Abstract
Background
We recognize an unprecedented opportunity to study the effects of withdrawing one or more chronic treatments in people with CF (PwCF) who benefit greatly from CFTR modulator therapy, but feasibility and acceptance of such a study within the community is unknown.
Methods
We surveyed PwCF, their families, and their acquaintances between November 16, 2018, and December 2, 2018, and CF clinicians between December 19, 2018, and January 2, 2019, about treatment withdrawal research. We sought feedback from these groups about their level of interest in this research, the consistency with which they were taking modulator and non-modulator treatments, the ways in which they conceptualized health changes, and what chronic non-modulator treatments they were most interested in stopping. We also asked for stakeholder perspectives on the design of a treatment withdrawal trial, but we intend to report these perspectives elsewhere.
Results
Eighty percent (541/675) of CF community respondents and 95% (206/218) of CF clinicians said that a trial of treatment simplification should be performed in the context of highly effective modulator therapy. Most current CFTR modulator users (292/359, 81%) have not stopped another chronic treatment. Worsening lung function by spirometry or increased daily symptoms were important health indicators. PwCF, their families, and/or their acquaintances ranked airway clearance techniques and inhaled antibiotics as the most burdensome treatments.
Conclusions
There is considerable support among the CF community and CF clinicians in the U.S. for controlled trials to assess the safety and impact of treatment simplification in patients taking highly effective modulator therapy.
Introduction
Drugs that effectively increase functioning of the cystic fibrosis transmembrane conductance regulator (CFTR) protein have revolutionized the treatment and anticipated health outcomes of many people with cystic fibrosis (PwCF). Collectively known as CFTR modulators, ivacaftor (IVA), lumacaftor/ivacaftor (LUM/IVA), and tezacaftor/ivacaftor (TEZ/IVA) improve lung function, reduce pulmonary exacerbation (PEx) risk, and alleviate respiratory symptoms of CF lung disease [1–3]. As evidence of a systemic biological effect, these drugs can reduce sweat chloride levels towards the normal range in some individuals. Researchers have reported other health benefits of CFTR modulators. In those with the G551D-CFTR mutation treated with IVA, Pseudomonas aeruginosa (P. aeruginosa) airway infection appears to lessen and even resolve in some individuals [4, 5]. IVA use has also been associated with weight gain, higher hemoglobin levels, better glycemic control, and less frequent need for antibiotics [4, 6–9].
Pivotal clinical trials of elexacaftor (VX-445) combined with TEZ/IVA [10, 11] have ushered in an era of CFTR modulator therapy that promises to yield results similar to or possibly better than IVA monotherapy. Importantly, elexacaftor plus TEZ/IVA was effective in those with one or two copies of the most common CFTR mutation, F508del; thus, it could become available for ≥ 90% of PwCF based on CFTR mutation profile. Although studies must still be done to understand the safety, tolerability, and efficacy of modulator therapy in young children and to determine the appropriate age for these treatments to begin, the introduction of highly effective and clinically beneficial modulator therapy for most PwCF could represent a landmark shift in clinical practice.
In light of the aforementioned progress toward normalizing the health of PwCF by restoring CFTR activity, the need for treatments that have been recommended for years [12, 13], including bronchodilators, mucolytic agents, chest physiotherapy, macrolides, and nebulized antibiotics, is being questioned. It is widely understood that: 1) even the most effective modulators do not fully normalize CFTR activity; 2) numerous PwCF have permanent structural lung damage resulting in physiologic impairment; and 3) individuals may experience more or less than average benefits reported in clinical trials. Notwithstanding these realities, many PwCF who take modulator therapy may find that the benefits of traditional strategies for symptom management are difficult to judge.
The pragmatic question raised by widespread adoption of modulator therapy is whether some fraction of PwCF will be able to abandon one or more burdensome treatments without incurring untoward health effects. Nebulized medications and chest physiotherapy are particularly time-consuming for PwCF [14, 15]. High treatment complexity tends to undermine quality of life and adherence and increases the potential for drug-related side effects [16–18]. If PwCF enjoy salubrious effects of modulator therapy, patients, families, and care teams will have an opportunity to reconsider the value proposition of existing therapies. Nonetheless, it is incumbent upon the CF care community to understand the potential health risks of simplifying treatment regimens for the sake of reducing the burden of daily care.
