Abstract
Objectives:
The aims of this observational study were to 1) accrue newly diagnosed patients with advanced-stage non-small cell lung cancer (NSCLC) awaiting the start of first-line treatment and identify those with moderate to severe depressive symptoms and, 2) provide a clinical description of the multiple, co-occurring psychological and behavioral difficulties and physical symptoms that potentially exacerbate and maintain depressive symptoms.
Materials and methods:
Patients with stage IV NSCLC (N = 186) were enrolled in an observational study (ClinicalTrials.gov Identifier: NCT03199651) and completed the American Society of Clinical Oncology-recommended screening measure for depression (Patient Health Questionnaire-9 [PHQ-9]). Individuals with none/mild (n = 119; 64 %), moderate (n = 52; 28 %), and severe (n = 15; 8 %) depressive symptoms were identified. Patients also completed measures of hopelessness, generalized anxiety disorder (GAD) symptoms, stress, illness perceptions, functional status, and symptoms.
Results:
Patients with severe depressive symptoms reported concomitant feelings of hopelessness (elevating risk for suicidal behavior), anxiety symptoms suggestive of GAD, and traumatic, cancer-specific stress. They perceived lung cancer as consequential for their lives and not controllable with treatment. Pain and multiple severe symptoms were present along with substantial functional impairment. Patients with moderate depressive symptoms had generally lower levels of disturbance, though still substantial. The most salient differences were low GAD symptom severity and fewer functional impairments for those with moderate symptoms.
Conclusions:
Depressive symptoms of moderate to severe levels co-occur in a matrix of clinical levels of anxiety symptoms, traumatic stress, impaired functional status, and pain and other physical symptoms. All of the latter factors have been shown, individually and collectively, to contribute to the maintenance or exacerbation of depressive symptoms. As life-extending targeted and immunotherapy use expands, prompt identification of patients with moderate to severe depressive symptoms, referral for evaluation, and psychological/behavioral treatment are key to maximizing treatment outcomes and quality of life for individuals with advanced NSCLC.
Keywords: Lung cancer, Depression, Anxiety, Quality of life, Illness perceptions
1. Introduction
Many facts about lung cancer are well-known: lung cancer persists as the number one cause of all cancer-related mortality worldwide [1]. The most prevalent type, non-small cell lung cancer (NSCLC), accounts for 85 % of all cases, and many individuals present with stage IV disease (44 %), with a 5-year survival estimate of 4.2 % [2]. What may be less well-known is that the case is compelling for patients with lung cancer being the most psychologically disabled of all cancer groups [3,4]. In fact, SEER registry data (3.5 million patients; 1973–2002) show the standardized suicide mortality ratio for patients with lung cancer is the highest of all cancer types, 5.74 (95 % CI = 5.30–6.22), with hazard ratios of 6.04 (95 % CI = 5.54–6.57) for men and 4.18 (95 % CI = 3.27–5.27) for women [5].
Studies comparing patients with cancer find that those with lung cancer have the greatest prevalence of mood disorders (est. 18 %) and anxiety disorders (est. 19 %) [6-8]. Studies assessing only patients with advanced NSCLC at diagnosis/prior to treatment find estimates of “probable cases” of depression to be 9 % in a patient sample (N = 461) from the United Kingdom [9], 17.9 % in a Canadian sample (N = 597; all types/stages) [6], and 32.9 % in a sample (N = 82) from Mexico [10]. Additional cases of “sub-clinical” or “borderline” symptom levels have ranged from 13 % [9] to 35.3 % [6]. Studies from the United States have predominantly assessed patients with lung cancer in the early weeks of treatment or thereafter and reveal estimates similar to those when patients are assessed at diagnosis. In them, rates of “severe” depressive symptoms have ranged from 14 % [11] to 41 % [12,13].
Irrespective of the occurrence of depressive symptoms, the days of diagnosis and awaiting treatment are unique. Reliably, cancer stress peaks during this period [14,15] and is associated with biobehavioral processes relevant to disease course, such as immunosuppression [16] and inflammation [17-19]. A meta-analytic study revealed stress assessed at some time after lung cancer diagnosis to heighten risk for premature cancer death (n = 23 studies; hazard ratio = 1.17; 95 % CI = 1.03–1.34) [20]. Potential contributors to the latter effects are the covariation of heightened stress with physical symptom exacerbation [21-26] and depressive symptoms [23,27].
With foundational data such as these, the American Society of Clinical Oncology (ASCO) recommended screening as the primary mechanism to determine the level/classification of symptom severity and asserted that screening should begin at diagnosis or start of treatment [28]. To date, the implementation of screening and use of the ASCO-recommended measures among patients with NSCLC is unknown. A comprehensive look at the characteristics of those with moderate to severe psychological symptoms among patients with NSCLC is needed. Even when patients are screened “positive,” the complete picture of difficulties and impairments that are likely to ac-company moderate to severe depressive symptoms is not clear. Research with other cancer types would suggest that there are co-occurring stressors and cognitive and behavioral disruptors, and they too may impede cancer patients’ coping with the diagnosis and decision-making as do depressive symptoms [29-32].
For this observational study, there were two aims. First, newly diagnosed patients with advanced-stage NSCLC who were awaiting the determination and start of treatment were accrued and administered the ASCO-recommended screening measure for depression (Patient Health Questionnaire-9 [PHQ-9] [28,33]) and supplementary measures. Individuals with symptoms of moderate (scores 15–19) and severe (scores 20–27) depressive levels were identified. Having identified these patients, the preeminent aim was to detail and discuss the co-occurring negative emotions (stress), impairments (quality of life, functional status, symptoms), and negative perceptions of one’s life and illness that co-occurred. To do this was significant because the latter factors foster the maintenance of depressive symptoms and, conversely, depressive symptoms increase the frequency/severity of these sources of impairment and disability [31,32,34-38]. Moving beyond PHQ-9 classification, the goal was provision of clinical descriptions for providers and researchers alike of the common psychological, behavioral, and symptom comorbidities experienced by such individuals, ones that, along with depressive symptoms, may impair patients’ coping and functioning when they are to make choices and begin lung cancer treatment [39].
