Abstract
Purpose:
Corneal collagen cross-linking by ultraviolet light activation of riboflavin has been used clinically to enhance corneal stiffness. We sought to determine if cross-linking differentially affects scleral regions.
Methods:
Adjacent, parallel strips of sclera were cut from superolateral, superomedial, inferolateral, and inferomedial quadrants of posterior and equatorial sclera of 12 human cadaver eyes. One of each pair served as control while the other was cross-linked by immersion in 0.1% riboflavin and 365 nm exposure at 6mW/cm2 irradiance for 30 minutes. Behavior of strips was characterized using a microtensile load cell. Preloaded strips were imaged using orthogonally mounted cameras and optical coherence tomography to determine specimen dimensions including cross-sectional area. Tension was measured during 0.1 mm/s constant rate elongation.
Results:
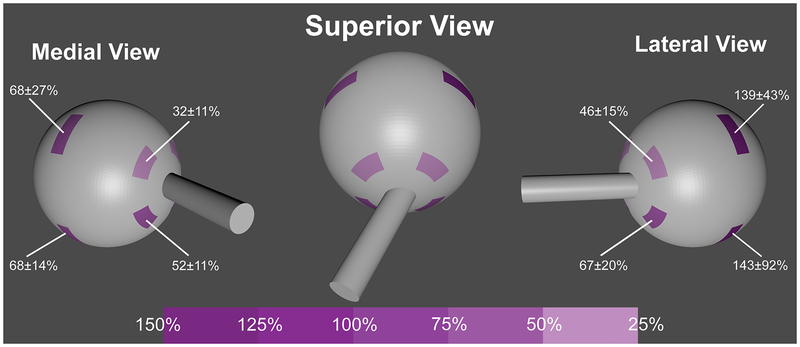
Young’s modulus (YM), the slope of the relationship relating tensile stress to strain, was calculated at 8% strain, and increased significantly after cross-linking (P<0.001). In posterior sclera, mean (± standard error of mean, SEM) YM increased in the superolateral, superomedial, inferolateral, and inferomedial quadrants by 46±15%, 32±11%, 67±20%, and 53±11%, respectively. In equatorial sclera, YM increased by 139± 43%, 68±27%, 143±92%, and 68±14%, respectively. The YM of pooled equatorial quadrants increased significantly more than that of the pooled posterior quadrants.
Conclusions:
Scleral collagen cross-linking by ultraviolet activation of riboflavin differentially increases scleral YM more in the equatorial than posterior sclera, and most in the lateral, equatorial sclera. Cross-linking might be used to arrest progressive myopia or to prevent staphyloma formation.
Keywords: biomechanics, collagen cross-linking, myopia, sclera, Young’s Modulus
Introduction
Myopia affects around 30% of the population in the United States and Europe, but up to 60% in Asian countries,1, 2 with myopic progression occurring in up to 50% of such cases.3 Severe myopia, a leading cause of blindness worldwide, is associated with sight-threatening consequences such as retinal detachment and macular choroidal degeneration.4 Pathological scleral thinning and localized ectasia often occur in high myopia.5 Because biomechanical properties of the sclera influence globe shape and size,6 scleral thinning may permit further globe elongation and progressive refractive error. Pathological scleral thinning involves structural scleral abnormalities such as decreased collagen fiber diameter,7 deficiencies in collagen fibrillogenesis,8 and impairment of collagen cross-linking (CXL).9 Reversing these abnormalities would beneficially change scleral biomechanical properties and might serve as a treatment for myopic progression.
Cross-linking induced by riboflavin and ultraviolet-A light (UVA) can increase corneal rigidity to treat keratoconus10 and has emerged as the favored initial treatment.11–14 Riboflavin serves as a photosensitizer in CXL, forming of intra- and interfibrillar covalent bonds between collagen fibers when activated by UVA.15, 16 The riboflavin also shields underlying tissues, such as the corneal endothelium and iris, from UV irradiation.13 Cross-linking increases corneal Young’s modulus (YM), a measure of stiffness, by about 4.5 fold and typically prevents progression of keratoconus.17,18
Since human sclera contains approximately 50% collagen by weight, primarily type I collagen19 much like cornea, CXL can also be used to increase scleral stiffness. Wollensak conducted CXL using riboflavin and UVA and noted a significant increase in the YM of porcine (145%) and human (31%) sclera in vitro,20 as well as in rabbit sclera (465%) in vivo.21 This study, however, did not consider possible regional variation as a result of CXL. In a study by Wang, CXL of anteriorly to posteriorly oriented scleral strips in the equatorial and posterior regions produced 185% and 201% increases in YM, respectively.22 However, since scleral thickness varies markedly between the equatorial and posterior regions,23 scleral strips in this orientation necessarily have nonuniform thickness, and so cannot reflect likely regional variations in mechanical effects of CXL. In the present study, we investigated the effect of CXL on biomechanical strength and volume of circumferentially cut scleral strips in 8 scleral regions.