Here, we present data from surveys of PwCF, families of PwCF, and CF clinicians designed to learn their perspectives on clinical research testing whether or not it is safe to withdraw one or more chronic treatments in the setting of highly effective modulator therapy. When applicable in our questionnaires, we used the perceived benefits typically associated with IVA monotherapy in people with the G551D-CFTR mutation as a benchmark to describe highly effective modulator therapy. We requested feedback about acceptability of several study design considerations. These findings will inform a future prospective clinical trial testing the impact of simplifying treatment regimens in PwCF who benefit from modulator therapy. Survey responses may also help CF clinical and research communities understand similarities and differences of opinion held by these representative groups. One message is clear from the results: this research is timely, of high priority, and if performed systematically, will provide data that are important to most patients and providers.
Methods
Focus Groups
We began with the goal of designing a survey about treatment simplification research for PwCF and their families and caregivers (hereafter, referred to as “community”) that was accessible and responsive to their priorities. The CF Foundation’s Community Partnerships team convened a virtual Community Voice (CV) focus group in September 2018 that included the investigators, two adults with CF, a parent of a child with CF, and CV facilitators.
Community Survey
Incorporating feedback from the focus group, we devised and refined a twenty question web-based survey using SurveyMonkey® about treatment simplification. Survey content is provided as an online supplement. The survey link was distributed via email and social media to members of the CF community, including members of CV. We asked respondents to share basic demographic information and their attitudes about treatment simplification research in the context of highly effective modulator therapy. We petitioned them to rank the importance of potential outcomes of a treatment simplification trial. We inquired about their level of interest in stopping specific chronic treatments as part of a study. Access to the survey link was not restricted to CV members. A unique respondent could take the survey only once. The community survey was available between November 16, 2018, and December 2, 2018. We provide a flow chart explaining survey distribution to and response rates from community members as supplementary material.
Clinician Survey
We designed a similar web-based electronic survey using SurveyMonkey® for CF care providers (hereafter, referred to as “clinicians”). Survey content is provided as an online supplement. We distributed the survey to 250 CF care center and/or program directors affiliated with the CF Foundation’s Therapeutics Development Network (TDN). The survey obtained basic demographic information and asked whether withdrawal of non-modulator treatments should be studied in a background of highly effective modulator therapy. We asked clinicians about their level of interest in contributing to this kind of trial and which treatments should be prioritized for withdrawal. Clinicians could share the survey link, but a unique respondent could take the survey only once. The clinician survey was available between December 19, 2018, and January 2, 2019. We provide a flow chart explaining survey distribution to and response rates from clinicians as supplementary material.
Regulatory Approval
The Institutional Review Board (IRB) of Seattle Children’s Research Institute approved the patient and community survey on 10/23/2018 and the clinician survey on 12/4/2018. The IRB registration number was 00001508.
Results
Survey Completion
We collected 812 surveys from the community, 117 of which came from CV and 695 of which came from outside CV. The response rate through CV was 15%. There were 667 completed surveys available for analysis. PwCF, parents of PwCF, and other acquaintances of PwCF generated 37% (247/667), 56% (371/667), and 7% (49/667) of completed surveys, respectively. The ages of PwCF who responded to the survey and those about whom parents, family members, or other acquaintances commented are listed in Table 1. We received 218 completed surveys from clinicians between December 19, 2018, and January 2, 2019. Most (185/218, 85%) clinician respondents were pulmonologists. Of the 121 clinicians who reported that they exclusively care for children with CF, 98 (81%) said that ≥ 25% of their professional effort focused on CF. Of the 81 clinicians who reported that they exclusively care for adults with CF, 65 (80%) said that ≥ 25% of their professional effort focused on CF.
Table 1.
