Abstract
The TNFR superfamily of receptors, the major focus of the recent TNFR Superfamily Conference held in June 2019, employ the TRAF family of adaptor proteins in key aspects of their signaling pathways. While many early studies investigated TRAF functions via exogenous overexpression in non-hematopoietic cell lines, it has subsequently become clear that although TRAFs share some overlap in function, each also plays unique biological roles, that can be highly context-dependent. This brief review summarizes the current state of knowledge of functions of each of the TRAF molecules that mediate important functions in T lymphocytes: TRAFs 1, 2, 3, 5 and 6. Due to our current appreciation of the contextual nature of TRAF function, our focus is upon findings made specifically in T lymphocytes. Key T cell functions for each TRAF are detailed, as well as future knowledge gaps of interest and importance.
Keywords: TNFR superfamily, T cell, signal transduction
Summary sentence:
Review of the multiple roles played by members of the TRAF family in the biology and functions of T lymphocytes.
INTRODUCTION
Members of the tumor necrosis factor receptor-associated factor (TRAF) family of adapter molecules were initially identified via their association with members of the TNFR superfamily of receptors [1]. Over the past approximately 25 years, their roles in multiple cell types are now appreciated to include many additional types of receptors, as well as non-receptor-mediated signaling pathways, in both the cell cytoplasm and nucleus. Many early studies of TRAF functions employed the technical approach of exogenous over-expression of TRAFs and proposed binding partners in easily-transfected transformed cell lines, of epithelial and/or fibroblastic origin. However, interpretation of such studies is complicated by several caveats. TRAFs 1, 2, 3 and 5 all bind a canonical overlapping recognition motif in the cytoplasmic domains of multiple TNFR superfamily receptors [2]. Thus, altering the relative amounts of any one TRAF necessarily impacts all the others in this group, so results attributed directly to the overexpressed TRAF may be due at least in part to consequent alterations in association of other TRAFs. While this is primarily a concern in TRAF overexpression studies using epithelial cell lines, it is possible that levels of expression of a given TRAF could alter the endogenous expression and/or receptor association of other TRAFs in various cell types of interest. Similarly, TRAFs can form heterotrimers and higher-order multimers with TRAFs of a different type [3], another complicating issue. Finally, it is now known that a given TRAF may have very distinct – even contrasting – roles when expressed at normal levels in different cell types [4], so extrapolating TRAF function from one cell type to another is inadvisable. For these reasons, in this brief review we will confine our discussion to results obtained in the cell type of focus, T lymphocytes.
When studies of TRAF function first began to move beyond transfected epithelial and fibroblast cell lines, initial work often focused upon B lymphocytes. These cells abundantly express the TNFR superfamily member CD40, and this receptor was the source of identification of several of the TRAF family of molecules with which it associates (CD40 binds TRAFs 1, 2, 3, 5, and 6) [5]. TRAF3 is also an important tumor suppressor specifically in B cells, [6]. Subsequently, TRAF6 was discovered to be an important participant in signaling by the Toll-like-receptor (TLR) family of innate immune receptors, most often studied in myeloid cells, and it was found that TRAF3 is also an important regulator of TLR signals in several cell types [7].
Studies of TRAF roles and functions in T lymphocytes have to date been much less frequent. The first two TRAFs identified, TRAFs 1 and 2, were found via their association with TNFR1 & 2 (aka CD120a and b) [1]. Paradoxically, while T cells express these receptors, all initial studies of TRAF1/2 regulation of TNFRs were conducted in non-T cells, most by over-expression in HEK293T adenovirally-transformed kidney epithelial cells. In fact, studies of endogenous TRAF1 and 2 functions in TNF signaling to normal T cells are still needed. However, over the past several decades, several labs have reported physiologically important roles for each of the endogenous lymphocyte-expressed TRAF molecules TRAFs 1, 2, 3, 5 and 6, in T lymphocytes. These roles are quite varied in regards to T cell subtypes, the TRAF-associating receptors involved, and the TRAF-mediated functions and signaling pathways impacted. Here, we summarize the current status of these studies and important future questions to be addressed.
TRAF1
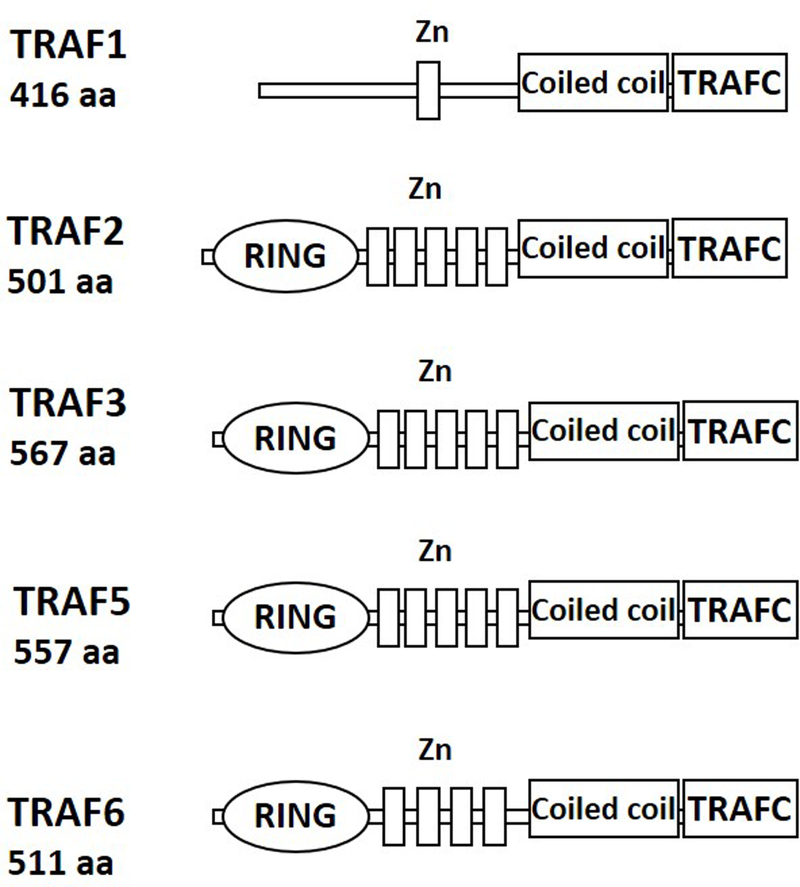
TRAF1 is an atypical member of the TRAF family, as it lacks a RING domain and has a single zinc finger (Figure 1). Although many early studies of TRAF1 were conducted in epithelial or fibroblast cell lines, its physiologic expression is restricted to spleen, lung, and testis, particularly to immune cells (reviewed in [8]). The first report to focus upon TRAF1 specifically in T cells studied a mouse strain in which transgenic TRAF1 expression is driven by an H-2K promoter. T cell proliferation and in vitro cytotoxic T lymphocyte (CTL) responses are not impacted by this whole-mouse constitutive TRAF1 over-expression, but a decrease in TCR-mediated apoptosis and peptide-induced tolerance is observed [9]. The authors attribute these findings to regulation of CD120b/TNFR2 signaling, but this signaling pathway was not investigated. In contrast, when TRAF1 is deleted from the whole mouse, an increase is seen in T cell proliferation in response to agonistic Abs to CD3 and CD28 [10]. T cells stimulated with anti-CD3 Ab and TNF in vitro display enhanced IκBα phosphorylation and binding to an NF-κB probe in electrophoretic mobility shift assays, as well as c-Jun kinase (JNK) phosphorylation and AP-1 activation [10].