Methods
Specimen Preparation.
Institutional review board review is not required for the cadaveric material studied here that was obtained in conformity with applicable local laws. Two eyes were harvested by eye banks within 48 hours of death and were obtained unfrozen but stored at just above 0° C. Ten eyes were obtained from cadavers donated to medical research and had been previously frozen. In all eyes, circumferentially oriented, parallel adjacent scleral strips measuring 2×8mm were trimmed by scalpel from the superolateral, superomedial, inferolateral, and inferomedial quadrants of the equatorial as well as posterior sclera. Equatorial strips were cut between the rectus muscle insertions, while posterior strips were cut 1 mm from edge of the optic nerve. The eye was initially hemisected sagitally, separating medial and lateral portions. Scleral strips were then excised from 4 different regions in each of the halves. (Fig. 1). One strip of each identically-prepared parallel pair was used as a control while the other underwent CXL. The retina, choroid, and episcleral tissue were removed before other procedures.
Fig. 1.
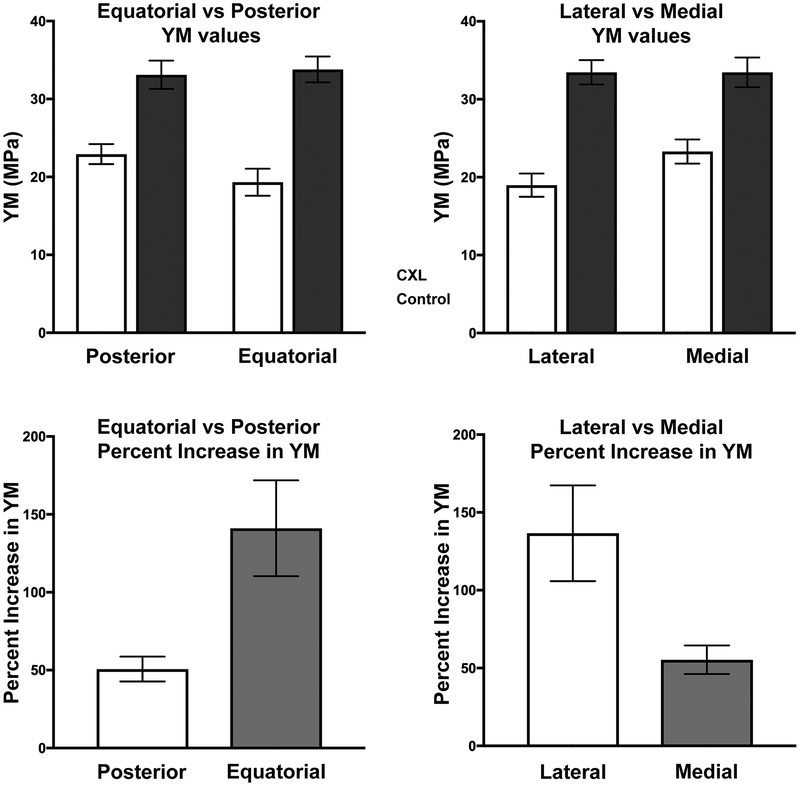
Scleral regions tested and mean percent increase in Young’s modulus (± standard error of mean) following cross-linking.
Cross-linking.
Each treated strip was immersed in 0.1% dextran-free riboflavin for 30 min, and then exposed to 365nm UVA light at 6mW/cm2 irradiance for 30 min using the LightLink CXL Corneal Cross-Link System (LightMed, San Clemente, CA). Riboflavin was applied drop wise onto the specimens every 30 s during CXL. To avoid dehydration, treated and control strips were kept in Ringer’s solution in petri dishes placed on ice until tensile testing.
Tensile Testing.
