Abstract
Purpose:
To identify predictors of favourable changes to postprandial insulin and glucose levels in response to interrupting prolonged sitting time with standing or light intensity physical activity.
Methods:
Data were combined from four similarly designed randomised acute cross-over trials (n=129; BMI range 19.6 to 44.6kg/m2; South Asian=31.0%; dysglycaemia=27.1%). Treatments included: prolonged sitting (6.5hours) or prolonged sitting broken-up with either standing or light-intensity physical activity (5 minutes every 30 minutes). Time-averaged postprandial responses for insulin and glucose were calculated for each treatment (mean±95% CI). Mutually adjusted interaction terms were used to examine whether anthropometric (BMI), demographic (age, sex, ethnicity (white European vs. South Asian)) and a cardiometabolic variable (HOMA-IR) modified responses.
Results:
Postprandial insulin and glucose were reduced when individuals interrupted prolonged sitting with bouts of light physical activity, but not with standing. Reductions in time-averaged postprandial insulin were more pronounced if individuals were South Asian compared with white European (−18.9mU/L (−23.5%) vs. −8.2mU/L (−9.3%)), female compared to male (−15.0mU/L (−21.2%) vs. −12.1mU/L (−17.6%)) or had a BMI ≥27.2kg/m2 (−20.9mU/L (−22.9%) vs. −8.7mU/L (−18.2%)). Similarly, being female (−0.4mmol/L (−0.6mmol/L, −0.2mmol/L) (−6.8%) vs. –0.1mmol/L (−0.3mmol/L, 1mmol/L) (−1.7%)) or having a BMI ≥27.2kg/m2 (−0.4mmol/L (−0.6mmol/L, −0.2mmol/L) (−6.7%) vs. –0.2mmol/L (−0.4mmol/L, 0.0mmol/L) (−3.4%)) modified the postprandial glucose response. No significant interactions were found for HOMA-IR or age.
Conclusion:
Being female, South Asian or having a higher BMI, all predicted greater reductions in postprandial insulin, while being female and having a higher BMI predicted greater reductions in postprandial glucose when sitting was interrupted with light physical activity. These results could help to guide personalised interventions in high-risk participants for whom breaking prolonged sitting time with light activity may yield the greatest therapeutic potential.
Keywords: postprandial, physical activity, sedentary behaviour, risk factors, insulin, glucose
Introduction
Postprandial hyperglycaemia plays a significant role in the development of cardiovascular disease (CVD) in people with type 2 diabetes mellitus (T2DM) (1). The postprandial phase is characterised by a rapid and large increase in blood glucose and insulin levels. Observational evidence suggests that postprandial hyperglycaemia, even in the absence of fasting hyperglycaemia, is associated with higher risks of future cardiometabolic disease (2, 3). Similarly, a hyperinsulinaemic response is closely associated with a number of CVD and T2DM related outcomes (4). Therefore, if these links are in part causal, establishing effective and pragmatic interventions that reduce post‐meal hyperglycaemic and hyperinsulinaemic excursions could be important therapeutic targets for the prevention of T2DM and CVD, particularly as individuals spend a large proportion of the day in a postprandial state (5).
Physical activity is known to enhance health and improve postprandial hyperglycaemia (6). Current physical activity guidelines recommend that adults engage in at least ≥150 minutes of moderate intensity physical activity or ≥75 minutes of vigorous activity and 2–3 resistance exercise sessions per week (7). In addition, current physical activity guidelines now include specific recommendations to reduce and interrupt prolonged sitting (6, 8). These guidelines have been informed by emerging research suggesting that sitting time per se is an independent risk factor for cardiometabolic morbidity and mortality (9, 10). Over recent years, epidemiological research has been complemented by acute experimental studies showing that breaking up bouts of prolonged sitting with standing or light intensity activity elicits significant benefits on markers of metabolic health (11–15).
These results are important as light intensity activities are behaviourally more ubiquitous than moderate to vigorous physical activity (MVPA) and may therefore be appealing interventional targets in the promotion of metabolic health, whilst also being more culturally acceptable to high risk groups (e.g. South Asian women). However, the inter-individual variability in the effectiveness of such interventions is likely to be large. For example, previous experimental research has shown that the magnitude of postprandial dysglycaemia in response to prolonged sitting and the subsequent reduction following breaks may differ considerably according to ethnicity or the degree of underlying insulin resistance (13, 16).
Therefore, in order to ensure future T2DM prevention strategies are stratified and targeted at those who could derive the greatest benefit, it is necessary to determine the factors that may predict a favourable response to breaking up prolonged sitting with a low intensity intervention. As such, the aim was to determine whether commonly measured demographic, anthropometric or clinical factors are associated with the postprandial insulin and glucose response when breaking up prolonged sitting, with short bouts of either standing or physical activity, at a light intensity.
Methods
Study design
We performed a pooled analysis of data collected from 129 individuals across four separate acute, randomised, crossover experimental studies conducted within the Leicester Diabetes Centre (University of Leicester) (n=99) and the University of Glasgow (n=30), UK (2015–2018); all of which followed the same protocols and standard operating procedures for data collection and the same treatment methodology of breaking sitting time with 5 minutes of standing or light physical activity every 30 minutes (see Figure, Supplemental Digital Content 1, protocols and standard operating procedures for data collection). The research design and methods have been published in detail elsewhere (11–14). Briefly, participants were recruited from studies previously conducted within the Leicester Diabetes Centre (ACUTE, ARMING HEALTH, STAND UP) or from the public via strategic placement and distribution of promotional materials (STAND UP, FIT2SIT). Detailed inclusion and exclusion criteria can be found in Supplementary Digital Content Table 1 (see Table, Supplemental Digital Content 2, Inclusion and exclusion criteria).
