no regions demonstrated a significant preference for two or more cognitive domains, consistent with non-overlapping cognitive specialisation outside the person knowledge network. Access to nominal knowledge is characterised by a broad pattern of activation, stronger in the left hemisphere than the right (Figure 6, Table 4). It encompasses left hemispheric sites associated with language production [reference?]and comprehension, as well as the posterior middle/inferior temporal gyrus [is this region not associated with language comprehension?]. Conversely, accessing physical knowledge is more pronounced in the right hemisphere, predominantly in the superior portion of the IFG. Social knowledge retrieval more strongly activated left ATL and an anterior patch of dmPFC. Recalling episodic memories involved the posterior cingulate cortex (PCC), angular gyrus as well as right lateral frontal pole and bilateral patches of the superior frontal gyrus. Semantic knowledge retrieval tasks did not selectivity recruit any region outside of the person-knowledge network.
Figure 6.
[BIGGER] Figure 6. Cognition specific brain activations. Whole brain map highlights peripheral, domain specific cognitive systems that are recruited during diverse kinds of person-knowledge retrieval. Semantic knowledge did not elicit significant clusters of activity.
Table 4.
Peak location, extent and cluster-level significance of whole brain analysis of the differential effects of each cognitive domain (see also: Figure 6). SFG - superior frontal gyrus, IPL - inferior parietal lobule. PMC - premotor cortex, PCC – posterioir cingulate cortex..
| Domain | Region | Hemisphere | cluster | Peak | |
|---|---|---|---|---|---|
| p (FWE) | size | T | |||
| Episodic | Angular | Right | < 0.001 | 507 | 8.06 |
| Angular | Left | < 0.001 | 264 | 6.57 | |
| SFG | Right | < 0.001 | 216 | 5.68 | |
| PCC | Medial | < 0.001 | 142 | 5.68 | |
| SFG | Left | 0.001 | 125 | 4.77 | |
| OFC | Right | 0.017 | 71 | 4.65 | |
| Precuneus | Medial | 0.032 | 61 | 4.16 | |
| Social | dmPFC | Medial | < 0.001 | 396 | 6.57 |
| ATL | Left | 0.001 | 130 | 4.58 | |
| Physical | IFG | Right | < 0.001 | 616 | 5.72 |
| FFA | Right | < 0.001 | 274 | 4.86 | |
| PMC | Right | 0.006 | 87 | 4.38 | |
| Amygdala | Right | 0.020 | 68 | 4.37 | |
| dmPFC | Medial | < 0.001 | 253 | 4.34 | |
| FFA | Left | 0.004 | 94 | 4.24 | |
| IPS | Right | 0.005 | 92 | 3.93 | |
| Nominal | IFG | Left | < 0.001 | 1188 | 6.99 |
| SFG | Medial | < 0.001 | 1670 | 6.40 | |
| Angular | Left | < 0.001 | 370 | 6.35 | |
| Angular | Right | < 0.001 | 265 | 5.31 | |
| FFA | Left | 0.003 | 97 | 4.70 | |
Discussion
Cognitive processes are distributed across networks of regions. By having our participants perform a broad range of cognitive tasks and exploiting data complexity through derivations of statistical tools such as RSA, we could focus on the subtle differences in inter-regional coordination that endow distributed cortical networks their considerable cognitive flexibility. By relating task activity not only to a baseline but to other tasks, we were able to establish regional preferences which allowed us to tackle specific hypotheses about regional function. Leveraging multivariate methods provided insight into regional coordination, uncovering principles of network organisation, as well as the similarity between different cognitive processes allowing us to build a cognitive taxonomy grounded in brain representation.
Accessing person related knowledge recruits extended, not core, components
***Even at the broadest level we see a clear division between core and extended system regions. Face-selective core system regions (OFA, FFA & pSTS) were not responsive to person knowledge tasks. This stands in contrast of the extended system regions which were strongly engaged by the experimental tasks, and particularly so in the left hemisphere. This is consistent with a predominant role of these regions in the extraction of perceptual information which is made available to the other elements of the system (c.f. Downing and Peelen 2011).