Tensile testing was performed in a horizontally mounted load cell incorporating a precise train gauge (LSB200, FUTEK, Irvine, CA) with 5mN force resolution attached to a linear motor (Ibex Engineering, Newbury Park, CA) having 20nm distance resolution. Scleral strips were tested in an environmental chamber simulating physiological conditions at 36°C and 100% humidity for 5 minutes. Excess moisture was wiped away. Scleral strips were anchored in serrated clamps separated by 5 mm to set initial specimen length, and preloaded to 0.05 N tension to eliminate slack. Preloaded strips were photographed with two orthogonally mounted digital cameras (Canon 70D and Canon 5D) to determine specimen dimensions, and also imaged using optical coherence tomography (OCT, Thorlabs Inc., Newton NJ) to measure cross-sectional area for stress calculation.
Young’s Modulus Calculation.
Tensile force was divided by mean specimen cross-sectional area in four OCT images obtained at 0.5 mm intervals along the specimen length to calculate stress. The YM, the ratio of tensile force to stress, was analyzed at 8% strain. In each of the 8 scleral regions, 10 control and 10 cross-linked samples were tested. Of the 12 specimens prepared for each region, two were typically damaged by ruptures due to preparation or clamping, so data from only the 10 specimens free of artifacts is included in the reported results. The sample size in the current study is similar to sample sizes in relevant literature20, 21, 24.
Cross-linking Shrinkage Analysis.
Twenty specimens, measuring approximately 3.5×3.5 mm, were excised from the posterior (10 specimens) and equatorial (10 specimens) regions. Excess moisture was removed, and the specimen equilibrated in the environmental chamber for 10 minutes prior to OCT imaging. The specimen was then immersed in 0.1% riboflavin for 30 minutes. Next, each specimen was placed on an inclined platform to prevent riboflavin pooling, and exposed to 365nm UVA at 6mW/cm2 irradiance for 30 minutes to produce CXL, during which specimens were dropwise irrigated with riboflavin every 30 seconds. After CXL, specimens were placed in the environmental chamber for 10 minutes, and were re-imaged by OCT.
Specimen thickness and volume were determined using OCT before and after CXL. The entire length of each specimen was cross-sectionally imaged at 125 μm intervals, requiring approximately 28 image planes in which specimen boundaries were then manually traced to determine cross-sectional areas. Summed areas were multiplied by 125 μm to calculate specimen volume. This current study only investigated CXL shrinkage in the posterior and equatorial sclera because we lacked sufficient scleral tissue to study CXL shrinkage in all 8 scleral regions individually.
Statistical Analysis.
In each region, paired t-tests were conducted for YM to compare control and cross-linked samples. An unpaired t-test was used to determine regional variation in YM. A secondary analysis was conducted to assess the effect of outlier values, with removal of all values more than 2 standard deviations (SD) from the mean. Since outlier removal did not change the overall conclusions, in the interest of rigor we report results of all data acquisitions that were free of obvious experimental artifacts.
Results
Stress-Strain Analysis.
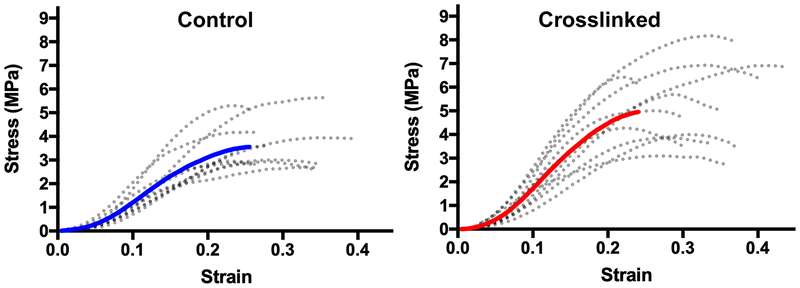
Significant increases in YM were evident after CXL in all 8 scleral regions. Figure 2 shows example stress-strain curves of individual samples (dotted curves) and their overall average (solid curve) in the posterior superolateral region.
Fig. 2.
Individual stress-strain curves of 10 samples from the posterior superolateral sclera (dotted curves), with average curves solid. Note greater stress for cross-linked specimens.