Participants attended up to four separate visits to their corresponding centre. One to two weeks after an initial familiarisation visit, participants were randomised to the following treatment conditions: 1) prolonged sitting (6.5 hours; plus 60 minute steady state); 2) prolonged sitting broken up with standing for 5 minutes every 30 minutes or 3) prolonged sitting broken up with physical activity (either walking or arm ergometry) for 5 minutes every 30 minutes. As an acute bout of physical activity may enhance insulin sensitivity for up to 48 hours, we used a minimum wash-out period of 7 days between each condition.
All studies were registered with clinicaltrials.gov (ACUTE: NCT02135172; STAND UP: NCT02453204; ARMING HEALTH: NCT02909894; FIT2SIT: NCT02493309). Written informed consent was obtained from all eligible participants and the individual studies had full ethical and governance approval.
Participants
In total, 147 participants were randomised. Causes of drop out between familiarisation and randomisation are detailed in Figure 1. A further 18 individuals were excluded after randomisation: due to cessation of the venous cannula line which resulted in less than 50% of data collection (n=11); illness (n=2); inability to tolerate the standardised meal (n=2), unable to commit time (n=2); or a change in personal circumstance (n=1). This left 129 participants that were included in the analysis.
Figure 1.
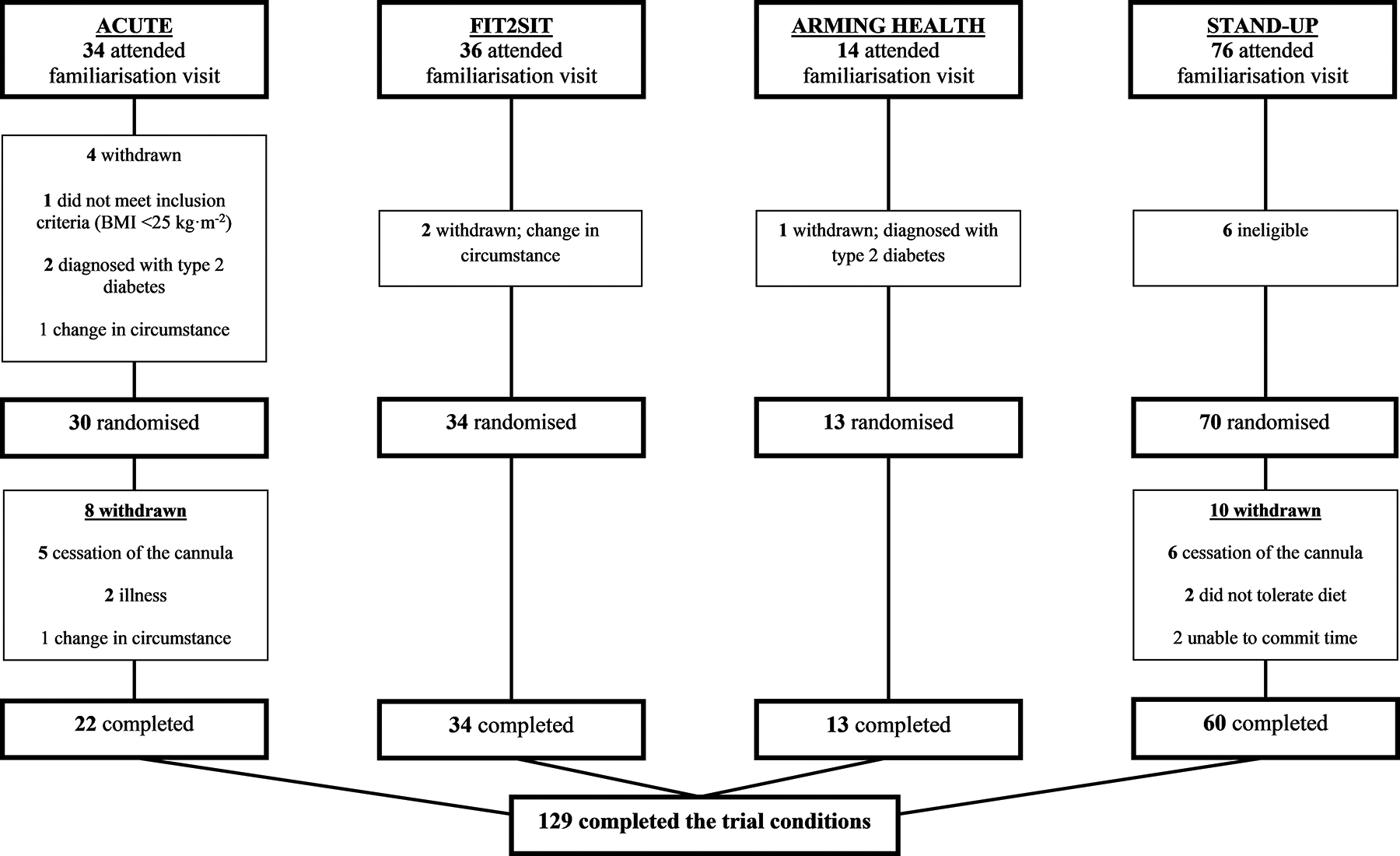
Study CONSORT Diagram.
Familiarisation visit
Before participating in the experimental protocol, participants visited the Leicester Diabetes Centre or University of Glasgow for a familiarisation visit in which they were accustomed to the required power output for the arm ergometry or walking speed (self-perceived light intensity). Participants were instructed to walk at a pace they felt was comfortable and registered between 10 and 12 on the Borg RPE scale (17). Body mass (Tanita TBE 611, Tanita, West Drayton, UK) and height were measured, to the nearest 0.1kg and 0.5cm respectively. Information regarding demographic variables (age and ethnicity) was collected following an interview administered protocol. For the ACUTE and ARMING HEALTH studies, non-diabetic hyperglycaemia was defined as 2-h post challenge glucose ≥7.8 mmol/L to <11.1 mmol/L after a standard oral glucose tolerance test or HbA1c 39–46 mmol/mol (5.7%−6.4%) inclusive (18), identified within the 12 months prior to the initial invitation letter being sent (see Table, SDC 1, inclusion and exclusion criteria).