Functional subdivisions between pSTS and Angular Gyrus
The pSTS was originally designated as part of the core system (Haxby, Hoffman, and Gobbini 2000b). Over the years this classification has become less clear and pSTS has been reclassified as part of both core and extended systems (Gobbini and Haxby 2007). The pSTS is part of a heterogeneous cortical region, the temporal parietal junction (TPJ), which includes pSTS, the angular gyrus and the supramarginal gyrus. In this study, we anatomically divided the person selective patch into pSTS and angular gyral components. We observed a pronounced dissociation across this subdivision. Firstly, compared to the face repetition-detection control task, AG exhibited a strong global response to access to person related knowledge while pSTS was either unresponsive (left) or supressed (right). Secondly, the response profile across cognitive tasks grouped them differently, with pSTS clustering with other core regions while AG clustered with regions of the extended system associated with intrinsic cognition.
These results supports the reclassification of ‘pSTS’ into face-selective pSTS and person-selective angular gyrus and indicate that the contiguous TPJ activation reported during the viewing of familiar faces is functionally heterogeneous, divided into an angular gyrus component that responds to non-visual theory of mind (Saxe and Powell 2006) and amodal access to person knowledge (Fairhall and Caramazza 2013b) and a pSTS component involved in face perception. [** This should be 2013*a* **] Future work will determine how these anatomically adjacent brain regions coordinate across other cognitive processes but within the context of person-related cognition, they appear to be highly distinct.
Regional tuning of ATL
Different hypotheses propose that ATL might mediate person-specific social knowledge (Olson et al. 2007) or proper naming (Semenza 2011). Here we directly contrasted these two hypotheses and observed that ATL responds strongly to social knowledge and is unresponsive to nominal knowledge retrieval. This is in apparent contrast to early positron emission studies which show the strong activation of the region during overt naming (Gorno-Tempini et al. 1998; Grabowski et al. 2001). However, it is consistent with the finding that the ATL responds equally to familiar people whether or not the name is known by the participant (Gesierich et al. 2011). The reason for these disparities may lie in fMRI signal drop-off in the ATL (Devlin et al. 2000) or in specific representational or phonological demands associated with overt speech production. Alternatively, it may be that ATL activation observed in earlier studies was not specific to nominal knowledge but rather, to generalised activation of person knowledge. Indeed, this generality is evident in the present study where, access to semantic, episodic and physical cognitive domains, as well as social, activated ATL. It is noteworthy that, while social knowledge did not produce a stronger response in the ATL ROI than these other three domains, the whole brain analysis (Figure 6) only social knowledge showed a significant cognitive selective response in adjacent the left ATL, consistent with the importance of social knowledge in this region.
Regional coordination across the person knowledge network
Elements of the network for person knowledge coordinate to form its diverse range of functions. Here, in contrast to investigating functional connectivity over time (e.g. Fairhall and Ishai 2007) we consider how these regions functionally coordinate over different tasks in response to their varied cognitive domains. Consistent with classic models (Haxby, Hoffman, and Gobbini 2000b) we observed that functional coordination between core perceptual (OFA, FFA and pSTS) regions was high. Interestingly, we observed that the fronto-lateral components of the classic extended system, IFG and OFC, coordinated more closely with these core regions rather than other elements of the classic extended system. It is notable that these fronto-lateral regions, particularly IFG, are closely related to extrinsic, task-activated, networks, distinguishing them from extended regions associated with the anti-correlated intrinsic resting state network (Fox et al. 2005). Additionally, during access to stored knowledge, the fronto-lateral IFG is implicated in guiding access to relevant information (Martin and Chao 2001; Wagner et al. 2001; Thompson-Schill 2003) suggesting a modulatory rather than representational role.
