Abstract
Purpose
The role of core stability in running and its influence on injury risk in runners is not well understood. The purpose of this study was to investigate the effect of core stability (and core fatigue) on running mechanics. We hypothesized that decreasing core stability in novice runners would result in altered running mechanics previously associated with increased risk for common lower extremity running injuries.
Methods
Three-dimensional running kinematics and kinetics and seated postural sway on an unstable surface were collected on 25 healthy, novice runners before and after they performed a core stability knockdown protocol (CSKP), designed to temporarily reduce participants’ core stability in a single testing session.
Results
Linear mixed models demonstrated that the CSKP resulted in an increased peak knee flexion moment (0.51 %BW·ht increase, ES=0.49, p=0.021) and a decreased vertical average loading rate (4.5 BW/s decrease, ES=0.44, p=0.037) during running, but no significant changes in peak knee adduction moment, knee adduction impulse, hip adduction moment, hip adduction impulse, or peak vertical ground reaction force (all p>0.05). Twenty of 25 runners demonstrated a measurable decrement in their core stability as defined by their seated postural sway center of pressure excursion changing more than the standard error of measurement of 76mm.
Conclusion
An experimentally induced decrement in core stability in novice runners caused an increased peak knee flexion moment during stance, which has previously been associated with increased patellofemoral contact pressure during running. Therefore, these results demonstrate that insufficient core stability in novice runners may be a risk factor for developing patellofemoral pain. Other results did not support a role of core stability in other common overuse running injuries in this population.
Keywords: running, lower extremity, knee, patella, trunk control, injury prevention
Introduction
Running is a popular physical activity despite the high annual rate of running-related injuries.(1) Novice runners may be most susceptible to these injuries, possibly due to a lack of training experience.(2) Literature on the prevention of running-related injuries is scarce and current injury prevention programs and products have been unsuccessful at lowering injury rates in runners.(3)
Several lower extremity (LE) biomechanical risk factors have been associated with common running-related injuries, such as patellofemoral pain syndrome (PFPS), iliotibial band syndrome (ITBS), and tibial stress fractures (TSF).(4) Very few intervention studies have targeted these parameters in an attempt to lower injury risk. Gait retraining may be beneficial as a biomechanical intervention paradigm;(5) however this approach may not be readily available to all runners. Identifying the fundamental contributors to these biomechanical risk factors may facilitate the design of improved treatment and prevention protocols.
Muscle activity of the trunk precedes dynamic movement of the extremities,(6) suggesting abdominal muscles may activate to provide a stable foundation for production, absorption, and control of force and motion to the extremities. Consequently, insufficient core stability is believed to lead to less efficient movements and ultimately musculoskeletal injury;(7) however, this theory has not been supported with sufficient scientific evidence. We define core stability herein as the body’s ability to control the torso and maintain or resume an equilibrium trunk and pelvis position or state following internal or external perturbations. Most studies that have examined the relationship between core stability and running injuries have used cross-sectional or retrospective designs.(8) Studies that directly manipulate core stability and determine the effects of that perturbation on running mechanics may facilitate a better understanding of the role that core stability plays during running and its influence on injury risk.
The purpose of this study was to identify any causal effects of reduced core stability on running mechanics in novice runners. Decreasing core stability in individual runners was hypothesized to result in altered running mechanics previously associated with increased risk for lower extremity musculoskeletal injury.
Methods
Thirteen novice female runners (23.9±8.8 years; 1.68±0.05 m; 62.4±9.51 kg; self-selected running speed 2.62±0.23 m·s−1) and 12 novice male runners (23.3±3.9 years; 1.80±0.07 m; 77.0±11.2 kg; 2.75±0.32 m·s−1) participated in the study after providing IRB-approved informed consent. Novice runners were defined as running <10 miles/week and not playing running sports (e.g., soccer) more than once a week. Other exclusion criteria for this study were: BMI>30; history of lower back pain; musculoskeletal injury within the past 3 months. All participants were recruited from the community using flyers posted at local running clubs, shoe stores, and fitness centers.
Core Stability Knockdown and Running Biomechanics
Three-dimensional overground running mechanics were collected before and after participants performed a novel core stability knockdown protocol (CSKP) designed to reduce a person’s core stability temporarily in a single testing session.(9) The CSKP consisted of 4 dynamic and 4 isometric exercises chosen based on preliminary investigations to target the superficial and deep core musculature with minimal activity of the lower extremity muscles (Figure 1). Participants completed all exercises consecutively, each to voluntary exhaustion or until proper form could not be maintained. Upon completion of the CSKP, any markers or electrodes that had been dislodged were replaced before proceeding with the post-CSKP core stability assessment and running biomechanics assessment. Replacing markers and electrodes took 4.5±1.2 min per participant.