Experimental treatment overview
Participants were asked to record all food and drink consumed the day before the first experimental condition. They were then asked to replicate this diet before subsequent treatments. Participants were also requested to avoid alcohol, caffeine and any MVPA for two days prior to each experimental condition (11–14).
Participants arrived at the laboratory after a 10-hour fast and had a cannula fitted into an accessible arm vein and then asked to sit quietly for 60 minutes. A fasting blood sample (9ml) was then taken (time point: 0 h) for the quantification of insulin and glucose. Participants were provided with a standardised breakfast that was typical of a westernised diet. Across the four studies, this consisted of 45.0±12.7% carbohydrate, 40.7±11.5% fat and 14.3±1.3% protein of energy intake (11–14). The time taken to consume the meal (≤15 minutes) was recorded and replicated in subsequent conditions. Blood was sampled at 30, 60, 120 and 180 minutes postprandially. Lunch, with an identical nutrient composition to breakfast, was consumed at 180 minutes with blood samples taken again at 30, 60, 120 and 210 minutes postprandially (see Figure, SDC 1, protocols for treatment conditions). The research staff supervised participants throughout each study cycle to ensure full compliance with the trial protocols. Participants consumed water ad libitum during the first of the experimental conditions and were asked to replicate the volume ingested in subsequent conditions.
Experimental conditions:
Figure SDC 1 highlights the experimental conditions undertaken during each of the four included studies (see Figure, SDC 1, protocols for treatment conditions).
Prolonged sitting (6.5 hours) (ACUTE, STAND UP, ARMING HEALTH, FIT2SIT)
All four studies included a prolonged sitting condition (11–14), where walking and standing was restricted (lavatory visits were conducted via a wheelchair). Participants sat in a designated room equipped with a chair, desk, laptop and access to books and magazines.
Standing: Sitting (total 5.5 hours) + Standing (total 60 minutes) (ACUTE, STAND UP)
Two studies employed a standing protocol (13, 14) which followed the same procedure as the sitting condition, except that participants were instructed to break their sitting time by standing close to their chair for 5 minutes, every 30 minutes. Individuals were asked to stand in the same, fixed position. In total, individuals accumulated 12 bouts (60 minutes) of standing.
Physical activity
Walking: Sitting (total 5.5 hours) + walking (total 60 minutes) (ACUTE, STAND UP, FIT2SIT)
Three studies employed a walking protocol (12–14) which was similar to the standing condition, but participants conducted 5-minute bouts of walking at a light intensity. Walking speed ranged from 1.5 to 4.4 km/h. In total, individuals accumulated 12 bouts (60 minutes) of walking. For the ACUTE and FIT2SIT trials, the walking breaks were carried out on a treadmill (Spazio Forma Folding Treadmill/Excite 700, TechnoGym U.K. Ltd., Bracknell, U.K). For the STAND UP trial participants were instructed to walk up and down a marked track in the laboratory.
Arm ergometry: Sitting (total 5.5 hours) + arm ergometry (total 60 minutes) (ARMING HEALTH)
One study employed upper body physical activity through arm ergometry (11). The power output (watts) necessary to elicit the desired energy expenditure during the main experimental condition (equivalent to walking at 3km/h) was established during the familiarisation visit (11). The subsequent power output was implemented for 5 minutes, every 30 minutes. In total, individuals accumulated 12 bouts (60 minutes) of arm ergometry.
Cardiometabolic variables
For the studies conducted solely at the Leicester Diabetes Centre (11, 12, 14), all samples were analysed within the same location. Plasma glucose was determined using standard enzymatic techniques with commercially available kits (Beckman, High Wycombe, UK) and using stable methodology standardized to external quality assurance reference values. Insulin and glucose samples underwent centrifugation to separate plasma within 15 minutes of collection. Plasma derived from insulin was stored at −80°C and analysed at the end of data collection using an enzyme immuno-assay (Mercodia, Uppsala, Sweden). Each sample was analysed in duplicate to ensure reliability of readings. Sample values with ≥20% variability were reanalysed.
All samples for STAND UP (13) were analysed at the University of Glasgow. Glucose was analysed using clinically validated automated biochemistry platforms (c311, Roche Diagnostics, Burgess Hill, UK). Insulin and glucose samples underwent identical preparation (centrifugation and storage) to the Leicester samples and were measured with an equivalent immunoassay platform (e411, Roche Diagnostics, Burgess Hill, UK). The analysers were calibrated and quality controlled using the manufacturer’s materials. Coefficient of variation over two levels of controls was less than 3% for biochemistry assays and less than 6% for insulin.
All measurements and analysis were undertaken by individuals blinded to experimental condition.
Statistical analyses
Missing outcome data for participants included in this analysis were imputed using a regression model with key predictor variables (baseline BMI, age, fasting values, ethnicity and treatment) for each time point and outcome. Imputation was used to correct for verification bias (19). Across all experimental conditions, 3.5% of data values (148/4248) were missing and imputed.
Generalised estimating equations (GEE) with an exchangeable correlation matrix were used, considering repeated measures across treatments. Due to the right-skewed distributions of positive values, insulin was analysed using a gamma distribution with an identity link. Total area under the curve (AUC) was first calculated by applying the trapezium rule and time-averaged AUC (i.e. AUC divided by the 6.5 hours, to give an average postprandial response) was then used as a summary measure for postprandial insulin and glucose, which can be interpreted as the average glucose or insulin concentration (not including the initial 60 minute steady state). Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated as fasting insulin (mU/L) x fasting glucose (mmol/L)/22.5, using baseline values. This model is commonly used as an index of insulin resistance and the validity of estimates in relation to gold standard measures has been examined in several epidemiological studies, in a wide variety of populations (20).
All models included, as independent variables, study and treatment (sitting, standing, light physical activity), along with age (continuous), sex, ethnicity, HOMA-IR (continuous) and BMI (continuous). In addition, interaction terms with treatment were entered simultaneously into the same model to investigate whether the effect of treatment was modified by anthropometric (BMI), demographic (age, sex, ethnicity) or cardiometabolic (HOMA-IR) variables independently to the other factors. Significant interactions were then stratified by dichotomous categories or using the median split.