Age of people with CF (PwCF) who responded to the survey and of those about whom family members and acquaintances commented in the survey.
| Age (years) | PwCF n (%) | Parent n (%) | n(%) | Other n (%) |
|---|---|---|---|---|
| <2 | — | 23 (6.2) | 0 (0) | 2 (6.7) |
| 2–5 | — | 84 (22.6) | 2 (10.5) | 8 (26.7) |
| 6–11 | — | 111 (29.9) | 7 (36.8) | 4 (13.3) |
| 12–17 | — | 87 (23.5) | 3 (15.8) | 4 (13.3) |
| 18–24 | 38 (15.4) | 46 (12.4) | 5 (26.3) | 6 (20.0) |
| 25–34 | 85 (34.4) | |||
| 35–44 | 59 (23.9) | |||
| 45–54 | 37 (15.0) | 20 (5.4)* | 2 (10.5)* | 6 (20.0)* |
| 55–64 | 16 (6.5) | |||
| ≥65 | 12 (4.9) | |||
| Total | 247 (100.0) | 371 (100.0) | 19 (100.0) | 30 (100.0) |
The highest selection for age range of PwCF about whom family members and acquaintances responded was “25 or older.”
Interest in CF treatment simplification research
Eighty percent (541/675) of the community and 94% (206/218) of the physicians completing the survey stated that treatment simplification should be studied in the context of highly effective modulator therapy. Similar proportions of PwCF (204/247, 83%) and parents of PwCF (294/371, 79%) and of adult (75/81, 93%) and pediatric (117/121, 97%) clinicians shared this sentiment. Very few PwCF (16/247, 6%), parents of PwCF (21/371, 6%), and clinicians (3/218, 1%) said that treatment simplification research should not be done.
Adherence to non-modulator therapies
Three hundred fifty-nine community members, all of whom recorded personal use of a modulator or use of a modulator by their acquaintance with CF, responded to a question asking whether they had reduced or stopped taking any chronic medication other than a modulator. Of these 359 respondents, 60 (17%) answered affirmatively. Clinicians offered similar estimates of the proportions of their modulator-treated patients they thought had stopped one or more chronic treatments. Clinician estimates of non-modulator treatment reduction and/or discontinuation by their patients are presented in Table 2.
Table 2.
Estimates from CF Clinicians of the proportions of their modulator-treated patients that have reduced and/or discontinued any other chronic treatment.
| Discontinued Other Treatments? | IVA n (%) | LUM/IVA n (%) | TEZ/IVA n (%) |
|---|---|---|---|
| Yes, <25% of patients | 61 (29) | 67 (33) | 53 (28) |
| Yes, 25–50% of patients | 39 (19) | 7 (3) | 7 (3.5) |
| Yes, >50% of patients | 18 (9) | 3 (1) | 1 (0.5) |
| No | 52 (25) | 91 (45) | 89 (47) |
| Do not know | 38 (18) | 36 (18) | 40 (21) |
| Total clinician respondents | 208 (100) | 204 (100) | 190 (100) |
IVA = ivacaftor; LUM/IVA = lumacaftor/ivacaftor; TEZ/IVA = tezacaftor/ivacaftor.
Goals of CF treatment simplification research
Most clinicians (206/215, 96%) and community members (533/632, 84%) felt that it would be important or very important for a study to determine whether “it is okay to stop other daily treatments” in the context of highly effective modulator therapy. Similar proportions of community members (505/633, 80%) and clinicians (162/215, 75%) felt that reducing the cost of CF treatments would be an important or very important primary goal of a treatment simplification study. Reduction of time and effort devoted to treatments was ranked as an important or very important study outcome by 572/631 (91%) of community respondents and 190/215 (88%) of clinicians.
Treatment candidates for withdrawal in a controlled trial
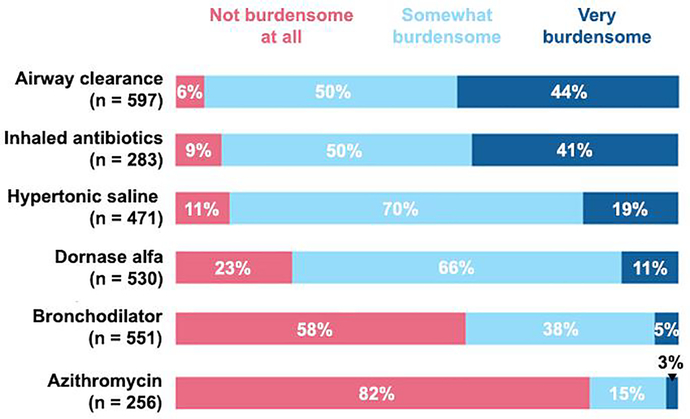
Community members ranked airway clearance techniques (ACT) and inhaled ABX as the most burdensome treatments (Fig. 1). Of the 586 community members who responded to a question about interest in stopping ACT for a study, 340 (58%) were very interested and 179 (30%) were somewhat interested. Of the 278 community members who answered a question about interest in stopping inhaled ABX for a study, 161 (58%) were very interested and 90 (32%) were somewhat interested. Clinicians were very interested in enrolling PwCF in a trial testing the effects of stopping inhaled hypertonic saline (140/215, 65%) and/or dornase alfa (136/215, 63%).