2. Materials & methods
From June 2017 to August 2019, individuals were accrued from the Thoracic Oncology clinics of an NCI-designated Comprehensive Cancer Center (CCC) for participation in an observational study (ClinicalTrials.gov Identifier: NCT03199651; see Fig. 1 for study flow). Consent was completed face-to-face by research personnel in the clinic at the time of first appointment with a thoracic oncologist. Within two weeks of enrollment, patients were contacted by telephone by trained interviewers who conducted the assessment of patient-reported outcomes (see below). Patients were also given a “hard copy” measure booklet to follow along with the interview and item responses. Each patient received $15 for participation.
Fig. 1.
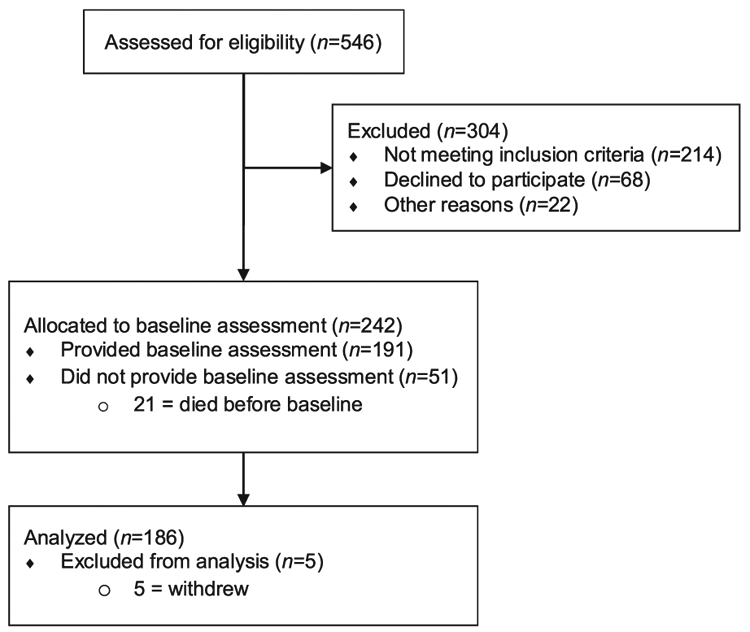
Flow diagram for the current observational study.
2.1. Eligibility criteria
Inclusion criteria were as follows: newly diagnosed stage IV NSCLC with pathological confirmation; any ECOG performance status and with any comorbidity; age > 18 years; English-speaking; and willing to provide access to medical records, provide biospecimens, and respond to self-report measures either in-person or by telephone interview. Exclusion criteria were as follows: patients to be treated with definitive chemo-radiotherapy; individuals age < 18 years; receiving treatment for advanced lung cancer for over one month before enrollment; and presence of disabling hearing, vision, or psychiatric impairments preventing consent or completion of self-report measures in English.
2.2. Variables and measures
2.2.1. Psychological symptoms
Three measures were used. 1) The Patient Health Questionnaire-9 (PHQ-9 [33]) is a 9-item self-report scale that assesses the frequency of symptoms of major depressive disorder, as defined by the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV [40]). Referencing the past two weeks, items were rated on a 4-point Likert scale (0=not at all to 3=nearly every day) and summed for a total score ranging from 0 to 27, with higher scores indicating higher depressive symptom severity. Cut-off values for the PHQ-9 are none/mild = 1–7, moderate = 8–14, moderate to severe = 15–19, and severe = 20–27. (Note: In this manuscript, moderate to severe and severe groups are combined and labeled “severe”). Internal consistency reliability was α = .806. Responses to two items were considered: a) number of individuals endorsing the suicidal ideation item (thinking one would be better off dead; thoughts of hurting oneself); b) score on an item assessing the impact of the depressive symptoms on working, home activities, and getting along with other people, rated from 0 (not difficult) to 3 (extremely difficult).
2) The Beck Hopelessness Scale (BHS [41]) was included, as it is a correlate of suicide [42,43]. Using 20 true–false items, the BHS assesses feelings and expectations about one’s future life and loss of motivation. Scores range from 0 to 20, with the following cut-offs: normal = 0–3, mild = 4–8, moderate = 9–14, and severe = 15–20. Normative data suggest a mean of 3.1 in non-illness samples [44], and scores ≥ 9 are associated with suicidal risk [42]. Internal consistency reliability was α=0.805.
3) The Generalized Anxiety Disorder-7 (GAD-7 [45]) is a 7-item measure that assesses the frequency of symptoms of generalized anxiety disorder (GAD) as defined by the DSM-IV [40]. Items were rated on a 4-point Likert scale (0=not at all to 3=nearly every day) and summed for a total score ranging from 0 to 21 with the following cutoffs: none/mild = 0–9, moderate = 10–14, and moderate to severe/severe = 15–21. Internal consistency reliability was α = .874. A final item assessed the impact of the symptoms on working, home activities, and getting along with other people, rated from 0 (not difficult at all) to 3 (extremely difficult).
2.2.2. Cancer stress and perceptions of lung cancer
Two measures were used. 1) The Impact of Events Scale-Revised (IES-R [46]), a 22-item measure, is widely used to assess subjective stress caused by traumatic events and has been adapted to measure cancer-specific stress [17,47]. Patients rate the frequency of intrusive thoughts (8 items; e.g., “Other things kept making me think about cancer”), avoidant thoughts and behaviors (8 items; e.g., “I tried not to talk about it”), and hyperarousal symptoms (6 items; e.g., “I felt irritable and angry”) over the past seven days on a 5-point Likert scale (0 = not at all to 4 = often). Total scores range 0–88, with higher scores indicating more cancer-specific stress. Internal consistency reliability was α = .902.
2) The Brief Illness Perception Questionnaire (BIPQ [48]) is a self-report measure used to assess eight mental representations of one’s illness (identity [symptoms], consequences, timeline, personal control, treatment control, coherence [understanding], concern, and emotional response), with one question for each. Patients responded to each item using a 0- to 10-point Likert scale, with higher scores reflecting stronger endorsement of the illness representation. As there are no cut-off values, scores were summarized by tertiles, with scores 0.00–3.33 viewed as low, 3.34–6.66 as moderate, and 6.67–10.00 as high.
2.2.3. Physical symptoms
Two measures were used. 1) The lung cancer-specific EORTC Quality of Life Questionnaire (QLQ-LC13 [49]) was used to assess health-related quality of life [50,51]. The QLQ-LC13 contains nine items that assess individual symptoms (e.g., pain, coughing) and one three-item symptom scale that assesses dyspnea. Items are rated on a 4-point Likert scale (1=not at all to 4=very much), and each is transformed to a score ranging 0–100, with lower scores indicating better health-related quality of life and lower symptoms. 2) Additionally, 20 common symptoms and treatment side effects (e.g., nausea, infection) were assessed. Rating scales were adapted from the QLQ-LC13, with items rated on a 4-point Likert scale (1=not at all to 4=very much). Scoring and interpretation are identical to the QLQ-LC13.