Figure 1.
Core Stability Knockdown Protocol (CSKP) designed to fatigue both the superficial and deep core musculature. The four dynamic and four isometric exercises were performed in the following order, each to voluntary exhaustion or until proper form could not be maintained. All exercises were completed consecutively with minimal to no rest between each.
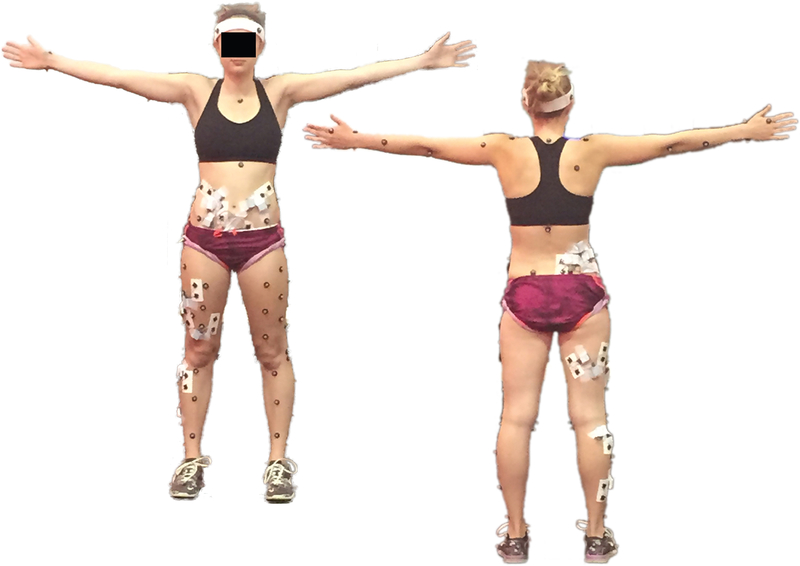
Marker data were collected at 300 Hz using a 9-camera optical system (MX-F40; Vicon Motion Systems; Oxford, UK) and filtered using a 4th-order Butterworth filter at 15 Hz. Ground reaction forces (GRF) were recorded at 1500 Hz from four force plates (4060–10; Bertec Corp; Columbus, OH, USA). Participants ran continuously around a 30 m loop with a straight section across the force plates. A trial was collected for each lap in which the feet cleanly landed entirely on force plates, until 10 trials had been collected. Running speed was monitored using timing gates (Fusion Sport; Sumner Park, QLD, Australia) to ensure all running trials were within ±10% of a self-selected, comfortable running speed. Retro-reflective markers were placed on the upper and lower body using a modified point cluster technique (Figure 2).(10) All moments presented are external joint moments, normalized to percent of body weight · height (%BW·ht). Ten overground running trials were collected for each leg, pre- and post-CSKP. Stance phase data from both the dominant and non-dominant legs were analyzed as described below in the Statistics section.
Figure 2.
Reflective marker and electromyography electrode placement. Note: only activations of core muscles were analyzed in this study.