Fig. 1.
Proportions of CF community members in the United States that ranked six chronic treatments with respect to perceived burden.
Relative value of health indicators to research stakeholders
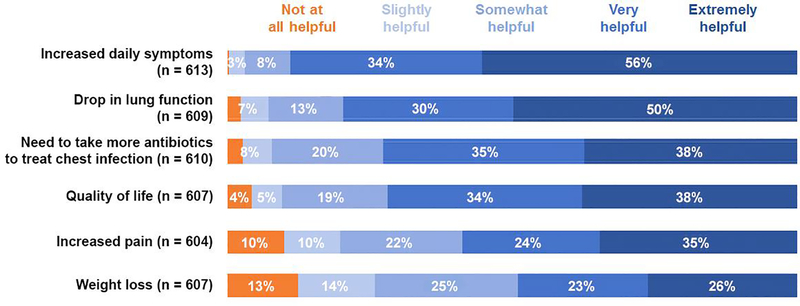
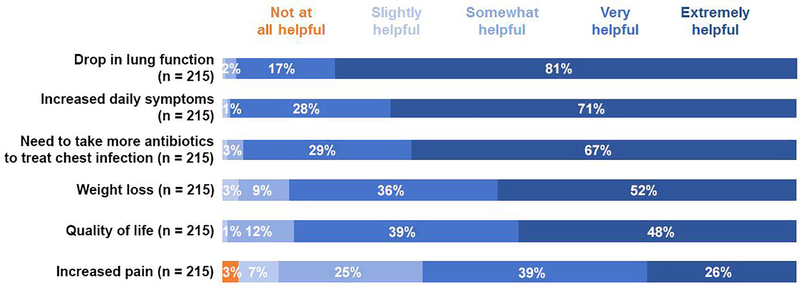
To understand the extent to which community members and clinicians valued certain health indicators when assessing the impact of treatment withdrawal, we asked these groups to assign a level of importance to each of six potential alterations in well-being. Of the 613 community members who offered an opinion about increased daily symptom burden, 546 (89%) thought that this was very or extremely helpful as an indicator of health deterioration (Fig. 2). Many community members (485/609, 80%) also believed that a drop in lung function was evidence of health deterioration. Clinicians ranked a drop in lung function (210/215, 98%) and increased daily symptom burden (212/215, 99%) as important or very important health indicators (Fig. 3). A higher proportion of clinicians (144/215, 67%) than community members (229/610, 38%) thought that the need to take more antibiotics to treat a chest infection (i.e., pulmonary exacerbation) was an extremely helpful health indicator.
Fig. 2.
Proportions of CF community respondents in the United States who ranked six contingencies with respect to how helpful they are as indicators of health deterioration.
Fig. 3.
Proportions of CF clinicians in the United States who ranked six contingencies with respect to how helpful they are as indicators of health deterioration in people with CF (PwCF).
Discussion
Herein, we have summarized input collected from the CF community and clinicians who care for PwCF in the U.S. about their level of interest in collaborating to study the effects of treatment withdrawal in the promising context of highly effective modulator therapy. Several important messages have emerged from these survey data.
First, widespread support exists in the U.S. for the conduct of prospective clinical trials designed to test the central hypothesis of whether PwCF whose health improves greatly from modulator therapy can safely discontinue one or more burdensome chronic therapies. This observation resonates with high global interest in developing strategies to reduce treatment burden for PwCF [19, 20], which is encouraging to see as we work to design and implement a timely and informative clinical trial of treatment simplification. We have heard from PwCF, their families, and CF clinicians in the U.S. that a decline in lung function and/or increased daily symptoms are warning signs of health deterioration. This will help to select appropriate outcome measures for a treatment withdrawal study conducted in the U.S.
