Abstract
Kidney transplant recipients (KTRs) and liver transplant recipients (LTRs) have significant post-transplant weight gain and low physical activity. We conducted a home-based, remotely-monitored intervention using wearable accelerometer devices to promote post-transplant physical activity. We randomized 61 KTRs and 66 LTRs within 24 months of transplant to: 1) control, 2) accelerometer, or 3) intervention: accelerometer paired with financial incentives and health engagement questions to increase steps by 15% from baseline every 2 weeks. The primary outcome was weight change. A co-primary outcome for the two accelerometer arms was steps. Participants were recruited at a median of 9.5 [3-17] months post-transplant. At 3 months, there were no significant differences in weight change across the 3 arms. The intervention arm was more likely to achieve ≥7000 steps compared to control with device (OR 1.99, 95% CI:1.03-3.87); effect remained significant after adjusting for demographics, allograft, time from transplant, and baseline weight. Adherence to target step goals was 74% in the intervention arm, 84% of health engagement questions were answered correctly. A pilot study with financial incentives and health engagement questions was feasible and led KTRs and LTRs to walk more, but did not affect weight. A definitive trial is warranted. (ClinicalTrials.gov number: NCT03221465).
Keywords: Physical activity, behavior change, remote monitoring, self-care, behavioral economics, exercise
INTRODUCTION
Post-transplant weight gain is highly prevalent and associated with adverse health outcomes among kidney transplant recipients (KTRs) and liver transplant recipients (LTRs) including a greater risk of graft loss, cardiovascular disease and new-onset diabetes after transplantation. The reasons for substantial weight gain stem from reduced physical activity after the development of end-stage organ disease that may further be impeded by the stresses of post-operative recovery. Additional contributors are increased post-transplant appetite as well as the obesity-promoting effects of calcineurin inhibitors and corticosteroids.(1-3) At one year post-transplant, KTRs and LTRs gain from 4-10 kg on average. Forty percent of LTRs with a normal body weight at transplantation become obese at one year.(4, 5) Among KTRs, weight gain doubles the risk of graft loss and is associated with reduced long-term survival.(5, 6) In LTRs, metabolic syndrome is twice as common as in the general population and is associated with cardiovascular events and new onset diabetes after transplantation.(7-10)
Despite the potential for positive behavior changes after the life-altering process of transplantation and close medical follow-up, weight gain and low physical activity have been the status quo. Intensive exercise interventions focused on aerobic and strength training have been studied, and, not surprisingly, improve muscle strength, exercise capacity, and health-related quality of life (11-17). However, despite the known benefits of physical activity after transplantation and guidelines recommending post-transplant exercise (18), durable behavior changes are difficult to maintain and intensive programs may be considered cost-prohibitive and not typically covered by healthcare plans.
From a behavioral economics standpoint, post-transplant weight gain and inactivity reflect a self-control burden on the patient who has to be adherent to medication as well as to diet, exercise and weight management. (19) Two concepts that informed the design of this study are 1) hyperbolic discounting, whereby patients place a disproportionately low value on future health outcomes at the expense of the immediately more pleasing alternatives (e.g. overeating and sedentary behavior), and 2) cognitive load, the perceived inconvenience of thinking of and remembering to follow all prescribed clinical recommendations. The problem of hyperbolic discounting with sedentary behavior can be addressed by making healthy choices more beneficial in the present via financial incentives, which also serve to focus the patient on a health behavior like walking. The problem of cognitive load can be addressed by “retrieval practice”, a structured process of training to recall and repeat health information with questions where correct answers are financially rewarded. This process induces a “testing effect” and leads to lasting retention of information. (20)
Despite the many challenges faced by transplant recipients, the post-transplant period whereby organ dysfunction is restored may be particularly salient in motivating individual behavior change. As substantial weight gain is expected in the post-transplant period, an intervention that succeeds in maintaining stable weight or preventing greater adiposity would be an improvement over typical outcomes. The objective of this randomized, controlled pilot study was to test the effectiveness of a home-based, low-impact exercise program using wearable devices, health engagement questions and financial incentives on post-transplant weight gain and walking among KTRs and LTRs. We hypothesized that a home-based physical activity program based on frequent feedback and financial incentives would mitigate post-transplant weight gain and increase walking.
PATIENTS AND METHODS
STUDY DESIGN
This was a block-randomized, controlled trial conducted for 18 weeks and consisted of a 2-week run-in period, a 12-week active intervention, and a 4-week follow-up. Patients were recruited at the Hospital of the University of Pennsylvania between March 2017 and January 2018. After confirming eligibility and obtaining informed consent, participants were randomized to one of three study arms. The three study arms were: Arm 1 – control, no device, Arm 2 – control with device only, and Arm 3 – intervention that included a device and an incentivized physical activity and health engagement program. This study was approved by the University of Pennsylvania Review Board (protocol # 825784; NCT03221465). The trial was initially planned to be conducted at 2 sites, however, due to rapid accrual, was conducted at a single site.
SETTING AND PARTICIPANTS
KTRs and LTRs were contacted by telephone by clinical research coordinators (CRCs) 1-2 weeks prior to their transplant clinic appointments to assess potential eligibility. Enrollment occurred in-person by the CRCs at transplant clinic appointments. The participants were followed remotely during the study through text, telephone calls, and email. At the end of the 12-week intervention period, participants were contacted and scheduled to complete an exit encounter.