Primary biomechanical kinetic variables of interest were those previously identified to be associated with running injury risk. Vertical GRF impact peak (VIMP) and average loading rate (VALR) were defined as by Davis et al., where VIMP is the local maximum in the vertical GRF between foot strike and the overall peak (11). The vertical GRF at 20% and 80% of the interval between foot strike and VIMP are then identified, and the slope between those points is defined as the VALR (11). Peak knee flexion (pKF) moment is the overall peak over stance phase of the knee flexion moment (12, 13). Peak knee adduction (pKAdd) moment is the overall peak over stance phase of the knee adduction moment, and impulse is the time integral over the interval when the frontal plane knee moment is adduction (12, 14). Peak hip adduction (pHAdd) moment is the overall peak over stance phase of the hip adduction moment, and impulse is the time integral over the interval when the frontal plane hip moment is adduction (15). Analyses were limited to kinetic variables rather than kinematic variables due to the hypothesized role of the upper body’s mass in altering the direction and magnitude of the ground reaction force.(10, 16) Wireless surface electromyography (EMG) (Telemyo DTS; Noraxon USA, Inc; Scottsdale, AZ) was measured during the CSKP using disposable surface electrodes (Figure 1), placed bilaterally on the external obliques (EO) and internal obliques (IO), and unilaterally on the dominant-side rectus abdominis (RA), and L5 lumbar extensor (L5). Dual electrodes were used for the EO, RA and L5 muscles and two single electrode discs were used for the IO muscles. Pre-gelled (Ag/AgCl), surface electrodes (A10011/A10005; Vermed, Inc; Bellows Falls, VT, USA) were placed as recommended by McGill to best reflect deep muscle activity(17) or directly on the most prominent aspect of the muscle belly and oriented parallel to the muscle fibers. Electrode locations were shaved, if necessary, and cleaned with alcohol pads. The median frequency (MedF) of the raw EMG signal for all core muscles was analyzed during the submaximal isometric exercises of the CSKP in order to obtain a measurement of core muscle fatigue induced by the CSKP. A decrease in the MedF of an EMG signal has been directly related to the level of muscle fatigability.(18)
Core Stability Assessment
A novel unstable quiet sitting test (QST) developed by our research team was used to quickly and objectively quantify each participant’s core stability in all planes of motion in a way that could also be replicated in a clinical setting (Figure 3). The QST is a modification of the quiet standing task that has been used for years to measure postural stability,(19) in order to isolate control of the lumbar spine and trunk from adjustments in the lower extremity joints. Postural control of the trunk has previously been investigated during sitting on an unstable chair with a rigid hemisphere beneath the seat,(20–23) however this chair is complex and may not be easily replicable by other researchers or clinicians. In the QST, participants sat on top of the rounded end of a BOSU® ball (BOSU®; Ashland, Ohio) placed on a rigid surface positioned on top of two force platforms and situated high enough so that their feet did not touch the ground. Participants sat as still as possible for 60 seconds with their eyes closed and arms crossed while performing a secondary task of counting backwards by 4’s, to simulate a real-life situation where attention is divided between multiple tasks. The starting number was incremented by one for each trial.
Figure 3.
Unstable quiet sitting task (QST) used to quantify core stability. Participants are instructed to sit as still as possible for 60 seconds with eyes closed while simultaneously counting down by 4’s. The chair is positioned on top of force platforms and center of pressure excursion (CoPexc) is recorded during the task.
Core stability was quantified during the QST through measurement of the total center of pressure (CoP) path length, also known as total CoP excursion (CoPexc) during the sitting task. CoPexc has been used frequently in previous research to quantify postural control.(20, 22, 24–26) Each participant performed one practice trial of the QST, then five trials before the CSKP and three trials after the CSKP. CoP data were collected at 1500 Hz and 4th-order lowpass Butterworth filtered with a 10 Hz cutoff frequency. The median CoPexc at each timepoint was used for analysis. The QST has very good intra- and inter-rater reliability (ICC(2,1): 0.877 and 0.813, respectively; SEM: 76 mm).(27) A meaningful decrease in core stability was defined as an increase or decrease in CoPexc ≥ 76 mm from baseline (pre-CSKP). An increase in CoPexc was considered as indicating a loss of ability to control sway, whereas a decrease in CoPexc was considered as indicating a greater effort to stiffen the torso due to reduced confidence in one’s ability to manage sway.
Statistics
A sample size of 25 was calculated a priori to provide at least 80% power to detect an effect size of 0.75 standard deviation change based on a two-sided paired t-test at an overall significance level of 0.05 (Bonferroni adjustment for 5 primary endpoints, at 0.01). Without multiple comparison adjustment, this sample size could provide at least 80% power to detect an effect size of 0.6 standard deviations based on a paired t-test. This is an appropriate and clinically-relevant effect size for the biomechanical variables of interest based on previous studies of these variables.(11, 14, 15, 28, 29)
Statistical analyses were performed in JMP® Pro 14.0.0 (SAS Institute Inc., Cary, NC). The mean and confidence interval of the change in median frequency were calculated to test for a significant change in core muscle fatigue following the CSKP. Linear mixed models were used to evaluate the effect of a change in core stability state (pre- or post-CSKP) on the primary variables of interest. Core stability state was treated as a fixed effect; participant was treated as a random effect. The use of a random participant effect allows for the examination of the effect of a change in core stability on running parameters within an individual.