2.2.4. Functional status and health evaluation
The EQ-5D [52] assesses five health status categories, with three relevant to functional status, i.e., mobility, self-care, and engagement in usual activities, with each rated on a 5-point Likert scale of performance difficulty (1=none, 2=slight, 3=moderate, 4=severe, 5=unable to perform). Population norms for the US have been published for the EQ-5D, and indicate that adults similar in age (55–64 years) have at least some problems with mobility=26.4 %, self-care=5.9 %, usual activities=27.0 %, pain/discomfort=58.8 %, and anxiety/depression= 31.3 % [53]. For health evaluation, the EQ VAS [52] is a single item asking the patient to rate his/her overall health, with anchors of 0 (the worst health you can imagine) and 100 (the best health you can imagine). The mean EQ VAS for adults aged 55–64 is 76.9 ± 21.0 [53].
2.2.5. Social connections
The Social Network Index (SNI [54]) is a 16-item measure of social contacts and involvement. Scores range from 1 to 12, with higher scores indicating more social connection; alternatively, social connection categories can be used: low (1), medium (2–5), medium-high (6–7), and high (8–12) social connectedness.
2.3. Statistical analysis
Descriptive statistics, including frequencies, means, standard deviations, and ranges, were used to summarize all sociodemographic and disease-related characteristics and measure responses. To determine depressive symptom severity groups, established cut-offs for the PHQ-9 were used [33]: none/mild (1–7), moderate (8–14), and severe (15–27). Analyses tested the hypothesis that overall group differences would be found, with negative functioning/symptoms outcomes ordered such that severe > moderate > none/mild. Analysis of variance for continuous measures was used to test for overall group differences, and when significant, Tukey’s tests were used for follow-up tests [55]. χ2 tests adjusted by Holm method were used to compare group differences for categorical measures [56].
3. Results
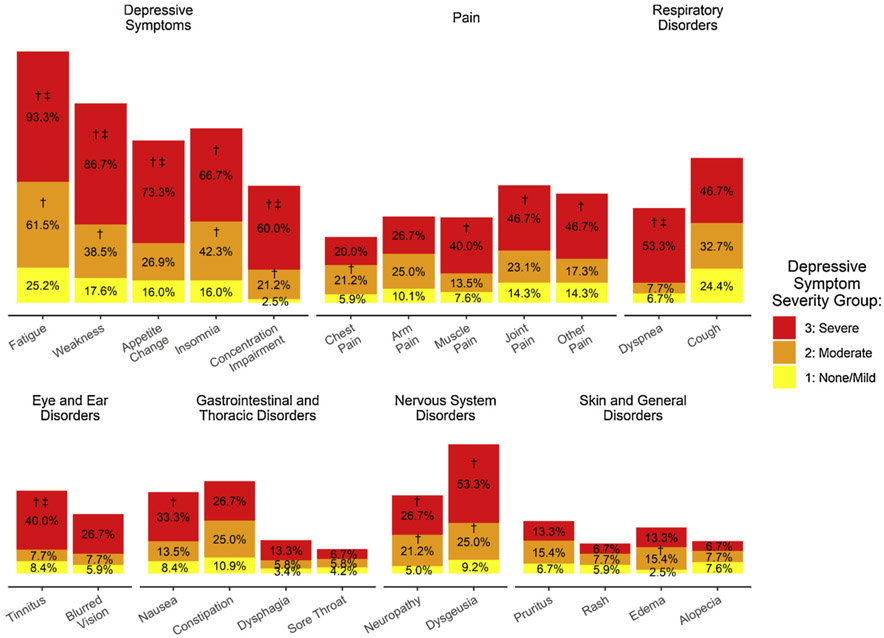
Two hundred and forty-two patients were enrolled in the study, 191 completed the baseline questionnaires (79 %), and 5 subsequently withdrew consent and were excluded from the analyses, yielding a final sample of 186 patients (77 % of total enrolled; see Fig. 1 for study flow). Sociodemographic and clinical characteristics of the sample are provided in Table 1. The sample was primarily older (M age = 63 years, SD = 12, range = 27–92), male (55 %), Caucasian (85 %), at least high school-educated (87 %), and married (58 %). Patients were diagnosed a median of 32 days before completing their baseline questionnaire, predominantly with adenocarcinoma (73 %). The depressive symptom (PHQ-9) severity groups included 119 (64 %) patients with none/mild depressive symptoms, 52 (28 %) with moderate depressive symptoms, and 15 (8 %) with severe depressive symptoms. Table 2 provides summary statistics for the psychological, behavioral, and symptom measures for each depressive symptom severity group, with significant differences between groups noted. Fig. 2 provides a graphical depiction of the percentage of patients in each severity group endorsing common symptoms/signs (depressive, pain, respiratory, eye/ear, gastrointestinal/thoracic, nervous system, skin/general), with group differences again noted.
Table 1.
Sociodemographic and clinical characteristics of sample (N = 186).
| n (%); Mean ± SD | |
|---|---|
| Sociodemographic: | |
| Age (years) | 62.5 ± 11.7 |
| Gender (male) | 103 (55.4 %) |
| Race: | |
| Caucasian/White | 158 (84.9 %) |
| African American/Black | 10 (5.4 %) |
| Other | 1 (0.5 %) |
| Multiracial | 17 (9.1 %) |
| Hispanic | 3 (1.6 %) |
| Education: | |
| < High school | 25 (13.4 %) |
| High school | 69 (37.1 %) |
| > High school | 92 (49.5 %) |
| Married | 107 (57.5 %) |
| Employed | 45 (24.2 %) |
| Income ≤ $25,000 (below Ohio poverty line for family of 4) | 40 (21.5 %) |
| Clinical: | |
| Lung cancer diagnosis: | |
| Adenocarcinoma | 135 (72.6 %) |
| Squamous | 28 (15.1 %) |
| Not otherwise specified | 11 (5.9 %) |
| Large cell | 4 (2.2 %) |
| Adenosquamous | 2 (1.1 %) |
| Missing/unknown | 6 (3.2 %) |
| Prior psychiatric diagnosis | 24 (12.9 %) |
| Prior psychological treatment for psychological distress | 58 (31.2 %) |
| Prior medical treatment for psychological distress | 107 (57.5 %) |
| Smoking status: | |
| Never | 16 (8.6 %) |
| Former | 137 (73.7 %) |
| Current | 33 (17.7 %) |
| Alcohol use: | |
| Never | 99 (53.2 %) |
| Monthly or less | 37 (19.9 %) |
| 2-4 times a month | 17 (9.1 %) |
| ≥ 2–3 times a week | 33 (17.7 %) |
Table 2.