Adults age 18or older who received KT, kidney/pancreas, LT or simultaneous liver kidney transplant (SLKT) from 2 - 24 months prior to screening were eligible for enrollment. The participants were included if they were English-speaking, able to provide informed consent, owned a smartphone compatible with the wearable accelerometer (iOS or Android), and were willing to walk and sync the wearable accelerometer daily as well as provide an end-of-study weight. Participants were excluded if they already used a wearable accelerometer, had a severe vision, hearing, or mobility impairment precluding participation, or if they were enrolled in another financial incentive-based exercise program.
ENROLLMENT AND RANDOMIZATION
The study employed the University of Pennsylvania’s Way to Health online platform (Supplementary Appendix 1) to facilitate enrollment, randomization, and subsequent tracking of step counts and bi-directional texting.(21, 22) Participants were told the investigators were studying the effects of a home-based walking program on their post-transplant health. Participants were randomized into one of three arms after consenting and completing the eligibility questionnaire. Block randomization was used with a block size of six, further stratified by organ (KT versus LT); patients who received SLKT were classified as LT as liver disease was the primary indication for transplant.
INTERVENTION
Participants in Arm 1 received standard instructions regarding healthy diet and physical activity that are provided after transplant and did not receive access to the online portal or health additional engagement questionsUpon enrollment, participants in Arms 2 and 3 received the same standard instructions as in Arm 1 and were also enrolled in a 2-week run-in period to get them accustomed to syncing the devices daily and to calculate baseline step counts. Participants in Arm 2 and 3 (those with wearable trackers) had access to an online portal with health information including answers to health engagement questions as well as links with educational online resources regarding healthy diet and physical activity. In addition, Arm 3 received step goals and health engagement questions sent via text messages with financial incentives. We included a device-only Arm 2 to be able to distinguish between the device effect and the additional incentive effects in Arm 3.
Those randomized to Arm 3 (intervention) were enrolled in a physical activity program that consisted of individualized bi-weekly walking goals with the baseline determined using their mean steps during the 2-week run-in period. The decision to individualize step goals was based on lack of literature regarding typical physical activity levels in transplantation. Using participants’ mean steps during the run-in as baseline, step goals were subsequently increased 15% every 2-weeks and were capped at a maximum goal 7,000 steps, which was chosen based on recommendations form the American College of Sports Medicine and exceeds the mean daily steps of about 5000 steps in the US population (23, 24).
Walking activity was promoted with financial incentives and rooted in the framework of behavioral economics, which recognizes that individuals often make inconsistent decisions over time about their health. Recent studies in non-transplant settings have effectively used financial incentives to make health benefits more salient and instant (21, 22, 25, 26). Financial incentives were “loss framed” since individuals tend to fear loss of rewards more than they value expected payouts of the same magnitude in the gain domain (27, 28). For this study, at the beginning of each 4-week study period, participants in Arm 3 were credited $54 to a virtual account. For each day that a participant failed to meet their step goal, he or she was informed that $3 was deducted from the virtual account balance.
Arm 3 participants were also financially incentivized to correctly answer two true/false transplant-specific health engagement questions each week during the intervention period (Supplementary Appendix 2). All participants in Arm 3 received paper and online copies of the questions and correct answers upon enrollment, since the objective was to test the retrieval practice effect rather than the effect of informing patients about specific recommendations. The questions were designed to give participants practice to more easily remember health information for when they make health-related decisions throughout the day. They included basic questions about exercise, healthful diet, and transplant food safety after transplantation (29, 30) Each participant was sent a true/false health engagement question twice a week; $3 was deducted from the virtual account if the questions were not answered or answered incorrectly and they received prompt feedback about the accuracy of their answers and any possible changes in their balance. Balances were disbursed on a monthly basis.
After 12 weeks of intervention, Arm 2 and 3 participants were instructed to continue to use their devices, which they kept after the active intervention was over with no further feedback or text messaging.
OUTCOMES
The primary study outcome was change in patient weight from enrollment to the end of the 4-month study period. End-of-study weight was obtained within a 5-week period of the completion of the active intervention (1 week before or 4 weeks after) and was obtained in-person by a research coordinator whenever possible (44/117, 38%) or by transplant clinic staff during a routine appointment (43/117, 37%).
In 30 (26%) cases where exit encounters were conducted over the telephone for patient convenience, weight was obtained by documentation from an outside physician’s office (14/117, 12%) or by having the participant text the study staff a photograph of their weight recorded while stepping on a scale (16/117, 14%). A secondary outcome was daily steps. Consistent with other studies, we analyzed the mean proportion of participant days that the target of 7000 steps was achieved, a previously studied goal in walking studies. (27) We also compared daily steps as a continuous outcome.
This was a single-blind study, where the participants and research staff could not be blinded. The investigators were blinded to study arm assignment and outcome measurement until all participants exited the study. After study completion, intervention fidelity in Arm 3 was assessed by measuring the percent adherence to step targets, the percent of health engagement questions answered via text message, and the percent of health engagement questions answered correctly.