Other components of the extended system appear to coordinate most closely with each other across different cognition domains. Hierarchical clustering revealed an apparent dissociation between medial temporal components (ATFP, amygdala) and those associated with internalised cognition (vmPFC, precuneus, ATL, AG). However, this was not confirmed by statistical analysis and future work will be needed to verify this grouping. A planned comparison of whether ATFP grouped more closely with core or extended systems revealed no evidence in either direction.
Cognitive taxonomy in person knowledge
By considering the similarity between the neural profile of different cognitive domains we can gain insight into the relationship between these cognitive processes. We implemented this approach within the network for perceiving and knowing about others by comparing the profile of each task across the 21 ROIs comprising this network. We observed that for each task, despite variations in reaction times and task structure, that the two task-exemplars for each of the five cognitive domains reliably clustered with their counterpart. Demonstrating that cognitive domain is the primary grouping factor of activation patterns across this network and validating our selection of tasks. The general pattern of cognitive clustering across the regions suggests that social and perceptual knowledge share similar neural patterns, as do semantic and episodic knowledge with nominal being represented somewhat distinctly. These findings fall broadly within hypothesised domain-specificity boundaries (Spunt and Adolphs 2017) suggesting that declarative memory (episodic, semantic tasks) and language (nominal tasks) are part of the ‘cognitive’ macro-domain, while facial reception (physical tasks) and theory of mind (social tasks) are part of the ‘social’ macro-domain. Of specific interest to us was the relationship between episodic, semantic and nominal knowledge. One classic distinction in forms of declarative memory is between episodic (personal experience) and semantic (general knowledge) (Tulving 1972). The term ‘semantic knowledge’ refers to a broad range of knowledge about objects, factual knowledge and linguistic access to word meaning. Here we specifically contrasted competing components comparing whether semantic memory in the form of factual knowledge about people was more closely related to our personal experience and memories about that person than access to their name, a task domain that strongly recruited language circuity (see next section). Model comparison revealed significantly greater evidence that semantic memory clustered more closely to episodic than nominal access. This grouping is roughly apparent in the tuning profiles presented in Figure 3, where episodic and semantic domains load most heavily on regions associated with intrinsic cognition while nominal knowledge follows a different topography - engaging mainly parts of the intrinsic network as well as regions outside of it (e.g. IFG). In a broad sense, this result suggests that within the context of the tasks used in this study, semantic access shares a mechanism with episodic rather than linguistic neural systems.
Cognitive domain across the whole brain
In the study, we sought to understand how different elements of the person knowledge network, active spontaneously when we view familiar people (Gobbini and Haxby 2007), contribute to our diverse array of person related knowledge. To this end, we perturbed the system towards five difference cognitive domains to understand regional processing bias. However, these cognitive domains are not manifest solely within the person knowledge network and a whole brain analysis revealed the cognitive-domain selective regions outside this network. Figure X shows the selective activation of cognitive domains across the brain (domain v. others).
Recruitment of language regions in the Nominal task: the supramarginal gyrus, lateral PFC and dorsomedial PFC (Price 2012), validate the importance of linguistic processes in the performance of this task. Similarly social knowledge tasks preferentially recruited regions of the left ATL and an anterior section of dorsomedial PFC, consistent with the social cognition network (Adolphs 2009). The engagement of broad parts of right lateral PFC as well as small clusters in medial temporal and occipital areas is consistent with the involvement of these regions in the retrieval of perceptual attributes (Kan et al. 2010). Episodic access generally recruited regions associated with autobiographical memory (Schacter et al, 2012, Neuron; Spreng and Grady, (2009), In particular, bilateral angular gyrus showed a strong modulation with episodic tasks and is an area associated with the strength of autobiographical recollection Rissman et al, 2016, JCON).. However, despite distinct of regions consistent with the episodic domain, the hippocampus was not recruited, even at uncorrected thresholds. The reason for this absence is uncertain but may relate to the use of a familiarity rather than recognition weighted task (Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci., 30, 123-152.) in our design or to other factors related to this kind of episodic access.