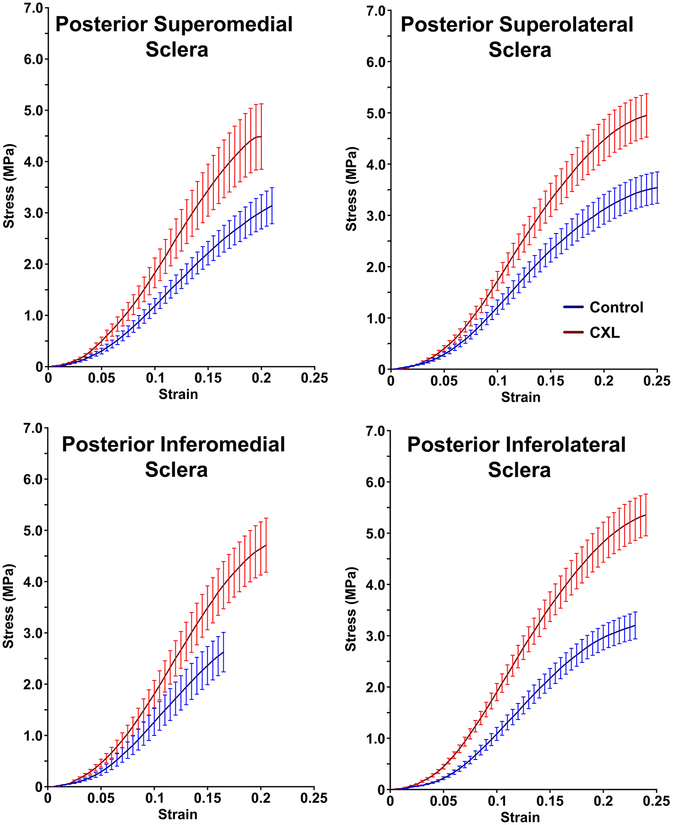
Average stress-strain curves for posterior sclera are shown in Fig. 3. In posterior superolateral sclera, mean YM (± SEM) increased 46±15% from 23.2±2.5 MPa to 32.4±2.9 MPa. In posterior superomedial sclera, YM increased 32±11% from 26.2±3.7 MPa to 34.7±5.8 MPa. In posterior inferolateral sclera, YM increased 67±20% from 22.1±2.0 MPa to 34.6±3.0 MPa, similar to the 53±11% increase in posterior inferomedial sclera from 26±4 MPa to 37±5 MPa. Data are summarized in Table 1 and Fig. 1.
Fig. 3.
Stress-strain curves in posterior superomedial, superolateral, inferomedial and inferolateral sclera for control and cross-linked (CXL) specimens. Error bands ± standard error of mean.
Table 1.
Effect of Cross-linking on Scleral Young’s Modulus
| Regions | Control YM (MPa, SEM) | Cross-linked YM (MPa, SEM) | Percent Increase (SEM) | Significance |
|---|---|---|---|---|
| Posterior Superolateral | 23.2±2.5 | 32.4±2.9 | 46±15% | 0.01 |
| Posterior Superomedial | 26.2±3.7 | 34.7±5.8 | 32±11% | 0.05 |
| Posterior Inferolateral | 22.1±2.0 | 34.6±3.0 | 67±20% | 0.01 |
| Posterior Inferomedial | 25.7±4.5 | 36.6±5.1 | 53±11% | 0.001 |
| Equatorial Superolateral | 17.1±3.8 | 30.8±3.5 | 139±43% | 0.001 |
| Equatorial Superomedial | 23.3±3.0 | 34.7±3.8 | 68±27% | 0.005 |
| Equatorial Inferolateral | 23.3±4.3 | 44.3±4.8 | 143±92% | 0.001 |
| Equatorial Inferomedial | 21.4±3.2 | 32.8±3.0 | 68±14% | 0.001 |
SEM – standard error of mean. Significance by 2-tail t-testing.