STATISTICAL ANALYSES
All participants that were initially randomized were compared on baseline characteristics using one-way analysis of variance for continuous and chi-squared tests for categorical variables. Step data were analyzed for 76 participants in Arms 1 and 3 using an intention-to-treat approach. We fit a logistic regression model for the physical activity outcome of proportion of days with ≥7000 steps. We fit a linear regression model for the outcome of weight change at 3 months from baseline in kilograms; models were not fit for steps as a continuous outcome as the difference in average step counts was not statistically significant in unadjusted analysis. For both outcomes, secondary analyses were performed adjusting for baseline weight, age, sex, race/ethnicity, time from transplantation and allograft type (kidney versus liver). We used robust standard errors for all models. For the physical activity outcome, additional sensitivity analyses were completed in which we: 1) excluded all days with less than 1000 steps; evidence from other studies suggests that this number of steps does not adequately reflect daily physical activity and may have resulted from device malfunction or misuse; 2) used multiple imputation to account for missing step counts assumed to be missing at random. The regression-based multiple imputation model (mi impute command in Stata) included age, gender, race, enrollment BMI, allograft type, time from transplant, participant, and study arm and included 20 imputations, which is considered more than sufficient to account for the 2% observed missing step data. Analyses were performed with Stata 15.0 (StataCorp, College Station, TX).
Sample size was constrained by the fact that this was a pilot study. Assuming 20% attrition and a sample size of 33 participants per arm (including Arms 2 and 3 with devices), the study had >90% power to detect a 6% difference in the proportion of days with ≥7000 steps and had 90% power to detect a difference of 2000 steps between the control and intervention arms with a type 1 error of 0.05.
RESULTS
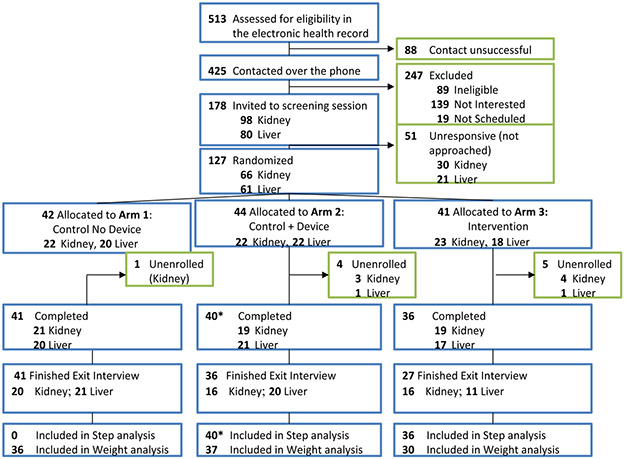
The study enrollment details are shown in Figure 1. A total of 513 participants who met initial criteria of being within 2-24 months from KT or LT were reviewed in the electronic health record and 425 were contacted by telephone. Among the 178 potentially eligible and interested patients, a total of 127 were randomized (n=41 to Arm 1: control, no device, n=44 to Arm 2: control with device, n=41 to Arm 3: intervention). The study retention rate was 117/127 participants (92.1%). Among the 117 retained in the study, a total of 103 (88.0%) provided end-of-study weight. Steps were analyzed among 76 participants in Arms 2 and 3; one participant in Arm 2 died 10 days prior to study completion, which was unrelated to the study. No other study-related adverse events occurred.
Figure 1.
Study flow diagram
* 1 patient died 10 days prior to study completion, steps were included in analysis. Patients in the Control No Device arm did not have measured steps. The Control + Device arm included an accelerometer to measure daily steps. The Intervention arm included an accelerometer, daily step goal targets with loss-framed financial incentives, and biweekly text messages with health engagement questions.
Table 1 shows baseline characteristics by study arm. The mean age was 52 (SD 13) years, 64% were male, 64% were white and 27% were black. The median baseline body mass index (BMI) at enrollment was 28 kg/m2 (IQR: 24,32). We did not observe clinically meaningful differences in participants at baseline across arms, except that participants in the intervention arm 3 were further from transplantation (median 13 months compared to 8.4 months in the control, no device arm and 6.5 months in the control with device arm). Participants in the intervention arm had a higher prevalence of new onset diabetes after transplant and higher estimated glomerular filtration rate (eGFR); these baseline differences were not statistically significant.
Table 1.