Figure 1. Domain organization of TRAFs expressed in T lymphocytes.
All TRAFs (with the exception of TRAF1) contain an N-terminal RING domain and one or more zinc finger motifs (labeled Zn). The C-terminus consists of the coiled coil domain and the TRAFC domain (also known as the MATH domain).
This same strain of TRAF1−/− mice was subsequently examined for their response to infection with influenza virus. Peptide-binding antigen-specific CD8 T cells from these mice are decreased in frequency in lungs and draining lymph nodes after a secondary antigen challenge. Interestingly, a normal frequency of interferon (IFN)γ+ CD8 T cells is seen during both 10 and 20 responses, and direct in vitro cytotoxicity towards target cells is also normal [11]. This report showed for the first time that TRAF1-deficient CD8 T cells have an increase in the pro-apoptotic protein Bim, and transduction of the cells with Bim siRNA rescues the phenotype of decreased T cell recovery [11]. This TRAF1-Bim regulatory association was further explored in CD8 T cells from chronically-infected HIV patients, and the TNFR superfamily member 4–1BB was introduced to the developing story. Stimulation of these T cells in vitro with 4–1BB ligand plus agonists to CD80 or CD70 expands CD8 effector T cells of these patients; 4–1BB engagement decreases both Bim and TRAF1 [12]. A return to this theme several years later demonstrated increased TRAF1 in CD8 T cells of so-called HIV ‘controllers’, whose infection does not apparently progress, and this is mirrored by levels of TRAF1 in CD8 T cells from mice acutely vs. chronically infected with a model virus [13]. Thus, TRAF1 appears to play an important role in promoting CD8 effector T cell maintenance in the setting of chronic viral infection.
There has been a strong focus upon the role of TRAF1 in T cell 4–1BB function in recent years. The evidence that TRAF1 serves to maintain CD8 effector T cells is reinforced by a new study in the mouse influenza virus model. This demonstrates an important, TRAF1-dependent role for 4–1BB in resident memory T cell formation, via promotion of lung CD8 T effector cell survival [14]. This role is also proving useful in the setting of therapeutic T cells with chimeric antigen receptors, (CARs) that contain an antigen-binding external domain fused to transmembrane and cytoplasmic domains of various costimulatory molecules. It appears that CAR T cells containing a 4–1BB domain show greater survival than those with a CD28 costimulatory domain; this 4–1BB function requires binding of the cytoplasmic domain by TRAFs 1, 2 and 3, and involves NF-κB activation [15].
It is clear from the summary above that TRAF1 is important to normal T cell functions, but the mechanisms by which it does so are only partially understood. Although early studies focused upon potential roles played by TRAF1 in CD120b/TNFR2 function, the mechanistic role of TRAF1 in signaling by this receptor in T cells has not been reported. In B cells, it was shown that TRAF1 inhibits CD40-mediated TRAF2 degradation to promote JNK and NF-κB activation [16], but whether this occurs for any TNFR superfamily members in T cells is unknown. Stimulation of primary mouse CD8 T cells via the TCR and 4–1BB activates the NF-κB1 pathway, and this is abrogated in TRAF1−/− T cells [17]. However, these T cells display enhanced NF-κB2 activation, implying contrasting roles for TRAF1 in the two pathways [17]. Sabbagh et al. performed proteomic analysis to identify proteins associating with both the 4–1BB cytoplasmic domain and TRAF1 in mouse CD8 T cells, and found a protein called leukocyte-specific protein 1 (LSP1) that fits this profile. The TRAF1-LSP1 complex is required for 4–1BB-mediated ERK activation, Bim downregulation, and CD8 T cell expansion mediated by CD3 + 4–1BB signals [18]. Further investigation of the mechanisms by which TRAF1 regulates the functions of multiple T cell subsets, as well as multiple TNFR superfamily members, will be valuable for a more comprehensive understanding of TRAF1 roles in T cells.
TRAF2
TRAF2 has a typical TRAF structure (Figure 1), including a RING domain that when deleted, creates a dominant negative form (TRAF2.DN) that interferes with downstream signaling [19]. TRAF2 was first identified as a component of TNFR1/CD120a and TNFR2/CD120b signaling complexes, and thus has been widely studied in the context of cell death pathways. Early experiments with purified proteins in vitro and in transfected HEK293 cells demonstrated that TRAF2 also associates, via the TRAF-C domain, with non-death domain-containing TNFR-SF members expressed by T cells, such as CD27, CD30, 4–1BB, and OX40 [19–22].
The bulk of the current knowledge of the role of TRAF2 in T cells is derived from the study of mouse models in which TRAF2 has been globally deleted, or TRAF2.DN has been overexpressed globally or specifically in the lymphocyte compartment [19, 23, 24]. Global TRAF2 deficiency (TRAF2−/−) leads to early lethality due to runting and an inflammatory phenotype characterized by high serum TNFα [23]. Survival can be improved by crossing these mice to TNFα−/− mice or deleting a single allele of the NF-κB inducing kinase (NIK), though it should be noted that TRAF2−/−TNFα−/− mice have elevated serum cytokines and develop a progressive inflammatory disease [23–25]. Analysis of thymocytes from TRAF2−/− animals, which have extensive lymphopenia, revealed that thymocyte development is normal, but thymocyte numbers are reduced due to increased sensitivity to TNFα-induced cell death mediated by TNFR1 [23]. Generation of the TRAF2.DN mouse, which lacks functional TRAF2, but does not have the survival defect observed in TRAF2−/− mice, revealed that total T cell numbers are normal, a finding that is corroborated by T cell-specific TRAF2 knockout mouse models [19, 26, 27].