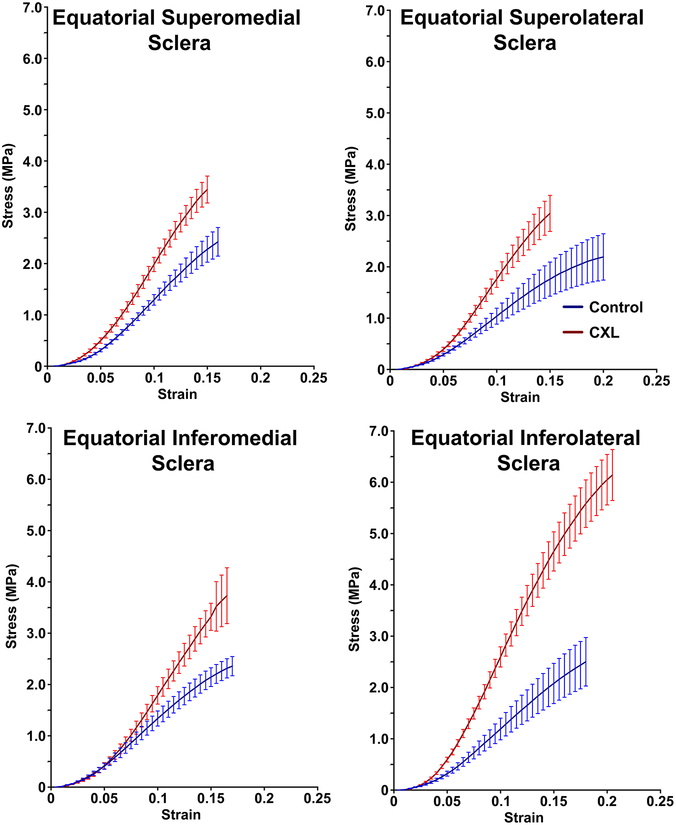
Average stress-strain graphs for equatorial sclera are shown in Fig. 4. The YM of equatorial superolateral and equatorial inferolateral sclera was similarly increased by CXL by 139± 43% and 143±92%, respectively. The YM of equatorial superolateral sclera was increased by CXL from 17.1±3.8 MPa to 30.8±3.5, while that of equatorial inferolateral sclera increased from 23.3±4.3 MPa to 44.3±4.8 MPa. Likewise, CXL increased the YM of superomedial and equatorial inferomedial sclera similarly by 68±27% and 68±14%, respectively. The YM of equatorial superomedial sclera was increased by CXL from 23.3±3.0 MPa to 34.7±3.8 MPa, while that of equatorial inferomedial sclera increased from 21.4±3.2 MPa to 32.8±3.0 MPa. Data are summarized in Table 1 and Fig. 5.
Fig. 4.
Stress-strain curves in equatorial sclera for control specimens and after cross-linking (CXL). Error bands ± standard error of mean.
Fig. 5.
Top. Young’s Modulus (YM, top row) and its percentage increase (bottom row) in control specimens, and after cross-linking (CXL), in posterior, equatorial, lateral, and medial sclera. Error bars – standard error of mean.
Un-paired t-testing showed that the increase in YM produced by CXL was significantly greater in the pooled equatorial than in the pooled posterior regions (P<0.01), and the increase in the pooled lateral regions was significantly greater than in the pooled medial regions (P<0.05, Fig. 5). However, CXL affected YM similarly in the pooled superior (71±14% increase) and inferior regions (118±29% increase) with P=0.14.
Scleral Shrinkage.
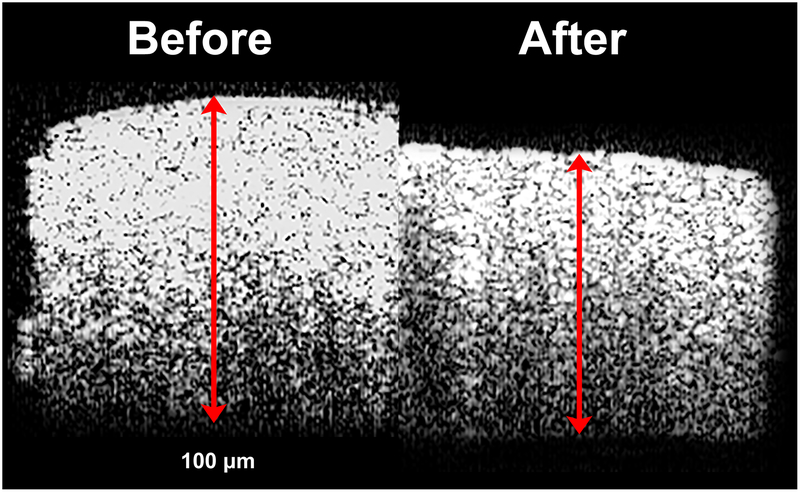
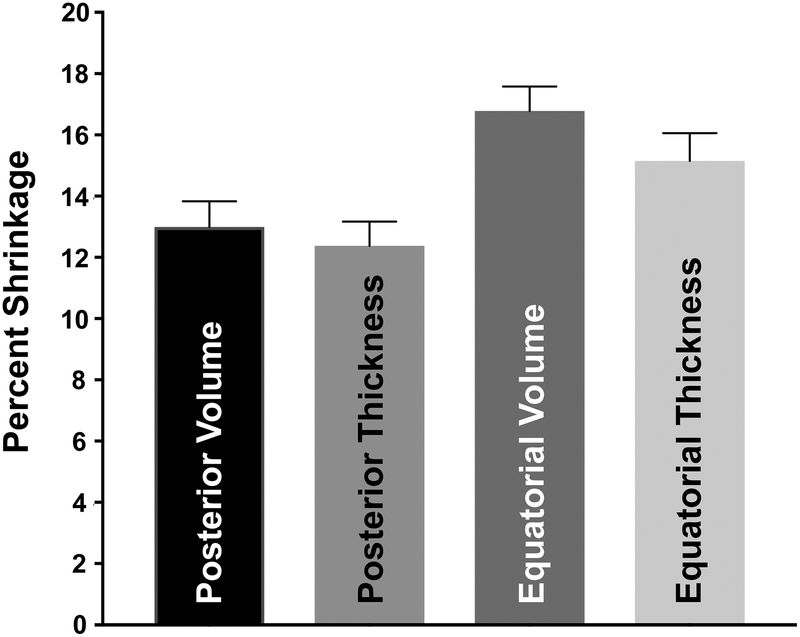
Posterior sclera was on average significantly thicker at 1.00±0.03mm than equatorial sclera at 0.77±0.03mm prior to CXL (P<0.001). Cross-linking generally reduced scleral thickness, as illustrated by the OCT cross section in Fig. 6. Pooling all quadrants, CXL reduced scleral volume 17±1% in the equatorial and 13±1% in posterior sclera (P<0.001).
Fig. 6.
Cross sectional optical coherence tomography of the same scleral specimen before and after cross-linking.
Figure 7 illustrates relative reduction of both thickness and volume in posterior and equatorial sclera. Correlation analysis indicated that 90% of the volume reduction was due to thickness reduction, with little change in length and width.
Fig. 7.
Shrinkage due to cross-linking in posterior and equatorial sclera.
Outliers.