Characteristics of Study Participants Initially Randomized
| Participant Characteristics at the time of study enrollment |
Total n=127 |
Control No device n=42 |
Control With Device n=44 |
Intervention n=41 |
P value |
|---|---|---|---|---|---|
| Age, mean ± SD | 52 ± 13 | 50 ± 15 | 53 ± 12 | 54 ± 13 | 0.42 |
| Male, n (%) | 81 (64) | 27 (64) | 30 (68) | 24 (58) | 0.65 |
| Race, n (%) | 0.97 | ||||
| White | 81 (64) | 28 (67) | 28 (63) | 25 (61) | |
| Black | 34 (27) | 10 (24) | 11 (25) | 13 (32) | |
| Hispanic/Asian/Other/Unknown | 12 (9) | 4 (10) | 5 (11) | 3 (7) | |
| Months from transplant, median (IQR) | 9.5 (3-17) | 8.4 (3.7-16) | 6.5 (3-13) | 13 (4-19) | 0.09 |
| Organ, n (%) | 0.73 | ||||
| Kidney | 65 (51) | 20 (48) | 22 (50) | 23 (56) | |
| Liver | 62 (49) | 22 (52) | 22 (50) | 18 (44) | |
| Pre-transplant diabetes, n (%) | 35 (28) | 14 (33) | 10 (23) | 11 (27) | 0.54 |
| NODAT, n (%) | 28 (22) | 7 (17) | 7 (16) | 14 (34) | 0.08 |
| eGFR , median (IQR) | 64 (47-80) | 57 (45-72) | 65 (46-79) | 68 (59-82) | 0.08 |
| Weight (kg), median (IQR) | 84 (70-97) | 84 (74-92) | 82 (67-94) | 83 (63-100) | 0.72 |
| Baseline BMI (kg/ m2), median (IQR) | 28 (24,32) | 28 (25,31) | 26 (23,33) | 29 (25,32) | 0.58 |
| Baseline systolic blood pressure (mm Hg), median (IQR) | 132 (119-143) | 125 (116-139) | 135 (121-143) | 132 (121-147) | 0.20 |
| Baseline diastolic blood pressure (mm Hg), median (IQR) | 75 (68-81) | 72 (66-78) | 76 (74-86) | 77 (70-84) | <.01 |
SD=standard deviation, IQR=interquartile range, BMI=body mass index, NODAT=new onset diabetes after transplant
Table 2 provides the unadjusted weight and step data for 117 study participants with complete weight data after 18 weeks, which included the 2-week run-in period, 12 weeks of active intervention, and 4 weeks of passive observation. The median overall weight gain was 0.91 kg (IQR: −0.91, 3.9). The median unadjusted weight gain was 0.91 kg (IQR:−1.0, 5.4) in the control, no device arm and 2.4 kg (IQR:−.45, 5.4) in the control with device arm. By contrast, the intervention arm had a median weight loss of −.45 kg compared to control [(IQR:−0.14, 3.4); p=0.05 for comparison across all arms].
Table 2.
Unadjusted weight and step data for study participants
| Variable | Total n=117 |
Control No Device n=41 |
Control With Device n=40 |
Intervention n=36 |
P value |
|---|---|---|---|---|---|
| Baseline weight (kg), mean (SD) | 84.5 (20.7) | 84.8 (21.7) | 82.5 (20.7) | 86.3 (19.5) | 0.54 |
| End of study weight (kg), mean (SD)a | 86.2 (21.1) | 86.0 (22.1) | 85.5 (20.6) | 87.1 (21.0) | 0.84 |
| Change in weight (kg), mean (SD)b | 1.5 (4.5) | 1.0 (3.9) | 2.7 (5.3) | 0.81 (4.0) | 0.07 |
| Change in weight (kg), Median [IQR]b |
0.91 [−0.91 to 3.9] |
0.91 [−1.0 to 5.4] |
2.4 [−0.45 to 5.4] |
−0.45 [−1.4 to 3.4] |
0.05 |
| Variable | Total n=76 |
Control No Device --- |
Control With Device n=40 |
Intervention n=36 |
P value |
| Proportion of participant-days with ≥ 7000 steps | 0.53 | --- | 0.45 | 0.62 | <0.001 |
| Proportion of days with ≥ 7000 steps at participant level mean (SD) Median [IQR] |
0.51 (0.35) 0.51 [0.14-0.85] |
--- | 0.43 (0.34) 0.36 (0.13-0.78) |
0.60 (0.34) 0.72 [0.28-0.87] |
<0.001 |
| Daily steps throughout study period, mean (SD) Median [IQR] |
7346 (3147) 6751 [4794-9920] |
--- | 7045 (3296) 6551 [4344-6551] |
7691 (2978) 8150 [5393-10000] |
0.30 |
| End of study steps, mean (SD) Median [IQR]c |
8439 (3736) 8455 [5551-10017] |
--- | 7337 (3494) 7121 [4853-10012] |
8532 (3907) 8754 [6560-12120] |
0.19 |
| Daily steps among those with | --- |
Abbreviations: IQR=interquartile range, Kg=kilogram, SD=standard deviation
N=116 with baseline weight data
N=103 with end of study weight data
Steps reported for the last 2-week period of the intervention
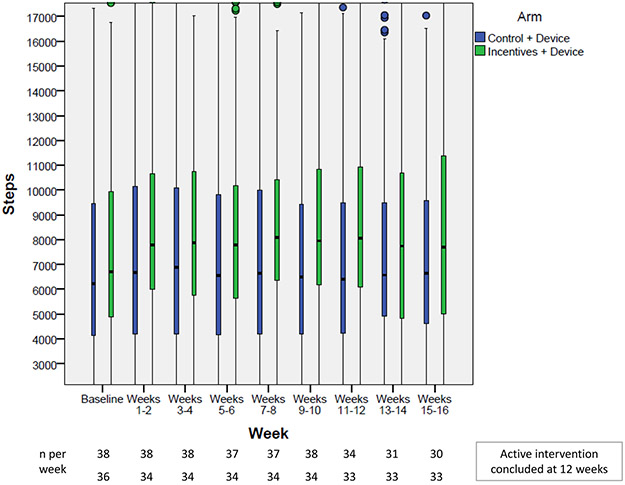
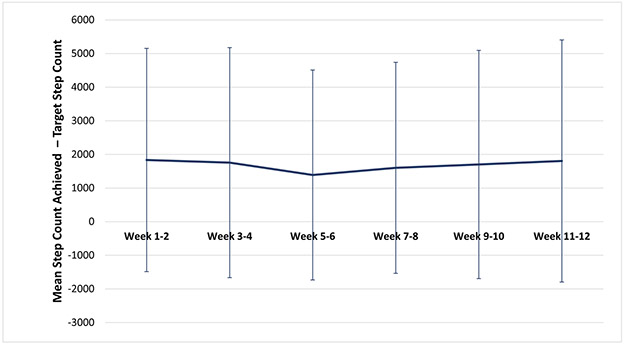
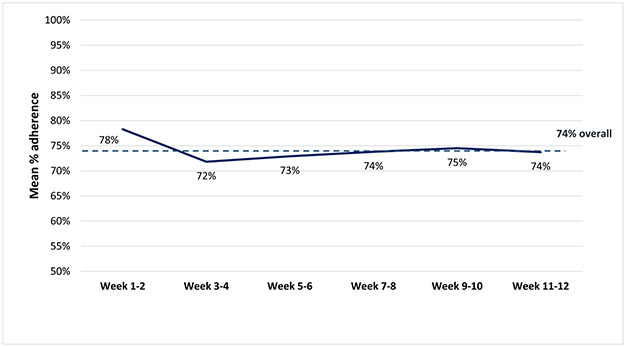