Collectively, these results indicate the separable nature of the cortical processes assoiated with our five cognitive domains and emphasises the coordination of regions of the person knowledge network with other brain regions in the perform of their tasks [poorly worded].
Conclusion
The current study examined how the distributed cortical network for perceiving and knowing about others coordinates to accomplish its diverse range of cognitive functions. By examining a range of tasks within a single study we were able to observe a clear preference for access to social over nominal knowledge in ATL and a clear division between a more perceptual pSTS and a more cognitive angular gyrus components of TPJ, both in terms of their global response to cognitive access and the profile of activation across different cognitive domains. Through further multivariate analysis of the cognitive response profile across the network, we observed that regional coordination profiles grouped core regions with the lateral frontal extended system regions, which functioned relatively independently of intrinsic components of the extended system. Moreover, we were able to reconstruct a taxonomy that reflected how cortically similar cognitive domains are to one another. Notably, we observed that access to factual semantic knowledge employs neural substrates more similar to episodic memory than to language-related nominal knowledge. Collectively, these results demonstrate the importance of network level dynamics in the instantiation of person related cognition and knowledge. Future work will determine whether these principles extend to the representation of knowledge in general.
Table 3. ROI sphere centre coordinates. Peak coordinates for regions active in the localiser experiment (N=42) and ROI sizes in voxels after thresholding. Coordinates are in MNI space.
| Region | Hemisphere | X | Y | Z | ROI size |
|---|---|---|---|---|---|
| Precuneus | Medial | 3 | -52 | 29 | 81 |
| OFA | Right | 30 | -91 | -10 | 65 |
| Left | -33 | -88 | -10 | 49 | |
| FFA | Right | 42 | -46 | -22 | 81 |
| Left | -39 | -46 | -22 | 30 | |
| IFG | Right | 39 | 17 | 23 | 44 |
| Left | -36 | 20 | 26 | 38 | |
| ATL | Left | -60 | -7 | -19 | 69 |
| Right | 57 | -7 | -19 | 81 | |
| Amygdala | Left | -21 | -10 | -13 | 62 |
| Right | 21 | -7 | -16 | 59 | |
| dmPFC | Medial | 6 | 59 | 23 | 59 |
| vmPFC | Medial | 3 | 50 | -19 | 66 |
| OFC | Right | 33 | 35 | -13 | 58 |
| Left | -33 | 35 | -13 | 27 | |
| ATFP | Right | 33 | -10 | -40 | 39 |
| Left | -36 | -10 | -34 | 24 | |
| Angular | Left | -48 | -67 | 35 | 68 |
| Right | 42 | -64 | 35 | 57 | |
| pSTS | Left | -48 | -49 | 14 | 54 |
| Right | 48 | -55 | 14 | 71 |
Acknowledgments
This project was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 640594).
References
- Adolphs R. Neural systems for recognizing emotion. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience: Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The Social Brain: Neural Basis of Social Knowledge. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolon C, Capdevielle D, Raffard S. Face recognition in schizophrenia disorder: A comprehensive review of behavioral, neuroimaging and neurophysiological studies. Neuroscience & Biobehavioral Reviews. 2015;53:79–107. doi: 10.1016/j.neubiorev.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, Laird A, Eickhoff SB. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Struct Funct. 2010;215:209–223. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nature Reviews Neuroscience. 2005;6:641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-Induced Loss of Signal: Comparing PET and fMRI on a Semantic Task. NeuroImage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Downing PE, Peelen MV. How might occipitotemporal body-selective regions interact with other brain areas to support person perception? Cognitive Neuroscience. 2011;2(3-4):216–226. doi: 10.1080/17588928.2011.613987. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Anzellotti S, Ubaldi S, Caramazza A. Person- and place-selective neural substrates for entity-specific semantic access. Cereb Cortex. 2014;24:1687–1696. doi: 10.1093/cercor/bht039. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Caramazza A. Category-selective neural substrates for person- and place-related concepts. Cortex. 2013a;49:2748–2757. doi: 10.1016/j.cortex.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Caramazza A. Brain regions that represent amodal conceptual knowledge. J Neurosci. 