After removal of YM values lying more than ±2SD from the mean of all sample results before and after CXL, average SEM for the remaining values decreased by 16% in posterior and 21% in equatorial sclera. Nevertheless, after outlier removal, the increase in YM due to CXL remained significantly greater in equatorial than posterior sclera (P< 0.01), and greater in lateral than medial sclera (P<0.05). These relative changes are the same as illustrated in Fig. 5 when considering the entire date set, an approach that avoids possible bias and which is therefore the basis for the principal data reported here.
Discussion
Cross-linking using riboflavin and UVA increased YM significantly in all regions of human sclera. The effect was varied topographically, being relatively greater in the equatorial than posterior region (Fig. 5). This difference is in part attributable to regional variation in scleral thickness. Consistent with previous studies,23 we found that the average posterior sclera thickness at 1.00mm to be significantly greater than the equatorial sclera thickness averaging at 0.77mm. Limited to a fixed tissue depth, UVA illumination therefore cross-linked a greater percentage of the total thickness of the equatorial than posterior sclera, and thus produced a greater increase in relative stiffness. In addition, we found that CXL increases YM significantly more in the lateral than in medial sclera. It should be noted that the irradiance and time of UV exposure were chosen to provide a stronger scleral crosslinking effect than the standard Dresden protocol for corneal cross-linking. Since other dosages of UV exposure were not investigated, specific protocols for in vivo scleral CXL would likely require optimization.
Another factor in differential regional effect of CXL may be a ceiling effect suggested by Fig. 5. After CXL, mean pooled YM similarly averaged about 33 MPa in posterior, equatorial, lateral, and medial regions. The differential effect of CXL might also be understood as increasing scleral YM to a maximum value regardless of reginal variation in untreated YM. Thus, the lower the untreated YM, the greater the relative increase in YM due to CXL.
Previous studies used calipers to measure volume changes post CXL21, 22, 24. In this study, we measured volume more accurately by OCT, showing that CXL significantly reduces equatorial and posterior sclera volume (Figs. 6 and 7), almost entirely due to thinning similar to that of corneal CXL25 In vivo, corneal thickness returned to baseline after CXL treatment for keratoconus26. It is unknown if similar reversal of scleral thinning might occur after CXL in vivo.