Among the 76 participants with step data, in univariable analysis, the overall proportion of participant-days achieving ≥7000 steps was 0.53; this was 0.17 higher in the intervention group compared to control (0.45 control with device group versus 0.62 in the intervention group [p<0.001]). On average throughout the entire study period, participants in the intervention group walked 646 steps per day more than in the control group. The mean of the last 2-week study period was 1195 steps higher in the intervention compared to the control group (p=0.19) (Figure 2); mean absolute differences between step counts achieved and step count targets are shown in Figure 3. With regards to intervention fidelity, the mean adherence to step targets in the intervention group was 74% (Figure 4). Eighty-four percent of health engagement questions were answered, and among those, 95% were answered correctly (Supplementary Appendix 2).
Figure 2.
Unadjusted distribution of step counts displayed by study arm for each 2-week study interval (n=40 control+device, n=36 intervention+device)
Figure 3.
Mean absolute differences between step counts achieved and step count targets (n=6) by 2-week period in the intervention (n=36)
Figure 4.
Mean percent adherence to step targets for each 2-week study interval in the incentives + device arm
The dashed line represents the mean percent adherence to step targets throughout the study period. The solid line represents the mean adherence to step targets within each 2-week interval.
In the primary model for the physical activity outcome (Table 3, Model 1), intervention arm 3 was associated with nearly twice the odds of achieving ≥7000 steps compared to the control with device arm (OR 1.99, 95% CI: 1.03 - 3.87). Results were similar in multiple secondary analyses excluding days with less than 1000 steps, multiple imputation of missing steps, and after adjustment for baseline characteristics (Table 3, Models 2-4). Among patient characteristics, compared to KT recipients, LT recipient status was associated with lower likelihood of achieving ≥7000 steps (OR 0.32, 95% CI :0.16-0.63).
Table 3.
Unadjusted and adjusted results for the outcome of proportion of participant-days that ≥7000 were reached among 76 participants and 5,857 participant-days with step data.
| Model 1 (primary model) |
Model 2 (days with <1000 steps excludeda) |
Model 3 (with imputed step countsb) |
Model 4 (Model 1 + baseline characteristics) |
|||||
|---|---|---|---|---|---|---|---|---|
| Participant-days | n=5,857 | n=5,549 | n=6,374 | n=5,857 | ||||
| Variable | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value |
| Intervention versus control with device | 1.99 (1.03-3.87) | 0.04 | 2.29 (1.56-4.52) | 0.02 | 1.97 (1.78-2.18) | <0.001 | 2.23 (1.06-4.71) | 0.04 |
| Age (years) | --- | --- | --- | --- | --- | --- | 1.00 (0.98-1.03) | 0.83 |
| Race | --- | --- | --- | --- | --- | --- | 0.57 | |
| White | --- | --- | --- | --- | --- | --- | Reference | |
| Black | --- | --- | --- | --- | --- | --- | 0.60 (0.29-1.22) | |
| Hispanic | --- | --- | --- | --- | --- | --- | 0.88 (0.31-2.41) | |
| Other | --- | --- | --- | --- | --- | --- | 1.04 (0.18-5.90) | |
| Months from transplant | --- | --- | --- | --- | --- | --- | 0.98 (0.93-1.03) | 0.46 |
| Allograft | ||||||||
| Kidney | --- | --- | --- | --- | --- | --- | Reference | |
| Liver/SLK | --- | --- | --- | --- | --- | --- | 0.32 (0.16-0.63) | 0.001 |
| Baseline weight (kg) | --- | --- | --- | --- | --- | --- | 1.00 (0.98-1.01) | 0.89 |
A total of 308 participant-days achieved less than 1000 steps representing 5.3% of total participant-days. Abbreviations: kg=kilogram, OR=odds ratio, CI=confidence interval, SLK=simultaneous liver/kidney.
For the outcome of change in weight from baseline (Table 4), no differences were noted by study arm. Older age and more time since transplant were associated with minimal, but statically significant weight loss from baseline with a 0.06 kg weight loss for every year increase in age (95% CI: 0.06 (−0.107 - 0.00) and a 0.24 kg weight loss with each additional month from transplant (95% CI: (−0.36 - −0.12).
Table 4.