To choose which leg to include in the analysis, the z-score for each variable across all 50 legs at baseline was calculated. The leg with the highest overall z-score across all seven kinetic variables was then chosen as the leg with the greatest a priori biomechanical injury risk and included in the mixed-effects model analysis. Effect sizes (ES) for all variables of interest were calculated using Cohen’s dZ for one-sample comparisons (30–32) based on the change values (post-pre). Standard benchmarks of small (ES = 0.2), medium (ES = 0.5), and large (ES = 0.8) effect sizes were used as suggested by Cohen (31) due to the novel nature of the study. Mixed model analyses were conducted on all 25 participants. Because the study hypothesized that decreasing core stability would result in altered running mechanics, we also performed a secondary subset analysis of the participants who exhibited a meaningful decrease in core stability using the same statistical approach, leaving out the participants who did not exhibit decreased core stability.
Results
Biomechanical Running Parameters
The mean changes in biomechanical running parameters for all 25 runners from pre- to post-CSKP and the mixed model effects are shown in Table 1.
Table 1.
Least-squares means for kinetic parameters of interest pre-CSKP and post-CSKP for all participants based on the mixed-effects model. Results are expressed as the mean (standard deviation). The p-value indicates the significance of the change. Effect sizes were estimated based on the change values (post-pre).
| ALL PARTICIPANTS (N=25) | Vertical Impact Peak [BW] | Vertical Average Loading Rate [BW/s] | Peak Knee Flexion Moment [%BW*h] | Peak Knee Adduction Moment [%BW*h] | Knee Adduction Impulse [%BW*h*s] | Peak Hip Adduction Moment [%BW*h] | Hip Adduction Impulse [%BW*h*s] |
|---|---|---|---|---|---|---|---|
| Pre-CSKP | 1.74 (0.32) | 59.6 (23.5) | 13.4 (2.65) | 7.52 (2.2) | 0.9 (0.3) | 10.93 (2.78) | 1.52 (0.51) |
| Post-CSKP | 1.74 (0.37) | 55.1 (16.9) | 13.92 (2.58) | 7.45 (2.17) | 0.87 (0.28) | 10.95 (2.96) | 1.49 (0.49) |
| p-value | 0.836 | 0.037 | 0.021 | 0.636 | 0.154 | 0.912 | 0.104 |
| Effect Size | 0.04 | −0.44 | 0.49 | −0.1 | −0.29 | 0.02 | −0.34 |
Significant effects (p<0.05) are bolded. All GRF loading variables were normalized to participant body weight (BW) and moments and impulses were normalized to BW and height (h).
A significant positive effect of the CSKP was found on peak knee flexion (pKF) moment during running, indicating that running in a state of reduced core stability and/or core muscle fatigue (post-CSKP) resulted in a small-to-medium increase in the pKF moment during stance. Additionally, the CSKP was found to have a significant negative effect on vertical average loading rate (VALR) during the weight acceptance phase of running, indicating that running in a state of reduced core stability and/or core muscle fatigue (post-CSKP) resulted in a small-to-medium reduction in VALR during early stance. There was no evidence of an effect of the CSKP on any of the other running parameters investigated (all p>0.05).
Muscle Fatigue and Core Stability
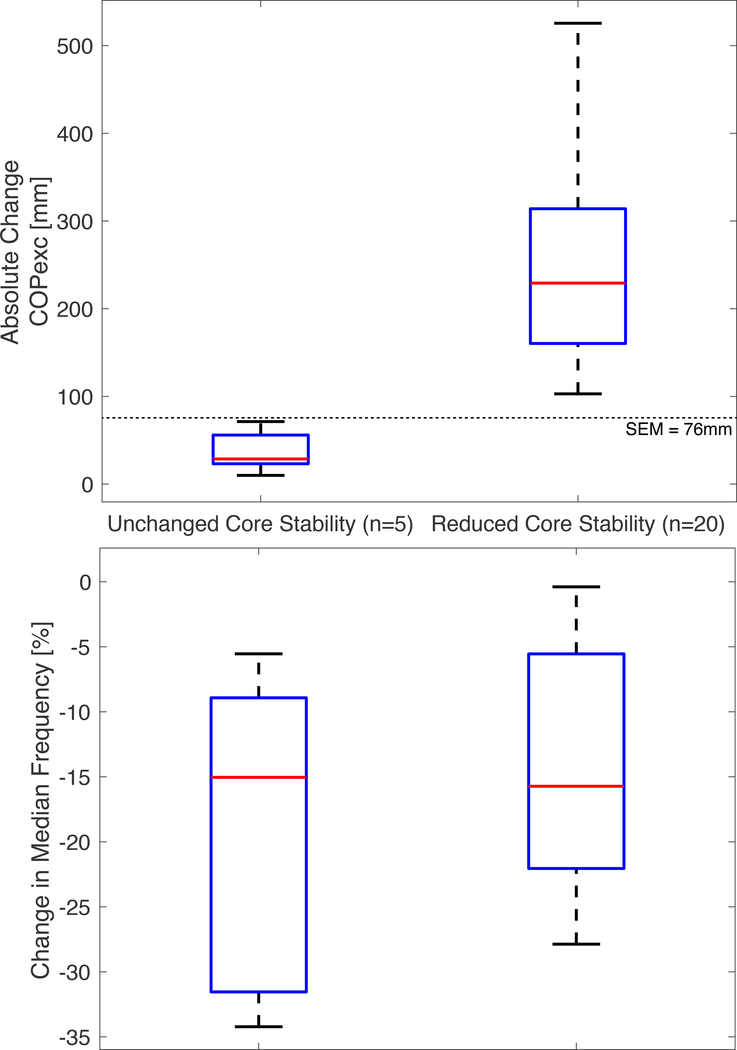