The current results differ substantially from those of Wang et al.22, the only other study to evaluate regional variations in human sclera tissue due to CXL. The Wang et al. study reported human YM in the quantitatively implausible range of 200–470 MPa,22 roughly an order of magnitude greater than typically observed in sclera. Such unrealistic scleral values are more typical of muscle at approximately 480 MPa,27, 28 or tendon, approximately 560 MPa.28–31 Our study of sclera yielded a mean YM of 23±1 (SEM) MPa, similar to the report of Wollensak, who did not investigate, however, possible regional variations.20 Prior studies of scleral YM20, 22 applied loading to scleral strips that had been cut sagitally from anterior to posterior, a direction in which systematic variation in specimen thickness compromises accurate computation of YM. Consistent with the anatomical literature23, we found equatorial to be significantly thinner than posterior sclera. Assuming that the thinnest cross-section is applicable to the entire specimen would exaggerate apparent YM by exaggerating computed stress, which is calculated as force divided by cross sectional area. Finally, in previous studies where CXL was performed in whole globes prior to specimen excision6, 22, control specimens were obtained from different eyes, introducing intersubject variability. The present approach mitigated interindividual variability by using adjacent scleral strips in the same eye as controls.
Diminished CXL is an important factor in the weakening process of myopic sclera9 as in myopic human eyes that have significantly lower scleral YM.32 There is a natural increase in CXL with advancing age9 and in diabetics.33 In older people, the natural increase in CXL is believed to slow myopic progression.34 Similarly, the increase glycation-induced CXL in diabetic patients has been proposed to explain their reduced axial myopia.33 It is therefore hypothesized that if natural CXL is associated with retardation of myopic progression, then artificially inducing CXL using UVA/riboflavin might be used therapeutically to retard axial elongation and reduce the risk of blindness.35 Other treatment options exist, such as scleral reinforcement surgery,36 in which a strip of cadaveric sclera is wrapped around the globe to prevent further elongation.37, 38 In one study, scleral reinforcement was usually successful in arresting the progression of myopia.39 However, such procedure is both highly invasive and relies on the availability of healthy scleral donor tissue, which is currently becoming scarcer with the increasing prevalence of myopia.
Complications of high myopia, such staphylomata, may be sight-threatening. Staphylomata form as localized scleral ectasia having a radius of curvature less than the surrounding scleral curvature.40 Localized posterior scleral ectasia is an important component of several vision-threatening myopic maculopathies.41–43 It is theorized that the prevention of posterior staphyloma could prevent further visual impairment related to maculopathies.41 Because the formation of staphylomata involves localized scleral weakening, CXL might be a prophylactic measure to reinforce scleral tissue. The topographically differential effect of CXL might be useful as a treatment for staphylomata formation in various regions of the globe.
Grant Support:
USPHS EY008313 and an Unrestricted Grant from Research to Prevent Blindness to the Department of Ophthalmology.
Footnotes
Disclosure Statement:
None of the authors has a financial interest in any material related to this paper.
References
- 1.Saw SM, Shih-Yen EC, Koh A, Tan D. Interventions to retard myopia progression in children: an evidence-based update. Ophthalmology. 2002;109:415–21. [DOI] [PubMed] [Google Scholar]
- 2.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res 2003;22:307–38. [DOI] [PubMed] [Google Scholar]
- 3.Bullimore MA, Jones LA, Moeschberger ML, Zadnik K, Payor RE. A retrospective study of myopia progression in adult contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43:2110–3. [PubMed] [Google Scholar]
- 4.Young TL. Molecular genetics of human myopia: an update. Optom Vis Sci. 2009;86:E8–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Zou Y, Zhang F, Zhang X, Wang M. Efficacy of blue-light cross-linking on human scleral Reinforcement. Optom Vis Sci. 2015;92:873–8. [DOI] [PubMed] [Google Scholar]
- 7.Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol. 1979;97:912–5. [DOI] [PubMed] [Google Scholar]
- 8.Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228:174–9. [DOI] [PubMed] [Google Scholar]
- 9.McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59:475–86. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. [DOI] [PubMed] [Google Scholar]
- 11.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–45. [DOI] [PubMed] [Google Scholar]