While TRAF2 deficiency has minimal impact on total T cell numbers and survival, there is abundant evidence to support a negative impact of TRAF2 loss upon T cell effector functions and the balance of T cell subsets. T cells from TRAF2.DN mice, TRAF2−/−TNFα−/− mice, and T-Traf2−/− mice are less responsive to expansion mediated through CD3 and CD28 than their wildtype counterparts [19, 24, 27]. Interestingly, TRAF2.DN T cells are hyperresponsive to stimulation by anti-CD3 Ab alone, and stimulation with soluble 4–1BB ligand dampens this response [20]. Defects in proliferation and production of IL-2, IL-4, and IFNγ become evident upon stimulation of TRAF2.DN T cells specifically through 4–1BB or OX40, in mixed leukocyte reactions, and with specific antigens in transgenic and allograft experimental models [20–22].These findings indicate that TRAF2 is important for the generation of effector T cell responses following costimulatory events.
In addition to primary T cell responses, the development of T cell memory is impaired in the absence of functional TRAF2 [22]. Adoptive transfer of OVA-specific transgenic TRAF2.DN T cells into recipient mice, followed by immunization with OVA and agonistic Ab to OX40, fails to generate a robust memory T cell population. The authors speculated that long term memory T cell survival is dependent upon TRAF2, but did not identify the required signals [22]. A T-Traf2−/− mouse model, generated by crossing the Traf2flox/flox mouse to the LckCre mouse, provided some insight into the observed defect in T memory cell formation, and revealed that T cell-specific deletion of TRAF2 results in constitutive NF-κB2 activation. T-Traf2−/− mice have normal numbers of splenic CD4 T cells. However, they have a 50% reduction in splenic CD8 T cells that is not due to reduced thymic cellularity nor decreased T cell proliferation [27]. CD44loCD62Lhi (naïve) CD8 T cells are reduced by 50%, CD44+CD62Llo (effector memory) CD8 T cells by 40%, and, most strikingly, CD4hiCD62Lhi (central memory, Tcm) CD8 T cells by 70%, with reduced Tcm cells also seen in the bone marrow. TRAF2−/− CD8 T cells are comparable to their wildtype counterparts in steady state turnover and expression of the pro-survival factor Bcl-2. However, they exhibit impaired expansion in response to IL-15, despite normal expression of IL-15Rα and IL-2Rcγ, and have hyperactive AKT at baseline and after IL-15 stimulation [27]. Exogenous administration of IL-15 can rescue the defect in numbers of Tcm cells, confirming that defective downstream IL-15R signaling is likely responsible for the alteration in Tcm in the absence of TRAF2. Additional studies are required to determine if TRAF2 interacts with the IL-15R.
While the importance of TRAF2 for T cell effector functions is evident, TRAF2 appears dispensable for T cell development and homeostatic survival [26, 27]. TRAF2 functions differently in CD4 versus CD8 T cells, with CD4 T cells more reliant upon TRAF2 for optimal activation and proliferation downstream of TCR signaling. TRAF2 also impacts Th subset differentiation, as T-TRAF2−/− mice have increased Th2 and Treg populations, but reduced Th17 cells, compared to their wildtype counterparts [28]. Additional studies with T-Traf2−/− mouse models are required for further evaluation of the role of TRAF2 in specific T cell subsets, and in functions of individual receptors expressed by T cells.
TRAF3
TRAF3 has a TRAF structure highly similar to that of TRAF2, and functions as an adaptor molecule in multiple signaling pathways (Figure 1). Unlike other RING domain-containing family members TRAFs 2 and 6, it lacks independent E3 ubiquitin ligase activity [29], instead recruiting TRAF2 into hetero-multimers for this purpose. Early studies in HEK293 cells revealed that overexpression of TRAF3, unlike that of TRAFs 2, 5, and 6, restrains, rather than enhances, NF-κB activation [30]. Subsequent studies revealed that the roles of TRAF3 are cell type and receptor-dependent [31].
The first indication that TRAF3 is important for T cell function came from characterization of the Traf3-null mouse. These mice appear normal at birth, but quickly become runted, exhibit progressive loss of peripheral leukocytes, and die within two weeks [32]. Chimeras generated by transferring TRAF3−/− fetal liver cells into lethally irradiated wildtype mice exhibit impaired Ab production following immunization with a T-dependent antigen, but normal Ab responses to a T-independent antigen, suggesting dysfunctional T cell help. The authors subsequently assessed the proliferation of primed T cells in response to antigen, finding TRAF3−/− T cells defective, thus suggesting that TRAF3 promotes normal T cell function [32].
Much of the current understanding of the roles that TRAF3 plays in T cells derives from studies performed with conditional knockout mouse models, which allow deletion of TRAF3 in specific cell types, without the early lethality associated with TRAF3 global deletion. In LckCreTraf3flox/flox mice, in which Traf3 is deleted at the double negative stage of T cell development, there is no alteration in T cell survival, despite constitutive NF-κB2 activation [26]. This is in contrast to the markedly enhanced survival of TRAF3−/− B cells [26, 33]. CD4CreTraf3flox/flox mice (henceforth referred to as T-Traf3−/− mice), have TRAF3 deleted at the double positive stage of T cell development, providing further insight into the impact of TRAF3 on mature T cell-mediated immunity. T-Traf3−/− mice have normal numbers of total CD4 and CD8 T cells, but a 2 to 3-fold increase in thymic-derived T regulatory cells (Tregs), also present in LckCreTRAF3flox/flox mice [33, 34]. Numbers of invariant natural killer T cells (iNKT) are reduced ~10-fold in the absence of TRAF3; this decrease is in part due to impaired IL-15 signaling [35].
The increased percentage and number of Tregs in T-Traf3−/− mice suggests that TRAF3 restrains Treg development. Treg-specific deletion of Traf3 (FoxP3CreTraf3flox/flox), in contrast, has minimal impact on Treg frequency, indicating that TRAF3 is not required for maintenance of mature Tregs [36]. Consistent with this, TRAF3−/− Tregs do not have a survival advantage over TRAF3+/+ Tregs, and express comparable levels of Treg signature proteins, including FoxP3, CTLA4, CD25, CD122, and GITR; thymic selection is also intact in T-Traf3−/− mice [34]. However, the transition from Treg precursor to mature Treg is enhanced, with increased IL-2-mediated phosphorylation of Stat5, which is required for FoxP3 gene expression [34]. Timing of Traf3 expression appears important for Treg function, as deletion of TRAF3 in mature Tregs results in impaired Treg suppressive capacity in vivo, but not in vitro. As such, the frequency of CD44hiCD62Llo effector/memory CD4 T cells is increased, as is the production of high-affinity Abs [36].