An absolute change in CoPexc > 76 mm corresponds to reduced stability and a negative change in MedF corresponds to muscle fatigue. Twenty of the 25 participants (21.9±3.1 years; 11 female; 1.73±0.09 m; 68.6±13.3 kg) exhibited a meaningful decrease in core stability post-CSKP, while five did not (30.4±12.5 years; 2 female; 1.78±0.06 m; 72.7±9.7 kg). Figure 4 shows the distribution of CoPexc and MedF data for the group that experienced a reduction in core stability post-CSKP (N=20) and the group that did not (N=5). All participants across both sub-groups experienced a significant decrease in the mean core MedF (−15.8± 9.7%, p<0.0001), indicating that the CSKP elicited a moderate level of muscle fatigue.(33)
Figure 4.
Distributions of CoPexc and MedF data for the group that experienced a reduction in core stability post-CSKP (N=20) and the group that did not (N=5).
Sub-Analysis of Reduced Core Stability Group
Mixed-effect models were also created for each running parameter separately for the group that experienced reduced core stability post-CSKP (N=20). Table 2 shows the mixed-model results for this sub-group, with only the peak knee flexion moment showing a significant increase (p=0.025). Vertical average loading rate and knee adduction impulse both showed a trend toward a reduction (both p<0.10).
Table 2.
Least-squares means for kinetic parameters of interest pre-CSKP and post-CSKP for the participants who experienced a decrease in core stability (N=20) based on the mixed-effects model. Results are expressed as the mean (standard deviation). The p-value indicates the significance of the change. Effect sizes were estimated based on the change values (post-pre).
| REDUCED CORE STABILITY PARTICIPANTS (N=20) | Vertical Impact Peak [BW] | Vertical Average Loading Rate [BW/s] | Peak Knee Flexion Moment [%BW*h] | Peak Knee Adduction Moment [%BW*h] | Knee Adduction Impulse [%BW*h*s] | Peak Hip Adduction Moment [%BW*h] | Hip Adduction Impulse [%BW*h*s] |
|---|---|---|---|---|---|---|---|
| Pre-CSKP | 1.72 (0.33) | 60.0 (21.0) | 13.61 (2.57) | 7.3 (2) | 0.87 (0.29) | 10.62 (2.95) | 1.49 (0.54) |
| Post-CSKP | 1.76 (0.38) | 55.8 (14.8) | 14.21 (2.53) | 7.18 (1.92) | 0.83 (0.27) | 10.74 (3.2) | 1.47 (0.54) |
| p-value | 0.213 | 0.083 | 0.025 | 0.311 | 0.058 | 0.582 | 0.209 |
| Effect Size | 0.29 | −0.41 | 0.54 | −0.23 | −0.45 | 0.13 | −0.29 |
Significant effects (p<0.05) are bolded. All GRF loading variables were normalized to participant body weight (BW) and moments and impulses were normalized to BW and height (h).
Discussion
In a group of novice runners, experimentally-induced core muscle fatigue and decreased core stability were associated with altered running mechanics previously associated with increased running injury risk (Table 2), with significant changes observed in both pKF moment (ES=0.49) and vertical average loading rate (ES=0.44). However, these changes were in opposite directions relative to previous reports of running injury risk. As the external pKF moment during stance must be balanced by the quadriceps femoris, an increase in this moment results in a compensatory increase in the quadriceps femoris workload directly increasing the load placed on the patellofemoral joint, which may consequently lead to PFPS.(34, 35) This effect was significant across the entire group of 25 subjects, showing that core muscle fatigue induced changes in the ground reaction force or in lower extremity kinematics that resulted in higher-risk patellofemoral joint loading.