To highlight the direction of significant interactions, modelling responses for insulin values were estimated in white European and South Asian males and females, aged 60, at BMI levels of 25kg/m2 (normal), 30kg/m2 (overweight) and 35kg/m2 (obese).
All data were analysed using SPSS (version 24.0). A p-value of <0.05 was considered statistically significant for main effects and p<0.1 for interactions. Descriptive data are reported as mean (95% CI) in text and tables, unless otherwise stated.
Sensitivity Analyses
In order to aid interpretation and assess the robustness of the outcome, we investigated whether results were affected by removing the ARMING HEALTH participants (n=13), as this protocol did not involve a change in posture. Furthermore, to ascertain whether factors that were found to modify the treatment effect for postprandial responses were driven by higher control values (postprandial response during the sitting condition), we repeated the main analysis after further adjusting for the postprandial response to prolonged sitting (categorised as low, medium or high derived through tertiles).
Results
129 participants were included in this analysis. Table SDC 3 shows the baseline anthropometric, cardiometabolic and demographic information. There were no significant differences in BMI, age, fasting or HOMA-IR values between those who dropped out and those who were included in this analysis (see Table, SDC 3, Metabolic, demographic and anthropometric characteristics).
Overall treatment effect
Table 1 displays the results for main effects of treatment. After adjustment for HOMA-IR, age, sex, BMI and ethnicity, the time-averaged insulin responses (reflecting average concentrations over the postprandial period) were 13.6mU/L ((95% CI) 9.5mU/L, 17.7mU/L) lower during light physical activity breaks compared with prolonged sitting. Similarly, time-averaged glucose responses were 0.3mmol/L (0.2mmol/L, 0.4mmol/L) lower in the light physical activity condition vs. prolonged sitting after adjustment for the same variables. There was no treatment effect for standing breaks compared to prolonged sitting for insulin or glucose.
Table 1.
Time-averaged area under the curve values (main effects and 95% CI) and outcome x interaction terms for insulin and glucose responses during each treatment condition
| Variable | Sitting | Standing | Light Physical Activity | Ethnicity x treatment | Sex x treatment | Age x treatment | BMI x treatment | HOMA-IR x treatment |
|---|---|---|---|---|---|---|---|---|
| Insulin (mU/L) | 69.9 (63.6, 76.3) | 75.9 (66.9, 84.9) | 56.4 (50.7, 62.0)** | <0.001 | 0.043 | 0.149 | <0.001 | 0.240 |
| Glucose (mmol/L) | 5.9 (5.7, 6.1) | 5.9 (5.6, 6.1) | 5.6 (5.4, 5.8)** | 0.354 | 0.018 | 0.811 | <0.001 | 0.549 |
Covariates to derive the estimated marginal means are fixed at the following values: Age= 63.3years; HOMA-IR=2.35; BMI=27.7kg/m2. Values displayed as time-averaged response (95% CI).
p=<0.001 compared to the prolonged sitting condition.
Impact of demographic (ethnicity, age, sex), anthropometric (BMI) and cardiometabolic (HOMA-IR) variables: Interaction and stratified analyses
The results for interactions are presented in Table 1. Figure 2a, 2b and Table SDC 4 display the stratified analysis for both insulin and glucose (see Table, SDC 4, stratified analysis for insulin and glucose responses during each treatment condition).
Figure 2.
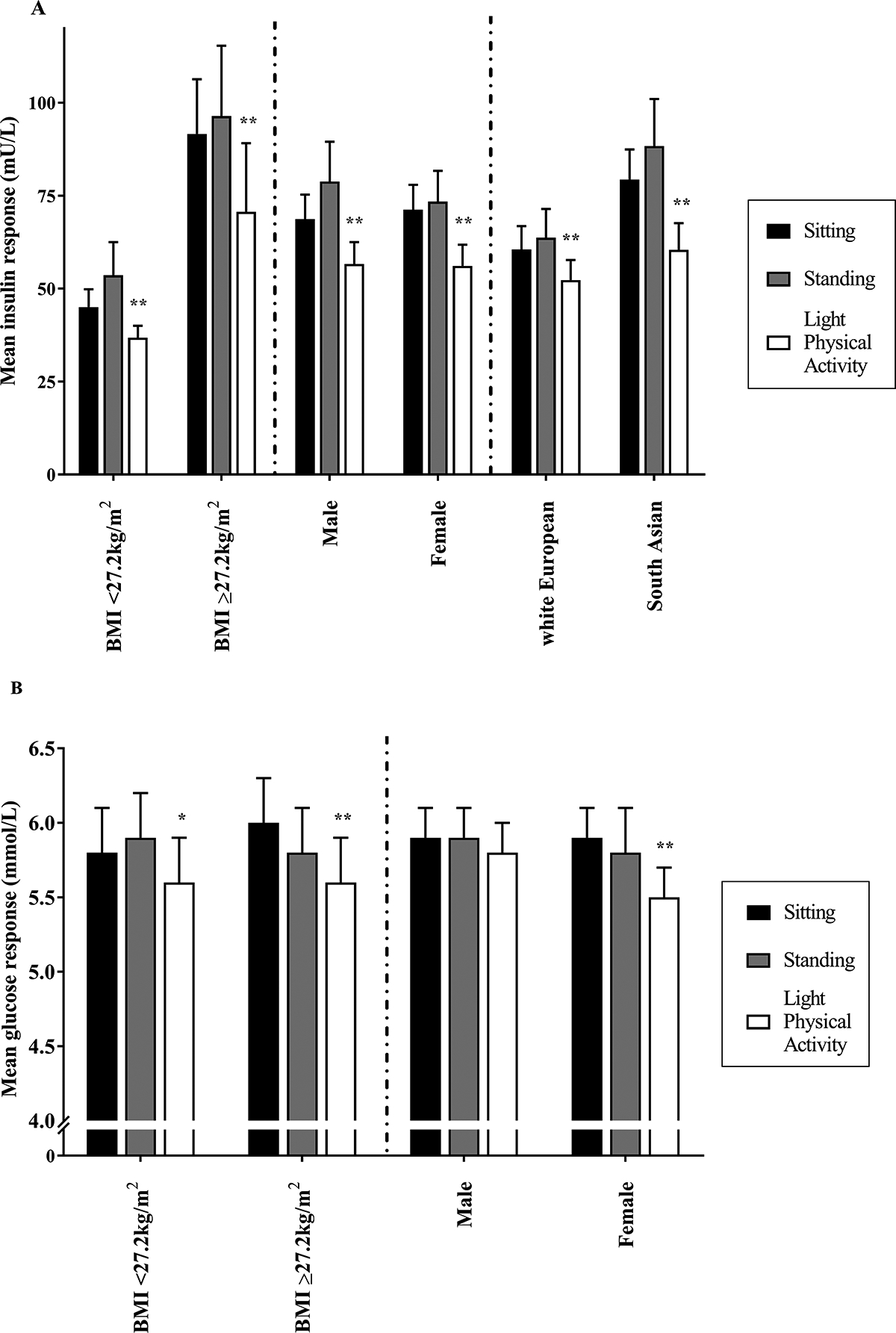
Stratified analysis for insulin (A) and glucose (B) responses during each treatment condition. **p=<0.001, *p=<0.05 compared to the prolonged sitting.