Unadjusted and adjusted results for the outcome of change in weight (kg) among 117 participants with complete weight data
| Model 1 | Model 2 (Model 1 + baseline characteristics) |
|||
|---|---|---|---|---|
| Variable | β (95% CI) | P value | β (95% CI) | P value |
| Study arm | 0.18 | 0.35 | ||
| Usual care control | Reference | Reference | ||
| Control with device | 1.70 (−0.35 - 3.75) | 1.38 (−0.51 - 3.27) | ||
| Intervention | −0.17 (−1.97 - 1.63) | 0.58 (−1.20 - 2.36) | ||
| Age (years) | --- | −0.06 (−0.107 - 0.00) | 0.03 | |
| Race | --- | 0.24 | ||
| White | --- | Reference | ||
| Black | --- | 1.06 (−1.00 - 3.13) | ||
| Hispanic | −1.88 (−4.39 - 0.62) | |||
| Other | --- | 0.50 (−1.99 - 2.98) | ||
| Months from transplant | --- | −0.24 (−0.36 - −0.12) | <0.01 | |
| Allograft | ||||
| Kidney | --- | Reference | ||
| Liver transplant | 1.39 (−0.20 - 2.98) | 0.09 | ||
| Baseline weight (kg) | --- | 0.001 (−0.031 - 0.034) | 0.12 | |
Interactions between study arm and organ and study arm and time from transplant were tested and were not significant. Model 1 is the primary pre-specified model. Model 2 is additionally adjusted for baseline weight, age race, organ, and months from transplant. Abbreviations: kg=kilogram, CI=confidence interval, Simultaneous liver/kidney transplant was evaluated as liver transplant
In exploratory analyses, we investigated whether the proportion of days that ≥7000 steps were achieved at the participant level was associated with changes in weight from baseline. Among the 76 participants with step data, the mean percentage of days ≥7000 steps were reached during the study period was 52% (SD: 36%). Although not statistically significant, there was a 2.2 kg lesser change in weightfrom baseline among participants who reached ≥7000 steps greater than 50% of the time compared to 50% or less (β= −2.2, 95% CI: - 4.50 - 0.09, p=0.06).
Exit survey data
In response to exit survey questions (Supplementary Appendix 3), most patients said they would be willing to participate in the study for greater than 9 months. A total of 89 (92%) enjoyed participating in the study. A total of 19 (56%) of patients in the control/no device arm strongly agreed/agreed that the study helped to increase their physical activity, versus 28 (78%) for the control with device arm and 18 (67)% for the incentives with device arm. A total of 38 (55%) of participants in the control or control with device arms strongly agreed/agreed that the study helped them keep a healthy diet compared to 20 (71%) in the intervention arm. A total of 50 (79%)of patients enrolled in device arms felt that the study helped improve their health and 51 (82%) overall said they were committed to walking for exercise every day. A total of 22 (81%)of patients strongly agreed/agreed that text messages received as part of the active intervention were helpful.
Notably, open-ended feedback (Supplementary Appendix 4) included comments that patients gained more stamina by walking more and the study increased motivation to weigh themselves daily and increase physical activity. A few patients noted that because of the study, walking was “always at the top of my mind”. A few patients in the control/no device arm were disappointed at their randomization assignment and either bought a wearable step tracker or started tracking steps on their phone. Participants made the following suggestions about improving the study: greater ease of technology use and accuracy of syncing; ability to track other types of exercise other than walking such as swimming or biking; and supplementary contacts by study staff to make sure devices were working well. A few participants reported wanting more specific exercise goals and thresholds beyond steps as well as more specific dietary goals.
DISCUSSION
In this randomized, controlled pilot study, we noted that a home-based exercise program using wearable devices, health engagement questions and loss-framed financial incentives increased walking among KTRs and LTRs who were within 2-24 months of transplant. The program was feasible with rapid recruitment and greater than 90% retention, carried out with high fidelity, and was favorably received by patients. This study suggests that a home-based exercise program combined with health engagement questions has the potential to change patient behavior in transplantation (22, 28, 31). Our study incorporated several key principles of behavioral economics – the desired behavior (walking, in this case) was reinforced with immediate feedback and its practice was aided by the memory-enhancing effect of health questions with feedback and frequent financial incentives. These incentives were framed as loss incentives as it has been shown that individuals are more motivated by regret aversion that comes with avoiding a loss compared to anticipating a financial gain.(27, 32) Several features of this pilot study suggest future scalability. Deploying text-message communications in larger populations is simple and low-cost as most patients now own cell phones with text messaging plans while recent innovations in wireless-enabled wearable device technology allow for accurate measurement of physical activity.(31, 33)
We observed that a short-duration, relatively low touch and low-cost intervention delivered with an online portal (Supplementary Appendix 1), the percent of patients reaching a 7000-step daily target was 17% higher in the intervention compared to the device control group. The absolute difference in mean steps during the last 2 weeks of the active study period was 1195 higher in the intervention group and in adjusted models the odds of reaching the 7000 daily step threshold were 2.24 when comparing intervention to control and adjusting for baseline characteristics such as race/ethnicity, allograft type, time from transplant and baseline weight. Interestingly, we noted that LTRs were less likely to reach the 7000 steps targets. Although data are limited, it is possible that liver transplant recipients may be more debilitated prior to transplantation given the nature of end stage liver disease with more sarcopenia, physical frailty, and malnutrition. Future studies should further investigate: 1) whether liver versus kidney transplant recipients should have different physical activity targets, 2) how pre-transplant body mass composition and physical activity affect post-transplant recovery and response to physical activity interventions, and 3) how physical activity interventions affect body mass composition in addition to weight. Although in multivariable models, no significant association was found between study arm and weight changes, unadjusted analyses showed that participants in the intervention arm gained 0.5 kg less weight, compared to about 1-2 kg gain in the control no device or control with device arms. It is not altogether surprising that a study of 12-week duration had modest effects on weight loss. However, given these promising early data, a larger multicomponent behavioral intervention focused on diet and lifestyle interventions combined with physical activity should be conducted.