Second, the CF community was somewhat keener than CF clinicians in the U.S. were to explore the effects of stopping time- and energy-consuming therapies, like ACT and inhaled antibiotics. Consistent with this perspective, community members were somewhat more likely than clinicians to prioritize reduction of time and effort devoted to treatments and treatment cost, although both groups recognized the importance of these goals. Inhaled mucolytic agents like dornase alfa and HS are favorable candidates based on widespread use, perceived burden, and overlapping effects (i.e. mucociliary clearance) with those observed for modulator therapy. The heterogeneity of ACT interventions and perceived importance of inhaled antibiotics for a subset of PwCF infected by P. aeruginosa [12] may challenge withdrawal studies testing these therapies, despite a recognized burden of care. Nonetheless, community input prioritizing ACT and inhaled antibiotics in this research context should be recognized going forward.
A third message from the survey data also addresses the feasibility of investigating treatment withdrawal. We learned that despite fostering high interest in such studies, most PwCF (>80%) currently taking modulators have not yet curtailed their use of non-modulator therapies and that clinicians’ estimates of this phenomenon occurring in their patients were comparable (Table 2). Using a survey to ask about treatment adherence, like we have done here, can introduce one or more biases to the responses, but identifying similitude between what the community and clinicians said about sustained use of non-modulator therapies bolsters our confidence about enrolling PwCF in a treatment withdrawal study.
We acknowledge several limitations of our work. The opinions voiced by American patients, families, and clinicians about treatment simplification research in CF may or may not be congruent with those held by individuals from other countries. This is an important realization because PwCF and clinicians residing outside the U.S. might approach treatment simplification research differently. For example, our surveys of stakeholders identified worsening of daily symptoms and a drop in lung function as helpful indicators of health deterioration, which from the standpoint of our study design deliberations, meant that these were logical outcome measures. Stakeholders in other parts of the world place considerable emphasis on changes in computed tomography (CT) of the chest as evidence of respiratory health status, which might inform their selection of this test as a primary outcome measure in treatment simplification research. We did not include chest CT scan as a choice in our surveys, so we cannot exclude the possibility that some of our respondents would have prioritized it higher than worsening of daily symptoms and/or a drop in lung function as helpful indicators of health deterioration. Nonetheless, we consider our findings informative and not necessarily prescriptive of how research in this field should proceed.
Our two surveys resulted in just under 900 completed responses from the U.S. CF patient/family and clinician provider communities. Seventy-five percent of these were from people with CF or their family members. This is the largest number of individual surveys collected on this topic in the U.S. and, to our knowledge, in the world. That said, our surveys were disseminated electronically to 725 CF clinicians and over 100,000 potential members of the CF patient/family community. Some may interpret our response rate as poor, although we are pleased with the number of completed surveys and grateful to the hundreds of individuals who provided their input on this important topic. It is possible that those not responding to this survey have different opinions, but we are unaware of any data suggesting that there is low interest in the U.S. to determine whether or not it is appropriate to withdraw chronic therapies in people with much improved health after starting CFTR modulator therapy. Continued discussion of this topic with as many stakeholders as possible will be important as we and others advance this field of study.
It is exciting and inspiring to members of this investigatory group that such immense progress has been made in the CF field. This now enables us to contemplate the design and conduct of a clinical trial of non-modulator treatment withdrawal. However, we appreciate the need to approach this kind of study with equipoise, ensuring that participants are monitored closely for objective and/or subjective evidence of clinical deterioration while they are without one or more non-modulator treatments. We may also need to balance concerns of safety and generalizability when determining the population eligible for withdrawal trials. Given that modulator therapy is likely to become available, albeit by degrees, to PwCF around the world, it would be prudent for the global CF research community to discuss elements of study design in order to optimize resource allocation and promote the generalizability of lessons learned. We recognize that the survey data presented here encompasses an American CF experience and are hopeful that the responses gathered will allow us to design the first large study of treatment withdrawal in the era of highly effective modulator therapy.
Supplementary Material
Highlights.
Stakeholders in the U.S. CF community support treatment simplification research.