Ethnicity
There was an ethnicity x treatment interaction for insulin (p=<0.001) but not glucose (p=0.354). For South Asians, the insulin time-averaged response was 18.9mU/L (13.8mU/L, 24.1mU/L) (23.5%) lower during physical activity breaks compared to prolonged sitting, whereas for white Europeans the insulin response was 8.2mU/L (3.5mU/L, 13.0mU/L) (9.3%) lower.
BMI
Interactions were seen for both insulin and glucose (both p=<0.001). For those with a BMI above the median split (≥27.2kg/m2), the insulin response was reduced by 20.9mU/L (11.7mU/L, 30.0mU/L) (22.9%) during physical activity breaks compared to prolonged sitting. Those with a BMI<27.2kg/m2 demonstrated an 8.7mU/L (4.7mU/L, 12.7mU/L) (18.2%) reduction in insulin. A similar pattern was observed for glucose, where those with a BMI≥27.2kg/m2 gained a greater metabolic benefit following regular light physical activity breaks [−0.4mmol/L (−0.6mmol/L, −0.2mmol/L) (−6.7%) vs. –0.2mmol/L (−0.4mmol/L, 0.0mmol/L) (−3.4%)].
Sex
A sex x treatment interaction was seen for insulin (p=0.043) and glucose (p=0.018). For the insulin response, females reported a greater metabolic benefit when breaking prolonged sitting with light physical activity [−15.0mU/L (−20.0mU/L, −10.0mU/L, (−21.2%)], compared to males [−12.1mU/L (−15.9mU/L, −8.4mU/L) (−17.6%)]. For glucose, females also displayed a greater reduction than men when breaking up prolonged sitting with light physical activity [(−0.4mmol/L (−0.6mmol/L, −0.2mmol/L) (−6.8%) vs. –0.1mmol/L (−0.3mmol/L, 1mmol/L) (−1.7%)].
Age
There was no age x treatment interaction for insulin (p=0.149) or glucose (p=0.811).
HOMA-IR
There was no HOMA-IR x treatment interaction for insulin (p=0.240) or glucose (p=0.549).
Predicted response
Figure 3 and Table SDC 5 display how the predicted average difference between conditions for insulin changes as BMI increases for white European and South Asian, males and females, using given values for HOMA-IR (2.0) and age (60 years) (see Table, SDC 5, redicted insulin response stratified by sex, ethnic and BMI categories for a 60-year-old individual). The results demonstrate that the average blood insulin response for a 60 year old, South Asian female with a BMI of 35kg/m2 and HOMA-IR of 2.0, decreased from 90.3mU/L to 58.2mU/L (35.2% reduction) (from prolonged sitting to light physical activity breaks, respectively), whereas average responses for a 60 year old, white European male, with a BMI of 25kg/m2 decreased from 49.5mU/L to 45.1mU/L (8.9% reduction).
Figure 3.