While total numbers of conventional T cells are unaffected by TRAF3 deficiency, the proportions of naïve, effector, and memory T cells are altered [37, 38]. The population of splenic effector/memory CD4 T cells is doubled in T-Traf3−/− mice, with a corresponding reduction in naïve CD4 T cells (CD44loCD62Lhi). Several groups suggest that this is due to a loss of Treg-mediated suppression of effector/memory CD4 T cells [36, 38]. Naïve and effector memory CD8 T cells are present in proportions comparable to wildtype mice, but the Tcm (CD44hiCD62Lhi) CD8 T cell population is reduced 5–10 fold [37, 38]. Following IL-15 stimulation, existing Tcm cells exhibit decreased proliferation and impaired activation of Stat5 and ERK pathways, indicating a role for TRAF3 in homeostatic survival of and cytokine production by Tcm CD8 T cells [38].
The altered phenotypes of existing CD4 and CD8 T cells impacts the ability of T-Traf3−/− mice to mount appropriate T cell responses. T-Traf3−/− mice fail to respond to challenge with a sublethal dose of L. monocytogenes, exhibiting decreased survival and delayed clearance of bacteria in the liver. A marked reduction in antigen-specific CD4 and CD8 T cells, and thus in IFNγ and TNFα production by these cells, contributes to the failure to clear the infection [37]. In agreement with the findings from early studies of globally TRAF3-deficient mice [32], T-Traf3−/− mice have defective T-dependent, but not T-independent Ab responses [37]
Investigation into the molecular mechanisms underlying the contribution of TRAF3 to T cell functions revealed an unexpected role for TRAF3 in early TCR/CD28 signaling. Upon stimulation through TCR and CD28, TRAF3-\- T cells exhibit reduced proliferation and enhanced apoptosis, independent of NF-κB activation or alterations in pro- and anti-apoptotic proteins [37]. Production of IL-2, IL-4, and IFNγ is decreased in CD4 and, to a lesser degree, CD8 T cells, and is not due to defective cytokine production machinery. Supporting a role for TRAF3 in TCR/CD28 signaling, TCR/CD28-induced phosphorylation of early signaling molecules, including Zap70, LAT, and PLCγ, as well as the downstream molecule ERK, is diminished in TRAF3−/− T cells [37]. The defect in signaling occurs early in T cell activation, beginning with altered activation of Lck. This is due to increased plasma membrane localization of the phosphatase PTPN22 and the inhibitory kinase Csk in TRAF3-deficient T cells. These two inhibitors of Lck interact with TRAF3 (via the TRAF-C domain) to prevent the inactivation of Lck [39].
TRAF3 can exert its effects on TCR/CD28 signaling, and thus T cell functions, in part because it is recruited to the TCR/CD28 complex upon engagement of both TCR and CD28 [37]. While it was initially hypothesized that CD28 recruits TRAF3 via cytoplasmic signaling motifs thought to contain a TRAF3-binding site, recent work demonstrated that TRAF3 is recruited to the TCR/CD28 complex through interactions between the TRAF-C domain and a TRAF3 binding motif in the cytoplasmic domain of the CD28-associated adapter protein LAT [40]. Investigation into the interactions between TRAF3 and Dok1, a LAT complex-associated negative regulatory protein, as well as regulators of Dok1, may provide further insights into how TRAF3 regulates this complex.
TRAF3 also regulates T cell cytokine receptor signaling. As mentioned above, TRAF3 appears to promote signaling to T cells via the IL-15R, although it is not known if TRAF3 interacts with IL-15R directly or indirectly [35, 38]. In contrast, TRAF3−/− conventional T cells exhibit enhanced IL-2R signaling, with increased phosphorylation of Jak1 and Jak3, the tyrosine kinases recruited to the IL-2R complex after IL-2 binding. This indicates that TRAF3 is involved early in IL-2R signaling [34]. TRAF3 associates with Jak3 upon IL-2 stimulation, and normally restrains further signaling via the recruitment of the phosphatase TCPTP (PTPN2). The interaction between TRAF3 and TCPTP requires the RING and zinc finger domains of TRAF3 [34].
It is clear that TRAF3 plays numerous key roles in T cell biology. Further investigations of its participation in signaling by both additional cytokine receptors, as well as members of the TNFR superfamily, should provide more potential important roles for this versatile TRAF.
TRAF5
TRAF5, another T cell-expressed TRAF with a typical TRAF structure (Figure 1), has been less-studied than TRAFs 2, 3 and 6, as unlike these TRAFs, deletion of TRAF5 in the whole mouse has a much more modest impact on phenotype. As with other TRAFs, the majority of published reports to date have not studied TRAF5 specifically in T cells; here we summarize the findings of those that have. The first TRAF5-deficient mouse was reported in 1999; the strain studied was an F1 hybrid between C57BL-6J and 129/J. Mice display no alteration in normal numbers of CD4 and CD8 T cells. The one notable finding specific to T cells in this mouse was that CD27-mediated proliferation in T cells provided with CD3 + costimulation signals is reduced [41]. Kraus et al. expanded upon the role of TRAF5 in T cell CD27 function in this TRAF5−/− mouse, fully backcrossed to C57BL/6. The mice display a defective response to in vivo infection with the bacterium Listeria monocytogenes, especially in the CD8 memory T cell compartment. This defect, which is T-cell intrinsic, is associated with normal proliferative capacity, but an increased tendency to undergo activation-induced cell death, and a failure of CD27-mediated signals to rescue the TRAF5−/− T cells from apoptosis [42].
T cells from the TRAF5−/− mouse stimulated in vitro via another TNFR superfamily receptor, OX40, display enhanced production of IL-4 and IL-5, and in vivo immunization that included an agonistic anti-OX40 Ab also resulted in increased Th2 cells, consistent with in vitro data. Lung inflammation in a mouse allergy-induction model is also increased, further supporting the concept that TRAF5 inhibits Th2 development in the mouse [43]. In addition to Th2 cells, TRAF5−/− mice develop increased Th17 cells following IL-6 treatment both in vitro and in vivo. T cell TRAF5 binds the IL-6R and suppresses IL-6 signaling by inhibiting recruitment of Stat3 [44]. TRAF3 in B cells also binds IL-6R to inhibit its function, but in this case does so by recruiting a phosphatase to the receptor [45], so the two TRAFs both inhibit IL-6R function by different mechanisms. It was subsequently shown that TRAF5 collaborates with TRAF2 in inhibiting IL6R-mediated Th17 development [46].
Another TNFR superfamily member regulated by TRAF5 in T cells is glucocorticoid-induced TNFR-related protein (GITR). In a second TRAF5−/− mouse strain (fully backcrossed to C57BL-6), TCR-mediated increases in surface GITR expression are normal, but GITR-mediated NF-κB1, p38, and ERK activation are markedly decreased, as is GITR-stimulated IL-2 production [47]. Subsequently, Snell et al. studied GITR specifically on CD8 T effector cells, finding that GITR-mediated NF-κB activation leading to enhanced Bcl-xL requires both TRAFs 2 and 5 [48]. Further understanding of how TRAF5 regulates T cell function, alone and in cooperation with other TRAF molecules, is an important goal of future studies.