Psychological and health characteristics by depressive symptom (PHQ-9) severity.
| Group 1: None/Mild (n = 119) |
Group 2: Moderate (n = 52) |
Group 3: Severe (n = 15) | Range | Comparisons of PHQ-9 severity groups:a |
|
|---|---|---|---|---|---|
| Depression: | |||||
| PHQ-9 score | 3.4 ± 2.3 | 10.0 ± 1.9 | 18.9 ± 3.4 | 0-24 | 1 < 2 < 3 |
| ≥ Some difficulties with function due to depression | 32 (26.9 %) | 32 (61.5 %) | 14 (93.3 %) | 1 < 2 < 3 | |
| Suicidal ideation | 0 (0.0 %) | 2 (3.8 %) | 5 (33.3 %) | 1,2 < 3; 1 = 2 | |
| Hopelessness | 3.4 ± 2.8 | 5.1 ± 3.0 | 8.1 ± 4.2 | 1-17 | 1 < 2 < 3 |
| Hopelessness ≥ 9 | 7 (5.9 %) | 9 (17.3 %) | 7 (46.7 %) | 1 < 2 < 3 | |
| Anxiety: | |||||
| GAD-7 score | 3.4 ± 3.5 | 7.2 ± 4.7 | 15.5 ± 5.2 | 0-21 | 1 < 2 < 3 |
| Moderate to severe/severe GAD symptoms | 2 (1.7 %) | 6 (11.5 %) | 11 (73.3 %) | 1 < 2 < 3 | |
| ≥ Some difficulties with function due to anxiety | 24 (20.2 %) | 29 (55.8 %) | 13 (86.7 %) | 1 < 2 < 3 | |
| Cancer stress: | |||||
| Cancer stress (IES) | 11.1 ± 10.0 | 21.8 ± 12.8 | 44.1 ± 16.4 | 0-80 | 1 < 2 < 3 |
| Perceptions of lung cancer: | |||||
| Consequences | 5.5 ± 2.8 | 6.3 ± 2.9 | 8.7 ± 2.7 | 0-10 | 1,2 < 3; 1 = 2 |
| Timeline | 6.4 ± 3.0 | 6.3 ± 2.6 | 7.2 ± 2.3 | 0-10 | 1 = 2 = 3 |
| Personal control | 5.5 ± 2.8 | 5.1 ± 2.9 | 3.0 ± 1.7 | 0-10 | 1,2 > 3; 1 = 2 |
| Treatment control | 8.4 ± 2.0 | 8.7 ± 1.6 | 6.9 ± 2.9 | 0-10 | 1,2 > 3; 1 = 2 |
| Identity | 3.7 ± 2.6 | 5.6 ± 2.9 | 8.7 ± 1.4 | 0-10 | 1 < 2 < 3 |
| Concern | 7.4 ± 2.9 | 8.1 ± 2.6 | 9.7 ± 0.7 | 0-10 | 1 < 3; 2 = 1,3 |
| Coherence | 8.1 ± 2.1 | 7.7 ± 2.5 | 7.4 ± 2.1 | 0-10 | 1 = 2 = 3 |
| Emotional response | 3.7 ± 2.3 | 5.7 ± 3.1 | 8.1 ± 2.0 | 0-10 | 1 < 2 < 3 |
| Functional status:b | |||||
| Mobility | 22 (18.5 %) | 17 (32.7 %) | 11 (73.3 %) | 1 > 2 > 3 | |
| Self-care | 2 (1.7 %) | 4 (7.7 %) | 5 (33.3 %) | 1 < 3; 2 = 1,3 | |
| Usual activities | 28 (23.5 %) | 20 (38.5 %) | 15 (100.0 %) | 1,2 > 3; 1 = 2 | |
| Pain & discomfort | 35 (29.4 %) | 28 (53.8 %) | 10 (66.7 %) | 1 < 2,3; 2 = 3 | |
| Anxiety/depression | 10 (8.4 %) | 11 (21.2 %) | 11 (73.3 %) | 1 > 2 > 3 | |
| Health evaluation: | |||||
| Perception of health | 66.9 ± 22.9 | 58.8 ± 21.9 | 40.1 ± 16.5 | 0-100 | 1,2 > 3; 1 = 2 |
Multiple comparisons were completed with Tukey’s test and χ2 tests adjusted by Holm method for continuous and categorical measures respectively.
≥ moderate problems with functional area.
Fig. 2.
Percentage of patients by depressive symptom (PHQ-9) severity groups (none/mild, moderate, severe) reporting symptoms/signs occurring quite a bit/very often in the last week.
Note: † Indicates significant difference (p < .05) between none/mild group and others;
‡ Indicates significant difference (p < .05) between moderate group and severe group.
3.1. Patients with severe depressive symptoms (n = 15)
3.1.1. Psychological symptoms
Fifteen patients (8.1 %) with newly diagnosed NSCLC scored at the severe depressive symptom level on the PHQ-9 (scores 15–27 of 27 possible; M = 18.9, SD = 3.4; see Table 2). Of them, all (100 %) reported depressed mood and 80.0 % reported anhedonia more days than not in the preceding two weeks. Patients’ responses on the QLQ-LC13 symptom questionnaire showed a majority reported experiencing vegetative and cognitive symptoms at level/frequency of “quite a bit” or “very much” (see Fig. 2), including 93.3 % with fatigue, 86.7 % with weakness, 73.3 % with appetite change, 66.7 % with insomnia, and 60.0 % with concentration impairment. Nearly all (93.3 %) reported that their depressive symptoms made it difficult to do their work, take care of things at home, and/or get along with other people. Patients with severe depressive symptoms also exhibited high levels of hopelessness (M = 8.1, SD = 4.2), with 46.7 % endorsing moderate or severe levels (scores 9–20). One third (33.3 %) reported suicidal ideation. A large majority of patients with severe depressive symptoms (73.3 %) also had moderate to severe/severe anxiety comorbidity (GAD-7 M = 15.5, SD = 5.2). Additionally, most (86.7 %) reported that their anxiety symptoms interfered with their occupational, household, and/ or social functioning.