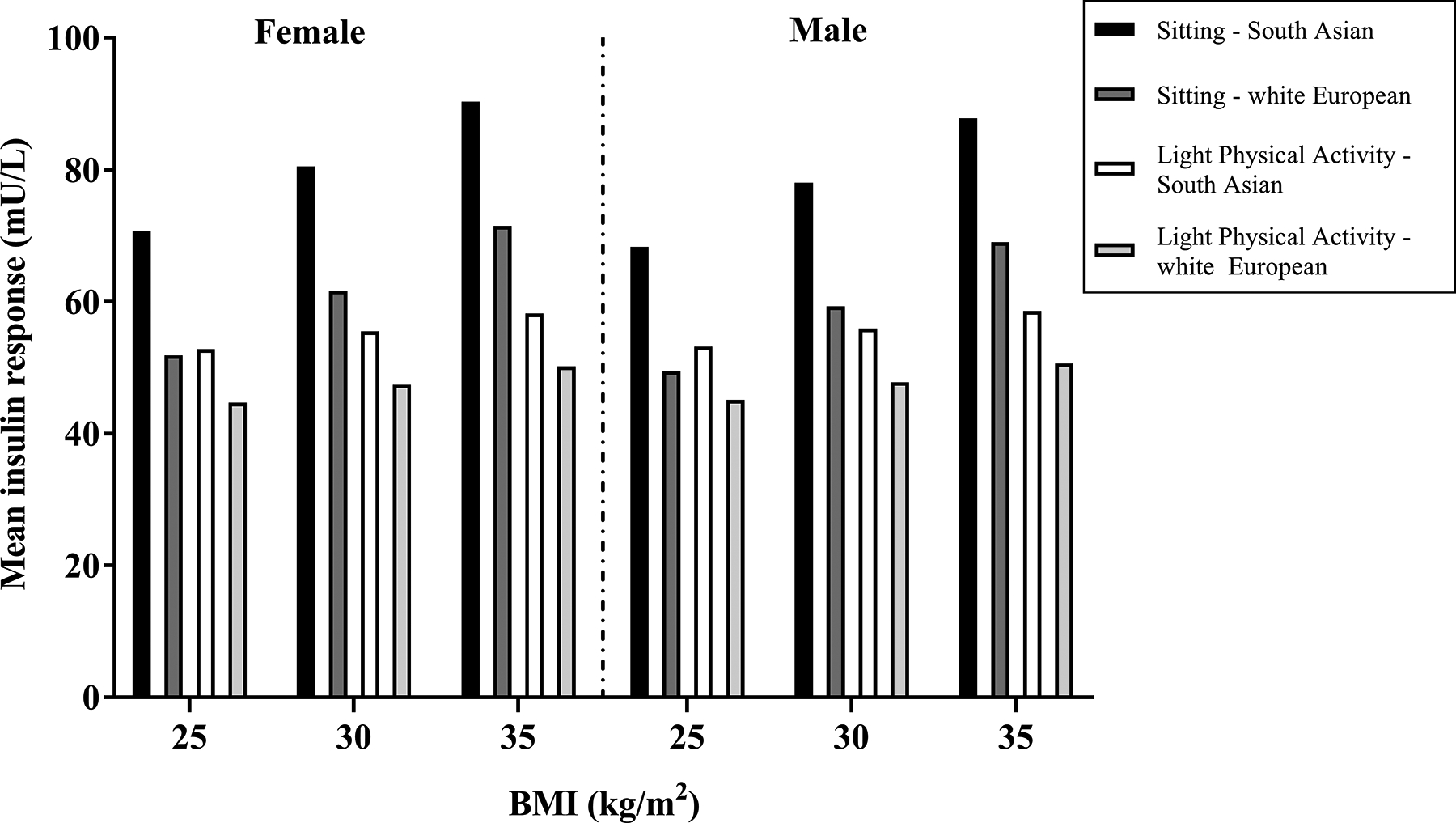
Predicted insulin response stratified by sex, ethnic and BMI categories for a 60-year-old individual.
Predicted insulin responses were calculated from the following, fully adjusted regression equation, derived from a single GEE model. The light-intensity physical activity condition includes a summation of the beta-coefficients for main outcomes and treatment x outcome interactions:
Sensitivity Analyses
The significance levels were largely unaffected when the ARMING HEALTH study was removed from the analysis. These results are presented in Table SDC 6 [see, Table, SDC 6, time-averaged area under the curve values (main effects) and outcome x interaction terms for insulin and glucose responses during each treatment condition – with the ARMING HEALTH participants removed (n=13)]. Furthermore, the pattern of results remained similar when additionally adjusting for the category of postprandial response during prolonged sitting. For insulin, the ethnicity (p=0.002) and BMI (p=0.021) x treatment interactions remained. However, the sex x treatment interaction was attenuated (p=0.124). For glucose, both the BMI (p=0.002) and sex (p=0.021) x treatment interactions persisted.
Discussion
This analysis demonstrates that laboratory studies regularly breaking prolonged sitting with light-intensity physical activity lead to acutely lower postprandial insulin and glucose levels. Furthermore, it illustrates that demographic (sex, ethnicity) and anthropometric (BMI) variables modify the insulin and glucose responses, with the results for ethnicity, BMI and sex (glucose only) being independent of the postprandial response to prolonged sitting. For insulin, being female, South Asian or having a higher BMI resulted in the greatest metabolic benefit when breaking prolonged sitting. For example, regular light intensity physical activity breaks for a 60-year-old South Asian female, with a BMI of 35kg/m2would lower insulin levels by more than a third (35.2%). In contrast, breaking prolonged sitting through regular physical activity breaks in a 60-year old white European male with a BMI of 25kg/m2 would only lower insulin levels by 8.9%.
These data build on previous work reporting potential differences in the postprandial response between white Europeans and South Asians and those with varying levels of underlying glycaemia (13). It has been well established that South Asians have a higher risk of cardiometabolic disease than white Europeans (21, 22), potentially driven by differences in body composition (23). For example, South Asians develop T2DM up to 12 years earlier than white Europeans and at lower BMI levels (24). Our results further illustrate that, a 60 year old South Asian female, with a BMI of 25kg/m2 would have a similar postprandial response during prolonged sitting to that of a 60-year-old white European female, with a BMI of 35kg/m2 (70.7mU/L vs. 71.5mU/L, respectively). Such findings are also broadly consistent with previous cross-sectional epidemiological data, which demonstrated that South Asians with a BMI of 22.6kg/m2 have equivalent prevalence of dysglycaemia to white Europeans with a BMI of 30kg/m2 (25). Nevertheless, despite South Asians having greater metabolic dysfunction, the results of our analysis suggest that they are likely to receive the greater absolute benefit per dose of light activity, which is also consistent with previous epidemiological and experimental work (13, 26).
In this analysis, females were also shown to derive the greatest metabolic benefit when breaking prolonged sitting with bouts of light physical activity. The sex difference observed in our results are broadly consistent with previous epidemiological work, which has demonstrated that associations between sedentary behaviour, total self-reported weekday sitting time and TV viewing time (a surrogate marker of total sitting time) with markers of cardiometabolic health are stronger in females (27, 28).
As all of the significant variables (sex, ethnicity, BMI) are central components to a number of inexpensive and easy to use risk assessment tools (29, 30), these variables may be used to further guide the identification of participants for whom breaking prolonged sitting time may yield the greatest benefit. Similar to individualised targets for HbA1c, these findings may also compliment a precision medicine approach, whereby T2DM prevention and treatment take into account individual variability in response to breaking prolonged sitting.