The CSKP also resulted in a decreased vertical average loading rate, which was contrary to our hypothesis. A higher VALR has been associated with an increased risk of tibial stress fractures, perhaps through a mechanism of increased tibial shock leading to bone microdamage.(11) Our results demonstrate that in the presence of core muscle fatigue, this group of healthy novice runners adopted running mechanics that could be protective of the tibia. However, it is important to note that the significance of this change was actually driven by the runners who did not experience a decrease in core stability. Within the 20 runners who experienced a meaningful decrease in core stability, the change in VALR was not significant, though it did demonstrate a trend towards a reduction with a medium effect size (ES=0.41, p=0.083).
While not statistically significant, the CSKP was also associated with a trend towards decreased KAdd impulse in the group of 20 participants whose core stability was reduced (ES=0.45, p=0.058). A decrease in the KAdd impulse is indicative of reduced cumulative frontal plane loading of the knee, possibly reducing PFPS injury risk.(14) Future study is necessary to examine whether participants adapt their gait to limit frontal plane knee loading or GRF loading characteristics during running with reduced core stability.
The specific magnitude of increased pKF moment during running that may elicit PFPS has not been established, but an increase in this biomechanical variable is known to increase patellofemoral joint stress. Since patellofemoral joint stress is difficult to measure in vivo, the external pKF moment is commonly used as a surrogate measure since the joint load directly increases with this moment.(36) There is in-vitro evidence that increased repetitive stress placed on the patella accelerates retropatellar cartilage damage.(37) A biomechanical model was used to estimate a 2x greater peak patellofemoral joint stress during walking in participants with PFP versus controls (6.61 MPa vs 3.13 MPa),(38) and the same model was used to estimate greater peak patellofemoral joint stress when shod versus barefoot (20.6 MPa vs 18.2 MPa).(29) Comparing to interventions designed to alter pKF moment, the CSKP used in the present study resulted in a medium effect size of 0.49, a larger effect than the 0.39 effect on pKF moment of decreasing cadence by 10% over preferred cadence as reported by Heiderscheit et al.(28). On the other hand, switching from shod to barefoot running has been observed to result in a larger effect size of 0.7 in pKF moment as reported by Bonacci et al.(29)
There are limitations that must be considered when interpreting the current results. The CSKP did not result in a meaningful decrease in core stability for five of our participants, possibly due to a reliance on volitional exhaustion during the CSKP causing greater-than-desired variability in core muscles’ force production or potential confounding by cognitive fatigue. An increased cognitive load has been associated with increased knee abduction during a landing task,(39) so cognitive fatigue induced by exhaustive physical exercise could play a role in the observed changes. Additionally, an individual’s response to fatigue may depend on many factors. Research has shown healthy individuals can maintain upper extremity movement stability in the presence of shoulder muscle fatigue.(40)
A second limitation to our CSKP was that muscle fatigue and changes in core stability happened together, so it is challenging to separate out the effect of muscle fatigue from the inability to stabilize the core. However, we believe that this limitation was minimized by the amount of time taken to perform the post-CSKP quiet sitting test and the running biomechanics measurement. As mentioned above, 4.5±1.2 min elapsed from the end of the last exercise repetition of the CSKP to the beginning of the QST, which lasted for 3 minutes. Several seconds subsequently elapsed while the participant was put in position to begin running again. Therefore, muscle recovery should have minimized the acute fatigability effects on muscle force production during the running trials, and remaining differences should be primarily attributable to altered motor control as a consequence of the CSKP rather than on reduced force generation capacity within the muscles.(41)
Lastly, the standard limitations of skin-based passive marker motion capture systems apply, though the use of a redundant marker set with the point-cluster algorithm to reduce the influence of soft tissue artifact(42) and a within-participant research design should have minimized the effects of these limitations.
Conclusion
An experimentally-induced decrease in core stability in novice runners caused an increased pKF moment during the stance phase of running (Table 2). Therefore, insufficient core stability in novice runners may be a risk factor for PFPS. This study’s results support the emphasis on core stability training interventions as a valuable adjunct for runners. While underpowered to make definitive conclusions, the result that some individuals can maintain core stability in the presence of core muscle fatigue while most do not supports the concept that merely increasing core muscle endurance may be inadequate to improve core stability. Moreover, it appears that some individuals’ running gait and seated balance may be more sensitive to fatigue than others. Further studies are needed to determine whether interventions aimed at improving core stability can alter the biomechanical variables in this study known to increase risk for PFPS.