3.1.2. Cancer stress and perceptions of lung cancer
Patients with newly diagnosed NSCLC and severe depressive symptoms reported extreme levels of cancer-related stress (M = 44.1, SD = 16.4). Patients with severe depressive symptoms, relative to all others, perceived the highest level of symptom burden (identity; M = 8.7, SD = 1.4), the greatest consequences for their lives (M = 8.7, SD = 2.7), the greatest emotional impact (emotional response; M = 8.1, SD = 2.0), the least personal control over their cancer (M = 3.0, SD = 1.7), and the least confidence that treatment would help (treatment control; M = 6.9, SD = 2.9). They reported the highest possible level of concern about their cancer (M = 9.7, SD = 0.7). These patients were no different than those with moderate and none/mild depressive symptoms in believing that they have a high level of understanding of their cancer (coherence; M = 7.4, SD = 2.1) and that their illness would last a long time (M = 7.2, SD = 2.3).
3.1.3. Physical symptoms
Patients with newly diagnosed NSCLC and severe depressive symptoms reported multiple physical symptoms of high intensity (see Fig. 2), including “quite a bit” or “very much” pain (73.3 %), loss of taste (53.3 %), dyspnea (53.3 %), and/or cough (46.7 %).
3.1.4. Functional status and health evaluation
Functional status was significantly impaired for those with severe depressive symptoms. The percentage reporting moderate or severe functional issues was 100 % for usual activities (e.g., work, study, housework, family or leisure activities), 73.3 % for mobility, and 33.3 % for self-care. In line with this level of disability, patients’ average self-rated health evaluation was 40.1 ± 16.5.
3.1.5. Social connections and other resources
These patients with severe depressive symptoms reported medium social connectedness (M = 2.5, SD = 2.7). Also, 46.7 % reported being unmarried. These patients reported limited financial resources, with 33.3 % earning an average annual income below the state poverty threshold for a family of four in the state they resided. Additionally, only 13.3 % were employed at the time of diagnosis.
3.2. Patients with moderate depressive symptoms (n = 52)
3.2.1. Psychological symptoms
Fifty-two patients (28 %) scored at the level of moderate depressive symptoms on the PHQ-9 (scores 8–14 of 27 possible; M = 10.0, SD = 1.9; see Table 2). Within this group, 15.4 % reported depressed mood and 34.6 % reported anhedonia more days than not in the preceding two weeks. Like those with severe depressive symptoms, patients in this group reported fatigue as their most common vegetative/cognitive depressive symptom (see Fig. 2), with 61.5 % reporting that this symptom affected them “quite a bit” or “very much.” Rates for other vegetative and cognitive symptoms of depression were 42.3 % for insomnia, 38.5 % for weakness, 26.9 % for appetite change, and 21.2 % for concentration impairment. The majority (61.5 %) reported that their depressive symptoms led to difficulties with occupational, household, and/or social functioning. Patients in this group had lower hopelessness scores (M = 5.1, SD = 3.0) than patients with severe depressive symptoms, but still a substantial percentage (17.3 %) had moderate to severe levels. Two individuals reported suicidal ideation. Of clinical importance, depression-anxiety comorbidity was notably lower, with only 11.5 % endorsing moderate to severe/severe anxiety symptoms (M = 7.2, SD = 4.7), although approximately half (55.8 %) reported anxiety-related impairment.
3.2.2. Cancer stress and perceptions of lung cancer
Though lower than the extreme scores of those with severe depressive symptoms, patients with moderate depressive symptoms endorsed a high level of cancer-related stress (M = 21.8, SD = 12.8). Patients with moderate depressive symptoms had similar illness perceptions to those with none/mild depressive symptoms in viewing their illness as moderately consequential for their lives (M = 6.3, SD = 2.9) and perceiving a moderate level of personal control over their cancer (M = 5.1, SD = 2.9) and a high level of treatment control (M = 8.7, SD = 1.6). However, they differed significantly from the none/mild depressive symptom group by reporting greater symptom burden (identity; M = 5.6, SD = 2.9) and viewing their lung cancer as having a greater emotional impact (emotional response; M = 5.7, SD = 3.1). They had a high level of concern about their cancer (M = 8.1, SD = 2.6).
3.2.3. Physical symptoms
Relative to those with severe depressive symptoms, patients with moderate depressive symptoms reported less intense (although still troublesome) physical symptoms (see Fig. 2), with fewer individuals endorsing “quite a bit” or “very much” pain (59.6 %), cough (32.7 %), and loss of taste (25.0 %). The groups differed substantially in their reports of dyspnea: 7.7 %, versus 53.3 % for those with severe depressive symptoms.
3.2.4. Functional status and health evaluation
Functional impairment was also a noteworthy problem for patients with moderate depressive symptoms, but the levels of impairments were lower than those reported by the severely depressed group. Among those with moderate depressive symptoms, 38.5 % reported at least moderate impairment in usual activities, 32.7 % in mobility, and 7.7 % in self-care. These patients rated their overall health as significantly better than that of the severe depressive symptom group (M = 58.8; SD = 21.9).
3.2.5. Social connections and other resources
Patients with moderate depressive symptoms reported a medium level of social connection (M = 3.9, SD = 2.5). More of these patients (51.9 %) were unmarried. Average annual income was similar, with 28.8 % reporting levels below the state poverty threshold for a family of four, despite a higher rate of employment (25.0 %) at the time of assessment.
4. Discussion
The American Society of Clinical Oncology (ASCO [28]) recommends that all patients with cancer be evaluated for symptoms of depression and anxiety at diagnosis/start of treatment. This study is an exemplar of the guideline’s edicts, i.e., using the recommended self-report measures to identify patients with moderate to severe depressive symptom levels. Beyond that, the aim was to expand providers’ and researchers’ perspective on the co-occurring difficulties and impairments that newly diagnosed advanced NSCLC patients with moderate to severe depressive symptoms are experiencing. That is, these patients’ depressive symptoms co-occur in a matrix which included clinical levels of anxiety symptoms, traumatic stress, impaired functional status, and significant pain and other physical symptoms. All of the latter factors have been shown—individually and collectively—to contribute to the exacerbation and/or maintenance of depressive symptoms. As a group, cancer patients’ stress declines and moods improve with the start of treatment [14,15,57,58], and this general observation would likely apply to the majority of the sample (64 %). However, its applicability to the patients described here, as discussed below, is limited.