With such a low attainment of current physical activity guidelines (5–10% achieve 30 minutes per day of at least moderate-intensity physical activity, on at least 5 days per week based on accelerometer data) (31, 32), a reasonable goal may be to first break up sitting time with light intensity physical activity and then eventually progress to higher activity intensities. The intensity of light breaks in this analysis ranged from 1.5–4.4km/h, with no adverse events, suggesting that the individuals included in this analysis are able to tolerate small activity doses on a regular basis. This also includes the arm ergometry experimental condition, where participants remained in a seated posture throughout, thus offering a potential alternative strategy to breaking sitting time in wheelchair users or those with peripheral neuropathy. In addition, although the beneficial effects of physical activity are generally attributed to intensity (33), evidence from acute, experimental studies demonstrate that higher intensities with increasing frequency in breaks in prolonged sitting are not necessarily a synonym of better postprandial control (15, 34). Indeed, high and low intensities and frequencies in breaks, when matched for energy cost, produce similar effects on postprandial concentrations (34, 35). The exact timing of the onset of postprandial physical activity to break sitting time may also be important. The first bout of light physical activity in this analysis took place 30 minutes after the first meal (breakfast), which has been proposed as the optimal timing for post meal exercise as peak post meal values typically occur within 90 minutes (36). Initiating activity during this time window may blunt peak excursions, even when performed at very light intensities and in small doses (15).
We found no change in the glucose or insulin postprandial values for the standing condition, which is consistent with other acute, experimental studies (37). Nevertheless, replacing sitting with standing may still yield other health benefits. For example, a recent randomised controlled trial demonstrated that a decrease in occupational sitting time (−83 minutes/workday vs. control) at 12 months had a positive impact on multiple subjective outcomes such as job performance, work engagement, occupational fatigue, sickness presenteeism, musculoskeletal problems and quality of life (38). Importantly, the time spent sitting was largely displaced with standing, as stepping time remained unchanged.
The current analysis has strengths and limitations. We were able to provide rigorous estimates of the postprandial responses to breaking prolonged sitting, by using data combined from four laboratory‐based, randomised cross-over treatments that used the same experimental protocols. For example, meal timing, frequency of blood samples and duration and frequency of light physical activity breaks were identical across studies (see Figure, SDC 1, protocols for treatment conditions). This current analysis also displays a reasonable degree of heterogeneity as it includes both men and women, white Europeans and South Asians, as well as individuals of normal-weight and individuals with overweight/obesity, encompassing a broad continuum of postprandial responses. By their nature, the studies were proof of concept experimental studies and utilised protocols that may have limited population generalisability. Future studies should focus on whether the effects observed in this analysis are replicable under free living scenarios over a longer observation period. Furthermore, as there was no formal sample size calculation, p values are to be viewed with caution and in relation to the overall pattern of results.
Conclusion
The present findings suggest that standard demographic and anthropometric outcomes may predict the postprandial response to breaking up prolonged sitting with regular bouts of light intensity physical activity. Being female, South Asian or having a higher BMI, all predicted greater reductions in postprandial insulin, while being female and having a higher BMI predicted greater reduction in postprandial glucose. These results may be used to guide individualised tailored interventions in high risk participants for whom breaking prolonged sitting time could be a viable and effective prevention strategy.
Supplementary Material
SDC 1-Supplementary Digital Content Figure 1.doc
SDC 2-Supplementary Digital Content Table 1.doc (Inclusion and exclusion criteria)
SDC 3-Supplementary Digital Content Table 2.doc (Metabolic, demographic and anthropometric characteristics)
SDC 4-Supplementary Digital Content Table 3.doc (Stratified analysis for insulin and glucose responses during each treatment condition)
SDC 5-Supplementary Digital Content Table 4.doc (Predicted insulin response stratified by sex, ethnic and BMI categories for a 60-year-old individual)
SDC 6-Supplementary Digital Content Table 5.doc [Time-averaged area under the curve values (main effects) and outcome x interaction terms for insulin and glucose responses during each treatment condition - with the ARMING HEALTH participants removed (n=13)]
Acknowledgements
We thank Prof. Stuart J.H. Biddle, Prof. Alan J. Sinclair, Dr Keith Tolfrey and Dr Danielle Bodicoat for their support and oversight into the individual studies. We also thank the participants for their time and commitment.