TRAF6
TRAF6 is distinguished by the E3 ubiquitin ligase activity associated with its RING domain (Figure 1), as it can attach K63-linked ubiquitin chains to itself and to other molecules [49]. The TRAF-C domain shares ~30% homology to the TRAF-C domains of TRAFs 1–5, and binds to consensus motifs that are distinct from consensus motifs bound by TRAFs 2, 3, and 5 [49–51]. TRAF6−/− mice, similar to TRAF2−/− and TRAF3−/− mice, become runted and die within two weeks of birth, but have unique abnormalities in skeletal development [51]. These were subsequently found attributable to important participation by TRAF6 in signaling during bone development by the TNFR SF member RANK [52–54]. Initial characterization of TRAF6−/− mice led to the conclusion that TRAF6 is not involved in T cell responses, as T cells from TRAF6−/− mice proliferate similarly to their wildtype counterparts in response to TCR and Concanavalin A stimulation [51]. More recently, TRAF6 has been revealed as an important player in T cell homeostasis and function.
TRAF6−/− chimeric mice were generated prior to availability of conditional gene deletion in mice, to circumvent the early lethality of the global TRAF6−/− mouse and facilitate study of the role of TRAF6 in the hematopoietic compartment. This was the first study to suggest that TRAF6 is a negative regulator of T cell activation [55]. TRAF6−/− chimeras develop a lethal inflammatory wasting disease, with evidence of Th2 cell infiltrates and production of the Th2 cytokines IL-4, IL-10, and TGFβ. TRAF6−/− T cells exhibit enhanced proliferation and Th2-type cytokine production in vitro that is most pronounced in response to stimulation through CD3 alone. These animals have atrophic thymi, small lymph nodes, and reduced splenic cellularity, but retain normal proportions of CD4 and CD8 T cells, suggesting that T cell maturation is intact. The CD4 T cell compartment skews towards effector and memory CD4 T cells, consistent with the activated phenotype observed [55]. King et al. confirmed that these findings are due to T cell-specific loss of TRAF6, using two T-cell specific TRAF6−/− mouse models (CD4Cre-Traf6flox/flox, designated here as T-Traf6−/−, and LckCre-Traf6flox/flox). Ablation of TRAF6 specifically in T cells revealed that, in addition to enhanced proliferation in the absence of costimulation, with a corresponding resistance to anergy, T-Traf6−/− T cells exhibit constitutive hyperactivation of the pro-survival kinases PI3K and AKT. They are also resistant to Treg-mediated suppression in a PI3K-dependent manner [56, 57]. The hyperactivity of the PI3K-AKT pathway may be due to loss of cooperation between TRAF6 and LAT following TCR stimulation, resulting in the loss of negative regulatory mechanisms intended to restrain downstream signaling [58].
T-Traf6−/− mice spontaneously develop Th2-like autoimmune disease, despite normal numbers and in vitro suppressive ability of Tregs [56]. A limitation of these early studies is that they did not assess in vivo suppressive ability of FoxP3+ T cells, rather than bulk activated CD4+CD25+ T cells. There is a striking defect in thymic FoxP3+ Treg development in TRAF6−/− mice, and subsequent work with Treg-Traf6−/− mice (FoxP3Cre-Traf6flox/flox) demonstrates that TRAF6 plays an important role in maintenance of peripheral FoxP3+ Tregs [59–61]. Treg-Traf6−/− mice develop the same Th2-like autoimmune disease as T-Traf6−/− mice, and have the same baseline immune hyperactivation [60, 61]. Treg-Traf6−/− Tregs fail to expand to the same degree as their wildtype counterparts, and are poised to more readily lose FoxP3 expression, becoming ex-FoxP3 T cells that produce effector cytokines IL-4 and IL-17 {Muto, 2013 #3508}. Normal CD4 T cells committed to become Tregs express more Traf6 transcripts than non-Treg-committed T cells. Increased Traf6 expression promotes the K63-linked ubiquitination of FoxP3, which is required for its nuclear localization. Experiments performed in exogenously-transfected HEK293 epithelial cells indicate that the RING domain is required for ubiquitination of FoxP3 at K262, and that FoxP3 associates with TRAF6 via its RING and zinc fingers [61]. Taken together, these findings indicate that TRAF6 is required for the development and maintenance of Tregs.
TRAF6 also negatively regulates T cell cytokine receptor signaling, sharing this property with TRAF3, as discussed above. T-Traf6−/− T cells exhibit a propensity to differentiate into Th17 cells in vitro; this is due to an increased sensitivity to TGFβ-induced signaling, which suppresses IL-2 production [62]. This finding is somewhat at odds with the increase in IL-2R signaling in TRAF6−/− thymocytes, and requires further investigation [63]. Treg-Traf6−/− Tregs are hyperresponsive to IL-2 stimulation, demonstrated by enhanced phosphorylation of Stat5 [60]. TRAF6 binds to IL-2Rβ, and potentially interferes with Jak1 activation by competing with Jak1 for this binding [63]. Further investigation into the mechanism by which TRAF6 inhibits Jak1 activation is required; perhaps, like TRAF3, TRAF6 recruits phosphatases to the IL-2R complex.
CONCLUSIONS
Studies of the TRAF family of molecules in chimeric and T cell-specific mouse models have revealed important roles for TRAFs in T cell homeostasis and function. As summarized in this review, TRAFs 1–3, 5, and 6 are involved in signaling through the TCR and cytokine receptors, in addition to their established roles in signaling through TNFR-SF members. The TRAF molecules are also important for the delicate balance of T cell activation and memory formation in the context of infection, with implications for enhancement of vaccination strategies and anti-tumor responses. It is thus very encouraging that recent work, including studies presented at the recent TNF Superfamily Conference at Asilomar in June 2019, highlight the important and varied roles played by T-cell-expressed TRAF molecules in the physiologic functions of this critical type of immune cell. The current state of knowledge of roles played by each of the T cell TRAFs discussed here is summarized in Table 1. Further studies of TRAFs in T cells are eagerly anticipated, to increase our understanding of how TRAFs regulate critical aspects of T cell biology.
Table 1. Impact of TRAF deficiency on T lymphocyte development and function.