Much evidence points to the likelihood that the patients reporting a severe level of depressive symptoms (n = 15) would be diagnosed with major depressive disorder (MDD). Positive responses to PHQ-9 items are endorsements of the DSM criteria symptoms for MDD [33,59]. Uniformly, patients endorsed depressed mood and/or anhedonia (the presence of either is necessary for a diagnosis of MDD [40]) as well as vegetative/cognitive symptoms at high rates (appetite changes [73 %], trouble sleeping [67 %], and trouble concentrating [60 %]). Virtually all patients in this group (93 %) felt their depressive symptoms made it difficult to work, take care of things at home, and/or get along with other people. Additionally, these patients’ levels of hopelessness were notably higher than those found in non-illness comparison samples [60]. Nearly half (47 %) reported moderate or severe hopelessness, signified by BHS scores ≥ 9; for comparison, Overholser et al. [61] and Fisher et al. [62] reported mean BHS scores of 10–11 among depressed psychiatric patients. Hopelessness is a particularly important reponse in this cancer group, as it predicts depressive symptoms throughout the disease trajectory [63] and suicidal ideation [64]. Indeed, a substantial proportion—one third—of patients with severe depressive symptoms in the sample reported suicidal ideation.
Importantly, once diagnosed, MDD is a psychiatric disorder which continues for months and may not remit [65-67]. Even with psychotherapy and/or pharmacotherapy, significant symptom remission is not typically observed until after 2–3 months of continuous treatment [68]. Quick resolution of symptoms is also unlikely because of the added vulnerabilities of the patients with severe symptom levels. These are vulnerabilities found previously to worsen and/or maintain depressive symptoms, even in the context of depression treatment.
First, more than 70 % of patients with newly diagnosed NSCLC and severe depressive symptoms also had moderate to severe/severe GAD symptoms. Depressive symptoms are known to co-occur with anxiety, with the majority (60 %) of those with a depressive disorder also having an anxiety disorder [69]. GAD worry or fear can be particularly toxic for lung cancer patients, as severe anxiety can worsen dyspnea and induce panic [70-72], and GAD can impede decision-making and participation in or continuation of treatment [10,73].
Second, the level of cancer-specific stress patients with severe depressive symptoms reported was extraordinary, far exceeding the IES-R cutoff of 24 for likely diagnosis of post-traumatic stress disorder (PTSD) [74], and so high that a search of the IES-R literature assessing patients with cancer at diagnosis (e.g., chronic lymphocytic leukemia, M = 13.6 [18]) found none comparable [75-79]. Likely contributing to their stress [80,81], patients with severe depressive symptoms, relative to all others, also endorsed the most negative perceptions of their illness. Appreciating patients’ illness perceptions at the time of diagnosis is important, as negative illness perceptions are associated with patients coping less effectively, especially when having to make treatment choices [82,83]. Patients with negative illness perceptions are more likely to delay seeking treatment [84], or conversely, pursue aggressive therapies at end of life that have detrimental effects on quality of life [85].
Third, while it is well-known that advanced-stage patients often experience significant physical symptoms [86], these data demonstrate that patients with severe depressive symptoms are particularly burdened, reporting their health status (M = 40.1) to be nearly two standard deviations below the US population norm (M = 76.9) for those of similar age (55–64) [53]. These patients reported the highest levels of dyspnea and cough—known correlates of poor quality of life [87] and functional impairment [88,89] in lung cancer patients. Upwards of 70 % of the patients reported one or more type of pain, suggesting a significant need for pain management (or referral for) at the point of diagnosis. Further, the co-occurrence of fatigue, weakness, and appetite changes for more than 70 % of the patients may be suggestive of cachexia. Oncology providers may not fully appreciate patients’ symptom experiences, as other data suggest a strikingly low concordance (i.e., 38 % agreement) between physician-rated and patient-reported lung cancer symptom burden [87,90].
Fourth, more than 30 % of these patients with severe depressive symptoms came with self-care impairments. Considering all the functional impairment data, the likelihood of additional patients becoming self-care impaired in the short term would be high. Functional status has obvious implications for day-to-day quality of life, but is additionally a prognostic factor in patients with lung cancer, predicting relative risk of death [91]. Data such as these illustrate the importance of determining patients’ functional status early and following with interventions, e.g., occupational therapy, to prevent further decline and disability [92,93].
Lastly, patients with severe depressive symptoms reported limited access to social and financial resources. Their level of social connectedness (M = 2.5 of 12 possible), combined with the fact that nearly half identified as unmarried, is concerning given the need for adequate social support when coping with cancer. Indeed, lower levels of social support from family and friends are associated with worse emotional and physical aspects of quality of life for patients with lung cancer [94], and unmarried patients are known to die earlier than married patients [95]. Of additional concern, one third of these patients with severe depressive symptoms reported income levels below the state poverty threshold for a family of four. The financial burden imposed by cancer is significant, with patients with cancer spending an estimated $976 to $1170 more on out-of-pocket treatment-related expenses in a given year than patients without cancer [81]. Moreover, in lung cancer specifically, financial strain is associated with higher symptom burden, reduced quality of life, and earlier mortality [96,97].
Patients having a moderate severity of depressive symptoms were, as expected, less symptomatic than those with severe depressive symptoms in the majority of the areas assessed. As noted above, 15.4% reported depressed mood and 34.6% reported anhedonia more days than not in the preceding two weeks on the first two PHQ-9 items. If only these two items were used as a screen [33] rather than the full measure, the majority of patients in the moderate group—roughly 70%—would have been missed. In other respects, there were two striking differences between the moderate and severe groups. First was that of GAD symptom severity, with 11.5 % of patients in the moderate depressive symptom group having a moderate to severe/severe GAD score versus 73.3 % of patients in the severe depressive symptom group. Second, many fewer of the patients with moderate depressive symptoms had impairments in self-care (7.7 % vs. 33.3 %), mobility (32.7 % vs. 73.3 %), and usual activities (38.5 % vs. 100.0 %). Considering the general observation of patients emotionally improving once cancer treatment begins, that may be more likely for individuals at the moderate depressive symptom level, though not certain, due to their co-occurring problems.