Conflicts of Interest and Source of Funding
The research was supported by the UK Research Councils’ Lifelong Health and Wellbeing Initiative in partnership with the Department of Health (grant number MR/K025090/1) (STAND UP); the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre; the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care-East Midlands (NIHR CLAHRC-EM); and the Leicester Clinical Trials Unit. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. D.W.D is supported by a NHMRC Senior Research Fellowship (NHMRC APP1078360) and the Victorian Government’s OIS Program. There are no other conflicts of interest. The results of the present study do not constitute endorsement by the ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014. August 15;5(4):444–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007. September 1;100(5):899–904. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999. February;22(2):233–40. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Reaven GM. Insulin resistance and hyperinsulinemia: You can’t have one without the other. Diabetes Care. 2008. July;31(7):1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon TPJ, Eves FF, Laye MJ. Targeting postprandial hyperglycemia with physical activity may reduce cardiovascular disease risk. but what should we do, and when is the right time to move? Front Cardiovasc Med. 2018. July 18;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: A position statement of the american diabetes association. Diabetes Care. 2016. November;39(11):2065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation. Global recommendations on physical activity for health [Internet]. World Health Organisation 2010 cited 11/07/2019] 2010. https://www.who.int/dietphysicalactivity/publications/9789241599979/en/
- 8.Office of Disease Prevention and Health Promotion. Physical Activity Guidlines for Americans: 2nd Edition [Internet]. https://health.gov/paguidelines/second-edition/
- 9.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia. 2012. November;55(11):2895–905. [DOI] [PubMed] [Google Scholar]
- 10.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann Intern Med. 2015. January 20;162(2):123–32. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M, Edwardson CL, Davies MJ, et al. Breaking up sedentary time with seated upper body activity can regulate metabolic health in obese high-risk adults: A randomized crossover trial. Diabetes Obes Metab. 2017. December;19(12):1732–9. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy M, Edwardson CL, Davies MJ, et al. Fitness moderates glycemic responses to sitting and light activity breaks. Med Sci Sports Exerc. 2017. November;49(11):2216–22. [DOI] [PubMed] [Google Scholar]
- 13.Yates T, Edwardson CL, Celis-Morales C, et al. Metabolic effects of breaking prolonged sitting with standing or light walking in older south asians and white europeans: A randomized acute study. J Gerontol A Biol Sci Med Sci. 2018. November 7 Available from: 10.1093/gerona/gly252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henson J, Davies MJ, Bodicoat DH, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: A randomized acute study. Diabetes Care. 2016. January;39(1):130–8. [DOI] [PubMed] [Google Scholar]
- 15.Chastin SFM, De Craemer M, De Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019. March;53(6):370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey PC, Larsen RN, Winkler EAH, Owen N, Kingwell BA, Dunstan DW. Prolonged uninterrupted sitting elevates postprandial hyperglycaemia proportional to degree of insulin resistance. Diabetes Obes Metab. 2018. June;20(6):1526–30. [DOI] [PubMed] [Google Scholar]
- 17.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012. January;35 Suppl 1:S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen KJ, Donders AR, Harrell FE Jr, et al. Missing covariate data in medical research: To impute is better than to ignore. J Clin Epidemiol. 2010. July;63(7):721–7. [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004. June;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 21.Gholap N, Davies M, Patel K, Sattar N, Khunti K. Type 2 diabetes and cardiovascular disease in south asians. Prim Care Diabetes. 2011. April;5(1):45–56. [DOI] [PubMed] [Google Scholar]
- 22.Barnett AH, Dixon AN, Bellary S, et al. Type 2 diabetes and cardiovascular risk in the UK south asian community. Diabetologia. 2006. October;49(10):2234–46. [DOI] [PubMed] [Google Scholar]
- 23.Lear SA, Kohli S, Bondy GP, Tchernof A, Sniderman AD. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. 2009. December;94(12):4696–702. [DOI] [PubMed] [Google Scholar]
- 24.Paul SK, Owusu Adjah ES, Samanta M, et al. Comparison of body mass index at diagnosis of diabetes in a multi-ethnic population: A case-control study with matched non-diabetic controls. Diabetes Obes Metab. 2017. July;19(7):1014–23. [DOI] [PubMed] [Google Scholar]
- 25.Gray LJ, Yates T, Davies MJ, et al. Defining obesity cut-off points for migrant south asians. PLoS One. 2011;6(10):e26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celis-Morales CA, Ghouri N, Bailey ME, Sattar N, Gill JM. Should physical activity recommendations be ethnicity-specific? evidence from a cross-sectional study of south asian and european men. PLoS One. 2013. December 11;8(12):e82568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yates T, Khunti K, Wilmot EG, et al. Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. Am J Prev Med. 2012. January;42(1):1–7. [DOI] [PubMed] [Google Scholar]
- 28.Dunstan DW, Salmon J, Healy GN, et al. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007. March;30(3):516–22. [DOI] [PubMed] [Google Scholar]
- 29.Gray LJ, Taub NA, Khunti K, et al. The leicester risk assessment score for detecting undiagnosed type 2 diabetes and impaired glucose regulation for use in a multiethnic UK setting. Diabetic medicine : a journal of the British Diabetic Association. 2010. 08;27(8):887–95. [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence. Preventing type 2 diabetes: risk identification and interventions for individuals at high risk [Internet]. https://www.nice.org.uk/guidance/ph38
- 31.Health Survey for England. 2008: Physical activity and fitness [Internet]. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2008-physical-activity-and-fitness
- 32.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: Adults compliance with the physical activity guidelines for americans. Am J Prev Med. 2011. April;40(4):454–61. [DOI] [PubMed] [Google Scholar]
- 33.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: A meta-analysis. Diabetes Care. 2006. November;29(11):2518–27. [DOI] [PubMed] [Google Scholar]
- 34.Thorsen IK, Johansen MY, Pilmark NS, et al. The effect of frequency of activity interruptions in prolonged sitting on postprandial glucose metabolism: A randomized crossover trial. Metabolism. 2019. July;96:1–7. [DOI] [PubMed] [Google Scholar]
- 35.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc. 2010. February;42(2):219–25. [DOI] [PubMed] [Google Scholar]
- 36.Haxhi J, Scotto di Palumbo A, Sacchetti M. Exercising for metabolic control: Is timing important? Ann Nutr Metab. 2013;62(1):14–25. [DOI] [PubMed] [Google Scholar]
- 37.Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015. May;18(3):294–8. [DOI] [PubMed] [Google Scholar]
- 38.Edwardson CL, Yates T, Biddle SJH, et al. Effectiveness of the stand more AT (SMArT) work intervention: Cluster randomised controlled trial. BMJ. 2018. October 10;363:k3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1-Supplementary Digital Content Figure 1.doc
SDC 2-Supplementary Digital Content Table 1.doc (Inclusion and exclusion criteria)
SDC 3-Supplementary Digital Content Table 2.doc (Metabolic, demographic and anthropometric characteristics)
SDC 4-Supplementary Digital Content Table 3.doc (Stratified analysis for insulin and glucose responses during each treatment condition)
SDC 5-Supplementary Digital Content Table 4.doc (Predicted insulin response stratified by sex, ethnic and BMI categories for a 60-year-old individual)
SDC 6-Supplementary Digital Content Table 5.doc [Time-averaged area under the curve values (main effects) and outcome x interaction terms for insulin and glucose responses during each treatment condition - with the ARMING HEALTH participants removed (n=13)]