Most modulator-treated people with CF in the U.S. have not stopped other therapies.
Airway clearance and inhaled antibiotics are burdensome to the U.S. CF community.
U.S. CF clinicians want to study withdrawal of hypertonic saline and dornase alfa.
Lung function and symptoms are useful health indicators to U.S. CF stakeholders.
Acknowledgments
The authors would like to thank the CF Foundation for facilitating research through Community Voice to support this work. Additionally, we would like to thank all of the people with CF and their family members across the United States for sharing their insights. Mr. Enid Aliaj and Ms. Christina Roman at the CF Foundation deserve special recognition for their efforts in support of this project.
FUNDING SOURCES: Research reported in this publication was supported by the CF Foundation (NICHOL18A0, GIFFOR17Y5), the Dartmouth Clinical and Translational Science Institute under award number KL2TR001088 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and Seattle Children’s Hospital under award number 5P30DK089507 from the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) of the NIH. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
Conflict of Interest Statement. Dr. Alex H. Gifford has received grant funding from the Cystic Fibrosis Foundation. Dr. Nicole Mayer-Hamblett has no conflicts of interest to declare. Ms. Kelsie Pearson has no conflicts of interest to declare. Dr. David P. Nichols has received grant funding from the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. The New England journal of medicine. 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. The New England journal of medicine. 2015;373:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. The New England journal of medicine. 2017;377:2013–23. [DOI] [PubMed] [Google Scholar]
- [4].Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. American journal of respiratory and critical care medicine. 2014;190:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. American journal of respiratory and critical care medicine. 2017;195:1617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hubert D, Dehillotte C, Munck A, David V, Baek J, Mely L, et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp-CFTR mutation after 1 and 2years of treatment with ivacaftor in a real-world setting. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2018;17:89–95. [DOI] [PubMed] [Google Scholar]
- [7].Kirwan L, Fletcher G, Harrington M, Jeleniewska P, Zhou S, Casserly B, et al. Longitudinal Trends in Real-World Outcomes Following Initiation of Ivacaftor: A Cohort Study from the Cystic Fibrosis Registry of Ireland. Annals of the American Thoracic Society. 2018. [DOI] [PubMed] [Google Scholar]
- [8].Gifford AH, Heltshe SL, Goss CH. CFTR Modulator Use Is Associated with Higher Hemoglobin Levels in Individuals with Cystic Fibrosis. Annals of the American Thoracic Society. 2019;16:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, et al. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2016;15:e25–7. [DOI] [PubMed] [Google Scholar]
- [10].Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. The New England journal of medicine. 2018;379:1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr., Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. American journal of respiratory and critical care medicine. 2007;176:957–69. [DOI] [PubMed] [Google Scholar]
- [13].Mogayzel PJ Jr., Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. American journal of respiratory and critical care medicine. 2013;187:680–9. [DOI] [PubMed] [Google Scholar]
- [14].Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2009;8:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dziuban EJ, Saab-Abazeed L, Chaudhry SR, Streetman DS, Nasr SZ. Identifying barriers to treatment adherence and related attitudinal patterns in adolescents with cystic fibrosis. Pediatric pulmonology. 2010;45:450–8. [DOI] [PubMed] [Google Scholar]
- [16].Nichols DP, Kuk KN, Nick JA. Drug interactions and treatment burden as survival improves. Current opinion in pulmonary medicine. 2015;21:617–25. [DOI] [PubMed] [Google Scholar]
- [17].George M, Rand-Giovannetti D, Eakin MN, Borrelli B, Zettler M, Riekert KA. Perceptions of barriers and facilitators: self-management decisions by older adolescents and adults with CF. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2010;9:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sawicki GS, Tiddens H. Managing treatment complexity in cystic fibrosis: challenges and opportunities. Pediatric pulmonology. 2012;47:523–33. [DOI] [PubMed] [Google Scholar]
- [19].Rowbotham NJ, Smith S, Leighton PA, Rayner OC, Gathercole K, Elliott ZC, et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax. 2018;73:388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kalaitzis IS, Rowbotham NJ, Smith SJ, Smyth AR. Do current clinical trials in cystic fibrosis match the priorities of patients and clinicans? A systematic review. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.