- 12.Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390–7. [DOI] [PubMed] [Google Scholar]
- 13.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. [DOI] [PubMed] [Google Scholar]
- 15.Ziaei M, Barsam A, Shamie N, Vroman D, Kim T, Donnenfeld ED, Holland EJ, Kanellopoulos J, Mah FS, Randleman JB, et al. Reshaping procedures for the surgical management of corneal ectasia. J Cataract Refract Surg. 2015;41:842–72. [DOI] [PubMed] [Google Scholar]
- 16.McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, Dedeic Z, Dionne MJ, Clement EM, Conrad GW. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest Ophthalmol Vis Sci. 2010;51:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. [DOI] [PubMed] [Google Scholar]
- 18.Henriquez MA, Villegas S, Rincon M, Maldonado C, Izquierdo L Jr., Long-term efficacy and safety after corneal collagen crosslinking in pediatric patients: Three-year follow-up. Eur J Ophthalmol. 2018;28:415–8. [DOI] [PubMed] [Google Scholar]
- 19.Keeley FW, Morin JD, Vesely S. Characterization of collagen from normal human sclera. Exp Eye Res. 1984;39:533–42. [DOI] [PubMed] [Google Scholar]
- 20.Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30:689–95. [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Iomdina E, Dittert DD, Salamatina O, Stoltenburg G. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand. 2005;83:477–82. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Zhang F, Qian X, Zhao X. Regional Biomechanical properties of human sclera after cross-linking by riboflavin/ultraviolet A. J Refract Surg. 2012;28:723–8. [DOI] [PubMed] [Google Scholar]
- 23.Vurgese S, Panda-Jonas S, Jonas JB. Scleral thickness in human eyes. PLoS One. 2012;7:e29692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit sclera after collagen crosslinking using riboflavin and ultraviolet A (UVA). Acta Ophthalmol. 2009;87:193–8. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblat E, Hersh PS. Intraoperative corneal thickness change and clinical outcomes after corneal collagen crosslinking: Standard crosslinking versus hypotonic riboflavin. J Cataract Refract Surg. 2016;42:596–605. [DOI] [PubMed] [Google Scholar]
- 26.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:691–700. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol (1985). 2001;90:164–71. [DOI] [PubMed] [Google Scholar]
- 28.McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng Part B Rev. 2011;17:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shadwick RE. Elastic energy storage in tendons: Mechanical differences related to function and age. J Appl Physiol (1985). 1990;68:1033–40. [DOI] [PubMed] [Google Scholar]
- 30.Azizi E, Halenda GM, Roberts TJ. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr Comp Biol. 2009;49:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wren TA, Yerby SA, Beaupre GS, Carter DR. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon). 2001;16:245–51. [DOI] [PubMed] [Google Scholar]
- 32.Awetissow ES. [The role of the sclera in the pathogenesis of progressive myopia (author’s transl)]. Klin Monbl Augenheilkd. 1980;176:777–81. [DOI] [PubMed] [Google Scholar]
- 33.Logstrup N, Sjolie AK, Kyvik KO, Green A. Long-term influence of insulin dependent diabetes mellitus on refraction and its components: a population based twin study. Br J Ophthalmol. 1997;81:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Research Council (U.S.). Working Group on Myopia Prevalence and Progression Myopia: Prevalence and progression. Washington, D.C.: National Academy Press, 1989; xii, 113 p. [Google Scholar]
- 35.Elsheikh A, Phillips JR. Is scleral cross-linking a feasible treatment for myopia control? Ophthalmic Physiol Opt. 2013;33:385–9. [DOI] [PubMed] [Google Scholar]
- 36.Autrata R, Rehurek J. [Scleroplasty surgery in the treatment of progressive myopia in children]. Cesk Slov Oftalmol. 1998;54:323–7. [PubMed] [Google Scholar]
- 37.Thompson FB. A simplified scleral reinforcement technique. Am J Ophthalmol. 1978;86:782–90. [DOI] [PubMed] [Google Scholar]
- 38.Snyder AA, Thompson FB. A simplified technique for surgical treatment of degenerative myopia. Am J Ophthalmol. 1972;74:273–7. [DOI] [PubMed] [Google Scholar]
- 39.Balashova NV, Ghaffariyeh A, Honarpisheh N. Scleroplasty in progressive myopia. Eye (Lond). 2010;24:1303. [DOI] [PubMed] [Google Scholar]
- 40.Spaide RF, Ohno-Matsui K, Yannuzzi LA. Pathologic myopia. New York: Springer, 2014; xvii, 376 pages. [Google Scholar]
- 41.Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–611, 611 e1–4. [DOI] [PubMed] [Google Scholar]
- 42.Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, Klaver CC, Moriyama M, Shinohara K, Kawasaki Y, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–83 e7. [DOI] [PubMed] [Google Scholar]
- 43.Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–87. [DOI] [PubMed] [Google Scholar]