In addition to financial incentives, a novel component of the design of this trial was the addition of health engagement questions. These questions were based on the principle of “retrieval practice”, which is rooted in educational psychology and assumes that memory improves with frequent testing making information more readily available. The health engagement questions in this trial (Supplementary Appendix 2) were designed to be simple and to keep the salience of both physical activity and healthful diet as “top of mind” for study participants; both behaviors are likely necessary to achieve positive changes in body composition. Although retrieval practice has shown to improve test performance in a classroom setting, applications of this paradigm to healthcare have not been widely investigated and warrant future study (29, 30, 36).
Our study has several limitations. This was a single-center pilot study with a relatively small sample size. Patients who were not smartphone users accounted for approximately one third of those ineligible for the study, potentially limiting generalizability. The study was brief and likely underpowered to show changes in weight. Patients were included beyond the first post-transplant year, when weight gain be less common than in the first year. Weight change may also not capture important facets of body composition, such as the gain of muscle or loss of fat that could be measured using psoas muscle thickness or bioimpedance in future studies. The participants may have been too far out from transplant to measure weight gain prevention. The study design did not include follow-up to measure the sustainability of walking or health behavior changes after interventions concluded. Several participants in the control, no device arm commented in exit interviews that they began to use smart phones to track steps outside of the study protocol. The intervention was not specifically designed to address weight loss via calorie restriction and did not identify which recipients might be in need of weight loss interventions. Rather, patients were given standard diet instructions (Supplementary Appendix 5). Future studies should tailor dietary recommendations based on enrollment weight and body mass composition. We did not measure aerobic fitness of participants in this pilot study; this will need to be performed in larger trials. We excluded one patient on the basis of being non-English speaking; larger studies should adapt intervention materials to non-English speakers. Finally, the trial was not designed to compare the relative effectiveness of the intervention components of financial incentives, reminders, and health engagement questions.
4.1. Conclusions:
A 12-week randomized, controlled pilot study of loss-framed financial incentives paired with frequent feedback and health engagement questions did not lead to weight loss but increased the proportion of days KTRs and LTRs walked ≥7000 daily steps. . The scalability and financing of monetary incentives to change behavior requires future study, however, models where employees and payers provide financial incentives for physical activity and biometric screening have been implemented (34, 35). It is, therefore, feasible to imagine such payer-based models to engage patients and promote healthy behaviors in the immediate post-transplant period. However, it will be important to consider the ethical implementation of these financial incentives prior to deploying them at a large scale. Future, larger, and longer studies should be conducted to test the effects of behavioral interventions pre- and post-transplant to promote physical activity, build strength, and minimize unhealthy weight gain.
Supplementary Material
Supplementary Appendix 1. Description of Way to Health Portal used for study enrollment and randomization
Supplementary Appendix 2. Health engagement questions and percent answered correctly in the devices + incentives arm
Supplementary Appendix 3. Answers to exit survey questions by study arm
Supplementary Appendix 4. Summary of selected open-ended participant feedback about the study intervention.
Supplementary Appendix 5. Sample recommendations for post-transplant nutrition after liver transplant (instructions are similar after kidney transplant)
Acknowledgments
Funding sources:
This study was supported by a pilot grant from the Translational Medicine and Therapeutics (ITMAT) and the Leonard Davis Institute (LDI) Center for Health Incentives and Behavioral Economics (CHIBE) Pilot Grant at the University of Pennsylvania. Marina Serper is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, award #1K23DK115897-01.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- IQR
interquartile range
- IS
immunosuppression
- KG
kilogram
- KT
kidney transplant
- LT
liver transplant
Footnotes
Conflicts of interest:
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Habedank D, Kung T, Karhausen T, von Haehling S, Doehner W, Schefold JC, et al. Exercise capacity and body composition in living-donor renal transplant recipients over time. Nephrol Dial Transplant. 2009;24(12):3854–60. [DOI] [PubMed] [Google Scholar]
- 2.Han SS, Hwang JH, Oh YJ, Cha RH, Ahn C, Kim YS. Change in body compositions of Asian recipients after kidney transplantation. Journal of Korean medical science. 2012;27(10):1182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchetti P New-onset diabetes after liver transplantation: from pathogenesis to management. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(6):612–20. [DOI] [PubMed] [Google Scholar]
- 4.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2005;18(4):461–6. [DOI] [PubMed] [Google Scholar]
- 5.Ducloux D, Kazory A, Simula-Faivre D, Chalopin JM. One-year post-transplant weight gain is a risk factor for graft loss. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(12):2922–8. [DOI] [PubMed] [Google Scholar]