2013b;33:10552–10558. doi: 10.1523/JNEUROSCI.0051-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective Connectivity within the Distributed Cortical Network for Face Perception. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of cognitive. 2000 doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Gesierich B, Jovicich J, Riello M, Adriani M, Monti A, Brentari V, Robinson SD, Wilson SM, Fairhall SL, Gorno-Tempini ML. Distinct Neural Substrates for Semantic Knowledge and Naming in the Temporoparietal Network. 2011;22:bhr286–bhr2226. doi: 10.1093/cercor/bhr286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS, Tempini ML. The neural systems sustaining face and proper-name processing. Brain. 1998;121(Pt 11):2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heide Von Der RJ, Skipper LM, Olson IR. Anterior temporal face patches: a meta-analysis and empirical study. 2013;7 doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior Temporal Sulcus—It's My Area: Or Is It? 2008;20:2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, Gallant JL. Natural speech reveals the semantic maps that tile human cerebral cortex. Nature. 2016;532:453–458. doi: 10.1038/nature17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, Thompson-Schill SL. Role of mental imagery in a property verification task: fmri evidence for perceptual representations of conceptual knowledge. Cognitive Neuropsychology. 2010;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel JL, Oedekoven CS, Tut AC, Bird CM. Multiscale integration of contextual information during a naturalistic task. Cerebral Cortex. 2017;1:9. doi: 10.1093/cercor/bhx218. [DOI] [PubMed] [Google Scholar]
- Koster-Hale J, Richardson H, Velez N, Asaba M, Young L, Saxe R. Mentalizing regions represent distributed, continuous, and abstract dimensions of others' beliefs. NeuroImage. 2017;161:9–18. doi: 10.1016/j.neuroimage.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Minnebusch DA, Daum I. Neuropsychological mechanisms of visual face and body perception. Neuroscience & Biobehavioral Reviews. 2009;33:1133–1144. doi: 10.1016/j.neubiorev.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Lee SM, Gabrieli JDE. Dissociable Neural Systems Supporting Knowledge about Human Character and Appearance in Ourselves and Others. 2011;23:2222–2230. doi: 10.1162/jocn.2010.21580. http://dxdoiorg/101162/jocn_a_00009. [DOI] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8:123–133. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajimehr R, Young JC, Tootell RBH. An anterior temporal face patch in human cortex, predicted by macaque maps. PNAS. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Olson IR. What's Unique about Unique Entities? An fMRI Investigation of the Semantics of Famous Faces and Landmarks. 2012;22:2005–2015. doi: 10.1093/cercor/bhr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London, Series B Biological Sciences. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza C. Naming with proper names: the left temporal pole theory. Behavioural Neurology. 2011;24:277–284. doi: 10.3233/BEN-2011-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Inter Neuropsych Soc. 2009;15:645. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R. A new look at domain specificity: insights from social neuroscience. Nature Reviews Neuroscience. 2017;18:559–567. doi: 10.1038/nrn.2017.76. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring "how" from "where". Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tippett LJ, Miller LA, Farah MJ. Prosopamnesia: a selective impairment in face learning. Cognitive Neuropsychology. 2000;17:241–255. doi: 10.1080/026432900380599. [DOI] [PubMed] [Google Scholar]
- Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. 2007;45:163–173. doi: 10.1016/j.neuropsychologia.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. Organization of memory; 1972. [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. NeuroImage. 2011;54:1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Viard A, Chételat G, Lebreton K, Desgranges B, Landeau B, La Sayette de V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: An fMRI study. Brain Cogn. 2011;75:1–9. doi: 10.1016/j.bandc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001 doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Werner NS, Kühnel S, Markowitsch HJ. The Neuroscience of Face Processing and Identification in Eyewitnesses and Offenders. Front Behav Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Brosch T. Faces in Context: A Review and Systematization of Contextual Influences on Affective Face Processing. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]