Aspects of study design and method are noted. This is a single institution study, but the case could be made that no single or even multi-institutional study is sufficiently generalizable. There may be differences across the United States, but lung cancer patients share common features, namely, it is largely a disease of smoking and aging. The state from which these patients came has among the highest smoking rates in the U.S. (21.1 % vs. 14 %) and is the 44th lowest in the nation for lung cancer mortality. Also, 50 % of the patients were from rural Appalachia counties. Unlike the majority of lung clinical trials [98], there were no age or functional status exclusions. Regarding the method, diagnostic interviews for depression and anxiety were not used. Yet, the items for both the PHQ-9 and GAD-7 are those of the DSM criteria and both have extensive literatures showing their convergence with interview determinations of MDD and GAD [33,45,99-101]. Supplementing measures for conceptually similar (though not overlapping) constructs—hopelessness and traumatic stress—added description and enhanced the validity of grouping patients into moderate and severe symptom groups. The remaining measures provided descriptive breadth to the difficult circumstances of these patients.
5. Conclusions
The present data speak to the immediate struggles facing patients with advanced NSCLC in the days of diagnosis and the critical importance of screening for depressive symptoms during this period. Patients with significant depressive symptoms display a constellation of anxiety symptoms, traumatic stress, impaired functional status, and physical symptoms—factors that may further exacerbate and/or maintain depression. Given the survival benefits of targeted and immunotherapies, it is crucially important to provide referral and follow-up mental health care to improve the quality of life of patients with depression and aid them to engage in and benefit from new therapies. Without appropriate referral and care, patients’ understanding of their disease will be suboptimal, decision-making and engagement in treatment will be impaired [10,102], tolerance of symptoms and treatment side effects will be lowered, and motivation and efforts to maintain functional status will decline [9,10,103,104].
Acknowledgements
This work was supported by The Ohio State University Comprehensive Cancer Center Pelotonia (BLA, SBL, DPC, PGS), the National Cancer Institute through The Ohio State University K12 Training Grant for Clinical Faculty Investigators (CJP, K12CA133250), and the Beating Lung Cancer in Ohio study. We thank the patients for their participation.
Footnotes
Declaration of Competing Interest
Barbara L. Andersen, Stephen B. Lo, and Peter G. Shields received funding from The Ohio State University Comprehensive Cancer Center Pelotonia. In addition to Peletonia, David P. Carbone reports personal fees from Abbvie, Adaptimmune, Agenus, Amgen, Ariad, AstraZeneca, Boehringer-Ingelheim, EMD Serono, Inc., Foundation Medicine, Genentech/Roche, Gritstone, Guardant Health, Helsinn, Incyte, Inivata, Inovio, Janssen, Kyowa Kirin, Loxo Oncology, Merck, MSD, Nexus Oncology, Novartis, Palobiofarma, Pfizer, prIME Oncology, Stemcentrx, and Takeda Oncology, and research funding and personal fees from Bristol Myers-Squibb (BMS). Carolyn J. Presley received funding from the National Cancer Institute through The Ohio State University K12 Training Grant for Clinical Faculty Investigators (K12CA133250). Thomas R. Valentine has no declarations of interest.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012, Int. J. Cancer 136 (2015) E359–E386, 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- [2].Cronin KA, Lake AJ, Scott S, Sherman RL, Noone A-M, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A, Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics, Cancer 124 (2018) 2785–2800, 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, MacRae JH, Martin M, Pelletier G, Robinson J, Simpson JSA, Speca M, Tillotson L, Bultz BD, High levels of untreated distress and fatigue in cancer patients, Br. J. Cancer 90 (2004) 2297–2304, 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S, The prevalence of psychological distress by cancer site, Psychooncology 10 (2001) 19–28, . [DOI] [PubMed] [Google Scholar]
- [5].Misono S, Weiss NS, Fann JR, Redman M, Yueh B, Incidence of suicide in persons with cancer, J. Clin. Oncol 26 (2008) 4731–4738, 10.1200/JCO.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Linden W, Vodermaier A, Mackenzie R, Greig D, Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age, J. Affect. Disord 141 (2012) 343–351, https://doi.Org/10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- [7].Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, Slatore CG, Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment, J. Clin. Oncol 34 (2016) 3984–3991, 10.1200/JCO.2016.66.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR, Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type, Psychosomatics 50 (2009) 383–391, https://doi.Org/10.1176/appi.psy.50.4.383. [DOI] [PubMed] [Google Scholar]
- [9].Hopwood P, Stephens RJ, Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data, J. Clin. Oncol 18 (2000) 893, 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- [10].Arrieta O, Angulo LP, Nunez-Valencia C, Dorantes-Gallareta Y, Macedo EO, Martinez-Lopez D, Alvarado S, Corona-Cruz JF, Onate-Ocana LF, Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer, Ann. Surg. Oncol 20 (2013) 1941–1948, 10.1245/s10434-012-2793-5. [DOI] [PubMed] [Google Scholar]
- [11].Pirl WF, Greer JA, Traeger L, Jackson V, Lennes IT, Gallagher ER, Perez-Cruz P, Heist RS, Temel JS, Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care, J. Clin. Oncol 30 (2012) 1310–1315, 10.1200/jco.2011.38.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B, Predictors of depressive symptomatology of geriatric patients with lung cancer - A longitudinal analysis, Psychooncology 11 (2002) 12–22, 10.1002/pon.545. [DOI] [PubMed] [Google Scholar]