Listed observations were published in one or more report, referenced here, and are organized by TRAF molecule and mouse model.
| TRAF | Mouse model | Observations | References |
|---|---|---|---|
| 1 | TRAF1.Tg | • Normal TCR-mediated proliferation and CTL responses in vitro • Decreased TCR-mediated apoptosis and peptide-induced tolerance |
9 |
| TRAF1−/− | • Viable mice with normal lymphocyte development • Increased TCR/CD28-mediated proliferation • Enhanced TCR/TNF-mediated phosphorylation of IκBα and JNK, and increased AP-1 activation • Decreased influenza-specific CD8 T cells due to increased Bim-mediated apoptosis • Decreased TCR/4–1BB-mediated NF-κB1 activation in CD8 T cells • Impaired resident memory CD8 T cell formation following influenza infection • Enhanced NF-κB2 activation |
10, 14, 17 | |
| 2 | TRAF2−/− | • Early lethality due to runting and inflammation • Increased sensitivity of thymocytes to TNFR1-mediated cell death |
23 |
| TRAF2−/−TNFα−/− | • Improved survival compared to Traf2−/− mice • Elevated serum cytokines leading to progressive inflammatory disease |
23–25 | |
| TRAF2.DN | • Viable mice with normal numbers of T cells • Hyperresponsive to CD3-mediated proliferation in the absence of costimulation • Decreased TCR/CD28-mediated proliferation • Decreased cytokine production upon 4–1BB or OX40 stimulation • Impaired memory T cell development |
19, 20, 22 | |
| LckCre-TRAF2fl/fl | • Normal numbers of total splenic CD4 T cells • Altered helper T cell differentiation (increased Th2 and Tregs, decreased Th17) • 50% reduction in total splenic CD8 T cells; 70% reduction in Tcm CD8 T cells • Impaired response of CD8 T cells to IL-15 signaling, restored by exogenous IL-15 • Constitutive NF-κB2 activation |
27 | |
| 3 | TRAF3−/− | • Early lethality due to runting and inflammation • Defective T cell proliferation • Defective Ab response to T-dependent Ag |
32 |
| LckCre-TRAF3fl/fl | • Normal T cell survival • Increased thymic Tregs • Constitutive NF-κB2 activation |
26 | |
| CD4Cre-TRAF3fl/fl | • Normal numbers of total CD4 and CD8 T cells • Increased IL-2-mediated thymic Treg maturation • Increased frequency of effector/memory CD4 T cells, and reduced naïve CD4 T cells • Impaired IL-15-mediated homeostatic survival and activation of central memory CD8 T cells • Decreased iNKT cells due to defective IL-15 signaling • Defective TCR/CD28 signaling, impacting proliferation and cytokine production • Enhanced IL-2R signaling • Constitutive NF-κB2 activation • Failure to clear L. monocytogenes infection • Defective Ab responses to T-dependent Ag |
34, 35, 37, 38 | |
| FoxP3Cre-TRAF3−/− | • Normal Treg frequencies • Impaired Treg function in vivo • Increased frequency of effector/memory CD4 T cells and production of high affinity Abs |
36 | |
| 5 | TRAF5−/−(F1 hybrid from C57BL/6J x 129/J cross) | • Normal numbers of CD4 and CD8 T cells • Reduced CD27-mediated proliferation in response to signals through CD3 and costimulation |
41 |
| TRAF5−/− (C57BL/6) | • Defective response to L. monocytogenes infection, mainly affecting memory CD8 T cells • Failure of CD27-mediated signaling to rescue CD8 T cells from apoptosis • Increased OX40-mediated Th2 cell differentiation and cytokine production • Enhanced IL-6R signaling • Enhanced IL-6-mediated Th17 differentiation • Decreased GITR-mediated NF-κB1, p38, and ERK activation • Decreased GITR-mediated IL-2 production |
42, 43, 44, 46, 47 | |
| 6 | TRAF6−/− | • Early lethality due to runting and skeletal abnormalities • Th2-like inflammatory phenotype • Normal proportions of CD4 and CD8 T cells, with skewing of CD4s toward an activated phenotype • Enhanced TCR-mediated proliferation |
51, 55 |
| CD4Cre-TRAF6fl/fl | • Enhanced TCR-mediated proliferation and resistance to anergy • Hyperactivation of PI3K and AKT • Increased Th17 differentiation • Defective thymic Treg development |
56, 57, 59–62 | |
| FoxP3Cre-TRAF6fl/fl | • Th2-like inflammatory phenotype • Impaired Treg proliferation • Increased conversion of FoxP3+ cells to to FoxP3- cells • Enhanced IL-2R signaling |
60, 61 |
Acknowledgements
Support for studies from the authors’ laboratory reviewed here was provided by grants from the NIH (AI123107 and P30CA086862) and the Veterans’ Administration (I01 BX001702) to GAB. TA received support from NIH T32 AI007485 and T32 GM007337.
Abbreviations:
- Ab
antibody
- AP-1
activated protein 1
- CAR
chimeric antigen receptor
- CTL
cytotoxic T lymphocyte
- DN
dominant negative
- ERK
extracellular regulated kinase
- GITR
glucocorticoid-induced TNFR-related protein
- IκBα
inhibitor of NF-κBa
- IFN
interferon
- iNKT
invariant natural killer T cells
- Jak
Janus kinase
- JNK
c-Jun kinase
- LAT
linker of activated T cells
- LSP1
leukocyte specific protein 1
- NF-κB
nuclear factor of kappa B
- NIK
NF-κB inducing kinase
- OVA
ovalbumin
- PLC
phospholipase C
- PTPN
protein tyrosin phosphatase number
- R
receptor
- RING
really interesting new gene
- Stat
signal transducer and activator of transcription
- Tcm
central memory T cells
- TCPTP
T cell protein tyrosine phosphatase
- TCR
T cell antigen receptor
- Th
T helper
- TLR
Toll-like receptor
- TNFR SF
tumor necrosis factor receptor superfamily
- TRAF
TNFR associated factor
- Treg
T regulatory cell
- T-Traf−/−
mice conditionally deficient in the indicated TRAF molecule in T cells
Footnotes
Competing financial interests
The authors declare no competing financial nor non-financial interests.
REFERENCES.