Acknowledgements
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. This work was supported by funding from NIH R03AR065215 & UL1TR002733 and NSF GRFP DGE-1343012. The results of the present study are solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor do they constitute endorsement by ACSM.
References
- 1.van Mechelen W Running injuries. A review of the epidemiological literature. Sports Med. 1992;14(5):320–35. [DOI] [PubMed] [Google Scholar]
- 2.Tonoli C, Cumps E, Aerts I, Verhagen E, Meeusen R. Incidence, risk factors and prevention of running related injuries in long-distance running: A systematic review injury, location and type. Sport Geneeskd. Sport en Geneeskunde. 2010;43(5):12–8. [Google Scholar]
- 3.Shirazi-Adl A Strain in fibers of a lumbar disc. Analysis of the role of lifting in producing disc prolapse. Spine (Phila Pa 1976). 1989;14(1):96–103. [DOI] [PubMed] [Google Scholar]
- 4.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. British Journal of Sports Medicine. 2003;37(3):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clinical Biomechanics. 2011;26(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodges PW, Richardson CA. Contraction of the Abdominal Muscles Associated with Movement of the Lower Limb. Physical Therapy. 1997;77(2):132–44. [DOI] [PubMed] [Google Scholar]
- 7.Willson J, Dougherty C, Ireland M, Davis I. Core stability and its relationship to lower extremity function and injury. The Journal of the American Academy of Orthopaedic Surgeons. 2005;13(5):316–25. [DOI] [PubMed] [Google Scholar]
- 8.Rojhani Shirazi Z, Biabani Moghaddam M, Motealleh A. Comparative evaluation of core muscle recruitment pattern in response to sudden external perturbations in patients with patellofemoral pain syndrome and healthy subjects. Arch Phys Med Rehabil. 2014;95(7):1383–9. [DOI] [PubMed] [Google Scholar]
- 9.Raabe ME, Monfort SM, Best TM, Onate J, Chaudhari AMW. Development of a Novel Protocol to Temporarily Reduce Core Stability. In: Proceedings of the 2015 American Society of Biomechanics Annual Meeting 2015: Columbus, OH, USA. [Google Scholar]
- 10.Jamison ST, Pan X, Chaudhari AM. Knee moments during run-to-cut maneuvers are associated with lateral trunk positioning. J Biomech. 2012;45(11):1881–5. [DOI] [PubMed] [Google Scholar]
- 11.Davis IS, Bowser BJ, Mullineaux DR. Greater vertical impact loading in female runners with medically diagnosed injuries: a prospective investigation. British Journal of Sports Medicine. 2015;50(14):887–92. [DOI] [PubMed] [Google Scholar]
- 12.James SL. Running injuries to the knee. Journal of the American Academy of Orthopaedic Surgeons. 1995;3(6):309–18. [DOI] [PubMed] [Google Scholar]
- 13.Andriacchi TP, Johnson TS, Hurwitz DE, Natarajan RN. Musculoskeletal Dynamics, Locomotion, and Clinical Applications In: Mow VC, Huiskes R editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Philadelphia, PA: Lippincott Williams & Wilkins; 2005, pp. 91–122. [Google Scholar]
- 14.Stefanyshyn DJ, Stergiou P, Lun VM, Meeuwisse WH, Worobets JT. Knee angular impulse as a predictor of patellofemoral pain in runners. Am J Sports Med. 2006;34(11):1844–51. [DOI] [PubMed] [Google Scholar]
- 15.MacMahon J, Chaudhari A, Andriacchi T. Biomechanical injury predictors for marathon runners: striding toward iliotibial band syndrome injury prevention. In: Proceedings of the XXVIII International Symposium of Biomechanics in Sports 2000: Hong Kong. [Google Scholar]
- 16.Chaudhari AM, Jamison ST, Best TM. Proximal risk factors for ACL injury: role of core stability In: FR Noyes, SD Barber-Westin editors. ACL Injuries in the Female Athlete: causes, impacts, and conditioning programs. Berlin: Springer; 2018, pp. 189–205. [Google Scholar]
- 17.McGill S, Juker D, Kropf P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. Journal of Biomechanics. 1996;29(11):1503–7. [DOI] [PubMed] [Google Scholar]
- 18.De Luca CJ. Myoelectrical manifestations of localized muscular fatigue in humans. Critical reviews in biomedical engineering. 1983;11(4):251–79. [PubMed] [Google Scholar]