- 6.el-Agroudy AE, Wafa EW, Gheith OE, Shehab el-Dein AB, Ghoneim MA. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation. 2004;77(9):1381–5. [DOI] [PubMed] [Google Scholar]
- 7.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(1):15–22. [DOI] [PubMed] [Google Scholar]
- 8.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35(1):105–9. [DOI] [PubMed] [Google Scholar]
- 9.Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2009;22(5):519–30. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HT, Lum E, Martin P, Bunnapradist S. Effect of diabetes and acute rejection on liver transplant outcomes, an analysis of the OPTN/UNOS database. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016. [DOI] [PubMed] [Google Scholar]
- 11.Galanti G, Stefani L, Mascherini G, Petri C, Corsani I, Francini L, et al. Short-term prospective study of prescribed physical activity in kidney transplant recipients. Intern Emerg Med. 2016;11(1):61–7. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood SA, Koufaki P, Mercer TH, Rush R, O’Connor E, Tuffnell R, et al. Aerobic or Resistance Training and Pulse Wave Velocity in Kidney Transplant Recipients: A 12-Week Pilot Randomized Controlled Trial (the Exercise in Renal Transplant [ExeRT] Trial). Am J Kidney Dis. 2015;66(4):689–98. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz EC, Amer H, Dean PG, Stegall MD, Cosio FG, Cheville AL. Adherence to a pedometer-based physical activity intervention following kidney transplant and impact on metabolic parameters. Clin Transplant. 2015;29(6):560–8. [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Johnson ST, Diehl-Jones W, Vallance JK. Walking, Sedentary Time and Health-Related Quality Life Among Kidney Transplant Recipients: An Exploratory Study. Transplant Proc. 2016;48(1):59–64. [DOI] [PubMed] [Google Scholar]
- 15.Garcia AM, Veneroso CE, Soares DD, Lima AS, Correia MI. Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant Proc. 2014;46(6):1807–8. [DOI] [PubMed] [Google Scholar]
- 16.Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7(3):213–9. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi A, Hu SL, Bostom A. Physical Activity in Kidney Transplant Recipients: A Review. Am J Kidney Dis. 2018;72(3):433–43. [DOI] [PubMed] [Google Scholar]
- 18.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberu J, Bakr MA, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duckworth AL, Milkman KL, Laibson D. Beyond Willpower: Strategies for Reducing Failures of Self-Control. Psychol Sci Public Interest. 2018;19(3):102–29. [DOI] [PubMed] [Google Scholar]
- 20.Karpicke JD, Blunt JR. Retrieval Practice Produces More Learning than Elaborative Studying with Concept Mapping. Science. 2011;331(6018):772–5. [DOI] [PubMed] [Google Scholar]
- 21.Reese PP, Bloom RD, Trofe-Clark J, Mussell A, Leidy D, Levsky S, et al. Automated Reminders and Physician Notification to Promote Immunosuppression Adherence Among Kidney Transplant Recipients: A Randomized Trial. Am J Kidney Dis. 2017;69(3):400–9. [DOI] [PubMed] [Google Scholar]
- 22.Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Eberbach K, et al. Individual Versus Team-Based Financial Incentives to Increase Physical Activity: A Randomized, Controlled Trial. J Gen Intern Med. 2016;31(7):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett DR Jr., Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42(10):1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 25.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel MS, Asch DA, Volpp KG. Framing Financial Incentives to Increase Physical Activity Among Overweight and Obese Adults. Ann Intern Med. 2016;165(8):600. [DOI] [PubMed] [Google Scholar]
- 28.Chokshi NP, Adusumalli S, Small DS, Morris A, Feingold J, Ha YP, et al. Loss-Framed Financial Incentives and Personalized Goal-Setting to Increase Physical Activity Among Ischemic Heart Disease Patients Using Wearable Devices: The ACTIVE REWARD Randomized Trial. J Am Heart Assoc. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpicke JD, Roediger HL 3rd, . The critical importance of retrieval for learning. Science. 2008;319(5865):966–8. [DOI] [PubMed] [Google Scholar]
- 30.Roediger HL 3rd, Butler AC. The critical role of retrieval practice in long-term retention. Trends Cogn Sci. 2011;15(1):20–7. [DOI] [PubMed] [Google Scholar]
- 31.Patel MS, Asch DA, Volpp KG. Use of wearable monitoring devices to change health behavior--reply. JAMA. 2015;313(18):1865–6. [DOI] [PubMed] [Google Scholar]
- 32.Zeelenberg M, Pieters R. Consequences of regret aversion in real life: The case of the Dutch postcode lottery. Organ Behav Hum December 2004;93(2):155–68. [Google Scholar]
- 33.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313(6):625–6. [DOI] [PubMed] [Google Scholar]
- 34.Medicaid Incentives for Prevention of Chronic Diseases. https://downloads.cms.gov/files/cmmi/mipcd-finalevalrpt.pdf, Accessed July 19th, 2019.
- 35.Cleveland Clinic Employee Health Plan. https://employeehealthplan.clevelandclinic.org/EHP-Wellness-Program/Physical-Activity/Activity-Device-Program.aspx, Accessed July 19th, 2019.
- 36.McDaniel MA, Agarwal PK, Huelser BJ, McDermott KB, Roediger Iii HL. Test-enhanced learning in a middle school science classroom: The effects of quiz frequency and placement. Journal of Educational Psychology. 2011;103(2):399–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix 1. Description of Way to Health Portal used for study enrollment and randomization
Supplementary Appendix 2. Health engagement questions and percent answered correctly in the devices + incentives arm
Supplementary Appendix 3. Answers to exit survey questions by study arm
Supplementary Appendix 4. Summary of selected open-ended participant feedback about the study intervention.
Supplementary Appendix 5. Sample recommendations for post-transplant nutrition after liver transplant (instructions are similar after kidney transplant)