- 1.Arch RH, Gedrich RW, Thompson CB (1998) TRAFs - a family of adapter proteins that regulates life and death. Genes Devel. 12, 2821–2830. [DOI] [PubMed] [Google Scholar]
- 2.Wajant H, Henkler F, Scheurich P (2001) The TRAF family: Scaffold molecules for cytokine receptors, kinases, and their regulators. Cell. Signaling 13, 389–400. [DOI] [PubMed] [Google Scholar]
- 3.Pullen SS, Miller HG, Everdeen DS, Dang TTA, Crute JJ, Kehry MR (1998) CD40-TRAF interactions: Regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochem. 37, 11836–11845. [DOI] [PubMed] [Google Scholar]
- 4.Bishop GA (2013) The many faces of TRAF molecules in immune regulation. J Immunol 191, 3483–3485. [DOI] [PubMed] [Google Scholar]
- 5.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ (2007) TRAF proteins in CD40 signaling. Adv Exp Biol Med 597, 131–151. [DOI] [PubMed] [Google Scholar]
- 6.Bishop GA, Stunz LL, Hostager BS (2018) TRAF3 as a multifaceted regulator of B lymphocyte survival and activation. Front Immunol 9, Article 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H (2013) Higher-order assemblies in a new paradigm of signal transduction. Cell 153, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edilova MI, Abdul-Sater AA, Watts TH (2018) TRAF1 signaling in human health and disease. Front Immunol 9, Article 2969, p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speiser DE, Lee SY, Wong B, Arron J, Santana A, Kong Y, Ohashi PS, Choi Y (1997) A regulatory role for TRAF1 in antigen-induced apoptosis of T cells. J. Exp. Med. 185, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsitsikov E, Laouini D, Dunn IF, Sannikova TY, Davidson L, Alt FW, Geha RS (2001) TRAF1 is a negative regulator of TNF signaling: enhanced TNF signaling in TRAF1-deficient mice. Immunity 15, 647–657. [DOI] [PubMed] [Google Scholar]
- 11.Sabbagh L, Srokowski CC, Pulle G, Snell LM, Sedgmen BJ, Liu Y, Tsitsikov EN, Watts TH (2006) A critical role for TRAF1 and Bim down-regulation in CD8 memory T cell survival. Proc Natl Acad Sci (USA) 103, 18703–18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Wen T, Routy J-P, Bernard NF, Sekaly R-P, Watts TH (2007) 4–1BBL induces TRAF1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol 179, 8252–8263. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GHY, Lang PA, Ambagala T, Pellegrini M, Calzascia T, Aidarus N, Elford AR, Yue FY, Kremmer E, Kovacs CM, Benko E, Tremblay C, Routy J-P, Bernard NF, Ostrowski MA, Ohashi PS, Watts TH (2011) Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J Exp Med 209, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou AC, Batista NV, Watts TH (2019) 4–1BB regulates effector CD8 T cell accumulation in the lung tissue through a TRAF1-, mTOR-, and antigen-dependent mechanism to enhance tissue-resident memory T cell formation during respiratory influenza infection. J Immunol 202, 2482–2492. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Boucher J, Kotani H, Park K, Zhang Y, Shrestha B, Wang X, Guan L, Beatty N, Abate-Daga D, Davila ML (2018) 4–1BB enhancement of CAR T function requires NF-κB and TRAFs. JCI Insight 3, e1211322 (1–18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA (2006) Cooperation between TRAFs 1 and 2 in CD40 signaling. J Immunol 176, 5388–5400. [DOI] [PubMed] [Google Scholar]
- 17.McPherson AJ, Snell LM, Mak TW, Watts TH (2012) Opposing roles for TRAF1 in the alternative versus classical NF-κB pathway in T cells. J Biol Chem 287, 23010–23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbagh L, Andreeva D, Laramee GD, Oussa NAE, Lew D, Bisson N, Soumounou Y, Pawson T, Watts TH (2013) Leukocyte-specific protein 1 links TRAF1 to survival signaling downstream of 4–1BB in T cells. J Leuk Biol 93, 713–721. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y (1997) TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity 7, 703–713. [DOI] [PubMed] [Google Scholar]
- 20.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH (1998) CD28-independent, TRAF2-dependent costimulation of resting T cells by 4–1BB ligand. J Exp Med 187, 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannons JL, Bertram EM, Watts TH (2002) Cutting Edge: Profound defect in T cell responses in TRAF2.DN mice. J Immunol 169, 2828–2831. [DOI] [PubMed] [Google Scholar]
- 22.Prell RA, Evans DE, Thalhofer CJ, Shi T, Funatake C, Weinberg AD (2003) OX40-mediated memory T cell generation is TRAF2 dependent. J Immunol 171, 5997–6005. [DOI] [PubMed] [Google Scholar]
- 23.Yeh W, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pampa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW (1997) Early lethality, functional NF−κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7, 715–725. [DOI] [PubMed] [Google Scholar]
- 24.Lin WJ, Su YW, Lu YC, Hao Z, Chio IIC, Chen NJ, Brustle A, Li WY, Mak TW (2011) Crucial fole for TRAF2 in regulating NFκB2 signaling that contributes to autoimmunity. Proc Natl Acad Sci (USA) 108, 18354–18359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen LT, Duncan GS, Mirtsos C, Ng M, Speiser DE, Shahinain A, Marino MW, Mak TW, Ohashi PS, Yeh W (1999) TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity 11, 379–389. [DOI] [PubMed] [Google Scholar]
- 26.Gardam S, Sierro S, Basten A, Mackay F, Brink R (2008) TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity 28, 391–401. [DOI] [PubMed] [Google Scholar]
- 27.Villanueva JE, Malle EK, Gardam S, Silveira PA, Zammit NW, Walters SN, Brink R, Grey ST (2015) TRAF2 regulates peripheral CD8+ and NKT cell homeostasis by modulating sensitivity to IL-15. Eur J Immunol 45, 1820–1831. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva JE, Walters SN, Saito M, Malle EK, Zammit NW, Watson KA, Brink R, La Gruta NL, Alexander SI, Grey ST (2017) Targeted deletion of Traf2 allows immunosuppression-free islet allograft survival in mice. Diabetol. 60, 679–689. [DOI] [PubMed] [Google Scholar]
- 29.Moore CR and Bishop GA (2005) Differential regulation of CD40-mediated TRAF degradation in B lymphocytes. J Immunol 175, 3780–3789. [DOI] [PubMed] [Google Scholar]
- 30.Devernge O, Hatzivassiliou E, Izumi KM, Kaye KM, Kleijnen MF, Kieff E, Mosialos G (1996) Association of TRAF1, TRAF2, and TRAF3 with an EBV LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16, 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop GA (2016) TRAF3 as a powerful and multi-talented regulator of lymphocyte functions. J Leuk Biol 100, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Cheng G, Baltimore D (1996) Targeted disruption of Traf3 leads to postnatal lethality and defective T-dependent immune responses. Immunity 5, 407–415. [DOI] [PubMed] [Google Scholar]