- 19.Madigan ML, Davidson BS, Nussbaum MA. Postural sway and joint kinematics during quiet standing are affected by lumbar extensor fatigue. Hum Mov Sci. 2006;25(6):788–99. [DOI] [PubMed] [Google Scholar]
- 20.Cholewicki J, Polzhofer GK, Radebold A. Postural control of trunk during unstable sitting. Journal of Biomechanics. 2000;33(12):1733–7. [DOI] [PubMed] [Google Scholar]
- 21.Hendershot BD, Nussbaum MA. Persons with lower-limb amputation have impaired trunk postural control while maintaining seated balance. Gait Posture. 2013;38(3):438–42. [DOI] [PubMed] [Google Scholar]
- 22.van der Burg JC, van Wegen EE, Rietberg MB, Kwakkel G, van Dieen JH. Postural control of the trunk during unstable sitting in Parkinson’s disease. Parkinsonism Relat Disord. 2006;12(8):492–8. [DOI] [PubMed] [Google Scholar]
- 23.van Dieen JH, Luger T, van der Eb J. Effects of fatigue on trunk stability in elite gymnasts. Eur J Appl Physiol. 2012;112(4):1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafond D, Corriveau H, Hebert R, Prince F. Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch Phys Med Rehabil. 2004;85(6):896–901. [DOI] [PubMed] [Google Scholar]
- 25.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions on Biomedical Engineering. 1996;43(9):956–66. [DOI] [PubMed] [Google Scholar]
- 26.Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724–30. [DOI] [PubMed] [Google Scholar]
- 27.Raabe ME, Teater R, Chaudhari AMW. Intra- and Inter-Rater Reliability of a Novel Core Stability Test. In: Proceedings of the 2016 American Society of Biomechanics Annual Meeting 2016: Raleigh, NC, USA. [Google Scholar]
- 28.Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc. 2011;43(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonacci J, Vicenzino B, Spratford W, Collins P. Take your shoes off to reduce patellofemoral joint stress during running. British Journal of Sports Medicine. 2014;48(6):425. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal R Meta-Analytic Procedures for Social Research. Thousand Oaks, California: Sage Publications, Inc.; 1991. [Google Scholar]
- 31.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988, 567 p. [Google Scholar]
- 32.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(863):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart JM, Fritz JM, Kerrigan DC, Saliba EN, Gansneder BM, Ingersoll CD. Reduced Quadriceps Activation After Lumbar Paraspinal Fatiguing Exercise. Journal of Athletic Training. 2006;41(1):79–86. [PMC free article] [PubMed] [Google Scholar]
- 34.Besier TF, Gold GE, Beaupre GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37(11):1924–30. [DOI] [PubMed] [Google Scholar]
- 35.Chen YJ, Powers CM. Comparison of three-dimensional patellofemoral joint reaction forces in persons with and without patellofemoral pain. J Appl Biomech. 2014;30(4):493–500. [DOI] [PubMed] [Google Scholar]
- 36.Scott SH, Winter DA. Internal forces of chronic running injury sites. Med Sci Sports Exerc. 1990;22(3):357–69. [PubMed] [Google Scholar]
- 37.Zimmerman NB, Smith DG, Pottenger LA, Cooperman DR. Mechanical disruption of human patellar cartilage by repetitive loading in vitro. Clin Orthop Relat Res. 1988;(229):302–7. [PubMed] [Google Scholar]
- 38.Heino Brechter J, Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Exerc. 2002;34(10):1582–93. [DOI] [PubMed] [Google Scholar]
- 39.Méjane J, Faubert J, Duchêne S, Labbe DR. Evaluating the effect of a perceptual-cognitive task on landing biomechanics of the lower limb. In: Proceedings of the XXXIV International Conference of Biomechanics in Sport 2016: Tsukuba, Japan. [Google Scholar]
- 40.Gates DH, Dingwell JB. The effects of muscle fatigue and movement height on movement stability and variability. Exp Brain Res. 2011;209(4):525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean SG, Samorezov JE. Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009;41(8):1661–72. [DOI] [PubMed] [Google Scholar]
- 42.Andriacchi TP, Alexander EJ, Toney MK, Dyrby C, Sum J. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. Journal of Biomechanical Engineering. 1998;120(6):743–9. [DOI] [PubMed] [Google Scholar]