- 33.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA (2007) TRAF3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity 27, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi Z, Lin WW, Stunz LL, Bishop GA (2014) The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol 15, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi Z, Stunz LL, Bishop GA (2013) TRAF3 plays a key role in the development and function of iNKT cells. J Exp Med 210, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang J-H, Hu H, Jin J, Puebla-Osorio N, Xiao Y, Gilbert BE, Brink R, Ullrich SE, Sun S-C (2014) TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med 211, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie P, Kraus ZJ, Stunz LL, Liu Y, Bishop GA (2011) TRAF3 is required for T cell-mediated immunity and TCR/CD28 signaling. J Immunol 186, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Z, Stunz LL, Lin WW, Bishop GA (2014) TRAF3 regulates homeostasis of CD8+ central memory T cells. PLOS One 9, e102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis AM, Wallace EC, Hostager BS, Yi Z, Houtman JCD, Bishop GA (2017) TRAF3 enhances TCR signaling by regulating the inhibitors Csk and PTPN22. Sci Reports 7, Article 2081, pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arkee T, Wallis AM, Bishop GA (2018) TRAF3-mediated regulation of the TCR complex. J Immunol 200, 112.1. [Google Scholar]
- 41.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, Munechika E, Sakai T, Shirasawa T, Akiba H, Kobata T, Santee SM, Ware CF, Rennert PD, Tanicuchi M, Yagita H, Okumura K (1999) Targeted disruption of Traf5 gene causes defects in CD40 and CD27-mediated lymphocyte activation. Proc. Natl. Acad. Sci. (USA) 96, 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraus ZJ, Haring JS, Bishop GA (2008) TRAF5 is required for optimal T cell expansion and survival in response to infection. J. Immunol. 181, 7800–7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So T, Salek-Ardakani S, Nakano H, Ware C, Croft M (2004) TRAF5 limits the induction of Th2 immune responses. J. Immunol. 172, 4292–4297. [DOI] [PubMed] [Google Scholar]
- 44.Nagashima H, Okuyama Y, Asao A, Kawabe T, Yamaki S, Nakano H, Croft M, Ishii N, So T (2014) The adaptor TRAF5 limits the differentiation of inflammatory CD4+ T cells by antagonizing signaling via the receptor for IL-6. Nat Immunol 15, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin WW, Yi Z, Stunz LL, Maine CJ, Sherman LA, Bishop GA (2015) The adaptor protein TRAF3 inhibits IL-6R signaling in B cells to limit plasma cell development. Sci Signal 8, ra88, pp. 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagashima H, Okuyama Y, Hayashi T, Ishii N, So T (2016) TRAFs 2 and 5 differentially regulate the instructive IL-6R signaling required for Th17 development. J. Immunol. 196, 4082–4089. [DOI] [PubMed] [Google Scholar]
- 47.Esparza EM, Lindsten T, Stockhausen JM, Arch RH (2006) TRAF5 is a critical intermediate of costimulatory signaling pathways triggered by GITR in T cells. J Biol Chem 281, 8559–8564. [DOI] [PubMed] [Google Scholar]
- 48.Snell LM, McPherson AJ, Lin GHY, Sakaguchi S, Pandolfi PP, Riccardi C, Watts TH (2010) CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J Immunol 185, 7223–7234. [DOI] [PubMed] [Google Scholar]
- 49.Walsh MC, Lee J, Choi Y (2015) TRAF6 regulation of development, function, and homeostasis of the immune system. Immunol Rev 266, 72–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV (1996) TRAF6 is a signal transducer for IL-1. Nature 383, 443–446. [DOI] [PubMed] [Google Scholar]
- 51.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, capparelli C, Van G, Kaufman S, van den Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW (1999) TRAF6 deficiency results in osteopetrosis and defective IL-1, CD40, and LPS signaling. Genes Devel. 13, 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, Nishikawa S, Inoue J (2002) TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci (USA) 99, 8766–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Jimi E, Bothwell ALM (2003) RANKL stimulates recruitment of SHP-1 to the complex containing TRAF6 that regulates osteoclastogenesis. J. Immunol. 171, 3620–3626. [DOI] [PubMed] [Google Scholar]
- 54.Bharti AC, Takada Y, Shishodia S, Aggarwal BB (2004) Evidence that RANKL can suppress cell proliferation and induce apoptosis through activation of a NF-kB-independent and TRAF6-dependent mechanism. J. Biol. Chem. 279, 6065–6076. [DOI] [PubMed] [Google Scholar]
- 55.Chiffoleau E, Kobayashi T, Walsh MC, King CG, Walsh PT, Hancock WW, Choi Y, Turka LA (2003) TRAF6 deficiency during hemopoiesis induces Th2-polarized inflammatory disease. J. Immunol. 171, 5751–5759. [DOI] [PubMed] [Google Scholar]
- 56.King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y (2006) TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Immunol 12, 1088–1092. [DOI] [PubMed] [Google Scholar]
- 57.King CG, Buckler JL, Kobayashi T, Hannah JR, Bassett G, Kim T, Pearce EL, Kim GG, Turka LA, Choi Y (2008) Cutting Edge: Requirement for TRAF6 in the induction of T cell anergy. J. Immunol. 180, 34–38. [DOI] [PubMed] [Google Scholar]
- 58.Xie J-J, Liang J-Q, Diao LH, Altman A, Li Y (2013) TRAF6 regulates TCR signaling via interaction with and modification of LAT adapter. J Immunol 190, 4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimo Y, Yanai H, Ohshima D, Qin J, Motegi H, Maruyama Y, Hori S, Inoue J, Akiyama T (2011) TRAF6 directs commitment to regulatory T cells in thymocytes. Genes to Cells 16, 437–447. [DOI] [PubMed] [Google Scholar]
- 60.Muto G, Kotani H, Kondo T, Morita R, Tsuruta S, Kobayashi T, Luche H, Fehling HJ, Walsh M, Choi Y, Yoshimura A (2013) TRAF6 is essential for maintenance of regulatory T cells that suppress Th2 type autoimmunity. PLOS One 8, e74639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ni X, Kou W, Gu JX, Wei P, Wu X, Peng H, Tao J, Yan W, Yang X, Lebid A, Park BV, Chen Z, Tian Y, Fu J, Newman S, Wang X, Shen H, Li B, Blazar BR, Wang X, Barbi J, Pan F, Lu L (2019) TRAF6 directs FOXP3 localization and facilitates regulatory T-cell function through K63-linked ubiquitination. EMBO J 38, e99766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cejas PJ, Walsh MC, Pearce EL, Han D, Harms GM, Artis D, Turka LA, Choi Y (2010) TRAF6 inhibits Th17 differentiation and TGF-b-mediated suppression of IL-2. Blood 115, 4750–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Motegi H, Shimo Y, Akiyama T, Inoue J (2011) TRAF6 negatively regulates the Jak1-Erk pathway in IL-2 signaling. Genes to Cells 16, 179–189. [DOI] [PubMed] [Google Scholar]