- [13].Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, Lyons KS, Slatore CG, Depression symptom trends and health domains among lung cancer patients in the CanCORS study, Lung Cancer 100 (2016) 102–109, 10.1016/j.lungcan.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andersen BL, Goyal NG, Westbrook TD, Bishop B, Carson WE, Trajectories of stress, depressive symptoms, and immunity in cancer survivors: diagnosis to 5 years, Clin. Cancer Res 23 (2017) 52–61, 10.1158/1078-0432.CCR-16-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV, Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis, Health Psychol. 29 (2010) 160–168, 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- [16].Kawamura N, Kim Y, Asukai N, Suppression of cellular immunity in men with a past history of posttraumatic stress disorder, Am. J. Psychiatry 158 (2001) 484–486, https://doi.Org/10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- [17].Andersen BL, Farrar WB, Golden-Kreutz DM, Kutz LA, MacCallum RM, Courtney ME, Glaser R, Stress and immune responses after surgical treatment for regional breast cancer, J. Natl. Cancer Inst 90 (1998) 30–36, 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andersen BL, Goyal NG, Weiss DM, Westbrook TD, Maddocks KJ, Byrd JC, Johnson AJ, Cells, cytokines, chemokines, and cancer stress: a biobehavioral study of patients with chronic lymphocytic leukemia, Cancer. 124 (2018) 3240–3248, 10.1002/cncr.31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Andersen BL, Kiecolt-Glaser JK, Glaser R, A biobehavioral model of cancer stress and disease course, Am. Psychol 49 (1994) 389–404, 10.1037/0003-066X.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chida Y, Hamer M, Wardle J, Steptoe A, Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol 5 (2008) 466–475, 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- [21].Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, Vlahos L, How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? Palliat. Med 23 (2009) 46–53, 10.1177/0269216308098088. [DOI] [PubMed] [Google Scholar]
- 22.[] Mykletun A, Dahl AA, Haaland CF, Bremnes R, Dahl O, Klepp O, Wist E, Fosså SD, Side effects and cancer-related stress determine quality of life in longterm survivors of testicular cancer, J. Clin. Oncol 23 (2005) 3061–3068, 10.1200/JCO.2005.08.048. [DOI] [PubMed] [Google Scholar]
- [23].Kang D-H, Park N-J, McArdle T, Cancer-specific stress and mood disturbance: implications for symptom perception, quality of life, and immune response in women shortly after diagnosis of breast cancer, ISRN Nurs. 2012 (2012) 608039,, 10.5402/2012/608039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thekdi SM, Milbury K, Spelman A, Wei Q, Wood C, Matin SF, Tannir N, Jonasch E, Pisters L, Cohen L, Posttraumatic stress and depressive symptoms in renal cell carcinoma: association with quality of life and utility of single-item distress screening, Psychooncology 24 (2015) 1477–1484, 10.1002/pon.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morrison EJ, Flynn JM, Jones J, Byrd JC, Andersen BL, Individual differences in physical symptom burden and psychological responses in individuals with chronic lymphocytic leukemia, Ann. Hematol. 95 (2016) 1989–1997, 10.1007/S00277-016-2790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mazor M, Paul SM, Chesney MA, Chen L-M, Smoot B, Topp K, Conley YP, Levine JD, Miaskowski C, Perceived stress is associated with a higher symptom burden in cancer survivors, Cancer (2019), 10.1002/cncr.32477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Norton TR, Manne SL, Rubin S, Carlson J, Hernandez E, Edelson MI, Rosenblum N, Warshal D, Bergman C, Prevalence and predictors of psychological distress among women with ovarian cancer, J. Clin. Oncol 22 (2004) 919–926, 10.1200/JCO.2004.07.028. [DOI] [PubMed] [Google Scholar]
- [28].Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Rowland JH, Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology Guideline adaptation, J. Clin. Oncol 32 (2014) 1605–1619, 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parpa E, Tsilika E, Galanos A, Nikoloudi M, Mystakidou K, Depression as mediator and or moderator on the relationship between hopelessness and patients’ desire for hastened death, Support. Care Cancer 27 (2019), 10.1007/S00520-019-04715-2. [DOI] [PubMed] [Google Scholar]
- [30].Kolva E, Hoffecker L, Cox-Martin E, Suicidal ideation in patients with cancer: a systematic review of prevalence, risk factors, intervention and assessment, Palliat. Support. Care (2019)1–14, 10.1017/S1478951519000610. [DOI] [PubMed] [Google Scholar]
- [31].Dempster M, Howell D, McCorry NK, Illness perceptions and coping in physical health conditions: a meta-analysis, J. Psychosom. Res. 79 (2015) 506–513, https://doi.Org/10.1016/j.jpsychores.2015.10.006. [DOI] [PubMed] [Google Scholar]
- [32].Schellekens MPJ, Wolvers MDJ, Schroevers MJ, Bootsma TI, Cramer AOJ, van der Lee ML, Exploring the interconnectedness of fatigue, depression, anxiety and potential risk and protective factors in cancer patients: a network approach, J. Behav. Med (2019), 10.1007/s10865-019-00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kroenke K, Spitzer RL, Williams JB, The PHQ-9, Validity of a brief depression severity measure, J. Gen. Intern. Med. 16 (2001) 606–613, 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen C-M, Mullan J, Su Y-Y, Griffiths D, Kreis IA, Chiu H-C, The longitudinal relationship between depressive symptoms and disability for older adults: a population-based study, J. Gerontol. A Biol. Sci. Med. Sci. 67 (2012) 1059–1067, 10.1093/gerona/gls074. [DOI] [PubMed] [Google Scholar]
- [35].Dauchy S, Dolbeault S, Reich M, Depression in cancer patients, EJC Suppl. 11 (2013) 205–215, https://doi.Org/10.1016/j.ejcsup.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ormel J, Rijsdijk FV, Sullivan M, van Sonderen E, Kempen GIJM, Temporal and reciprocal relationship between IADL/ADL disability and depressive symptoms in late life, J. Gerontol. B Psychol. Sci. Soc. Sci 57 (2002) P338–347, 10.1093/geronb/57.4.p338. [DOI] [PubMed] [Google Scholar]
- [37].Grotmol KS, Lie HC, Hjermstad MJ, Aass N, Currow D, Kaasa S, Moum TÅ, Pigni A, Loge JH, European Palliative Care Research Collaborative (EPCRC), Depression - A major contributor to poor quality of life in patients with advanced cancer, J. Pain Symptom Manage 54 (2017) 889–897, 10.1016/j.jpainsymman.2017.04.010. [DOI] [PubMed] [Google Scholar]
- [38].Wen S, Xiao H, Yang Y, The risk factors for depression in cancer patients undergoing chemotherapy: a systematic review, Support. Care Cancer 27 (2019) 57–67, 10.1007/s00520-018-4466-9. [DOI] [PubMed] [Google Scholar]
- [39].McKay C, Burke T, Cao X, Abernethy AP, Carbone DP, Treatment patterns for advanced non-small-cell lung cancer after platinum-containing therapy in U.S. community oncology clinical practice, Clin. Lung Cancer 17 (2016) 449–460, 10.1016/j.cllc.2016.03.008 e7. [DOI] [PubMed] [Google Scholar]
- [40].American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th ed., American Psychiatric Publishing, Arlington, VA, 2013. [Google Scholar]
- [41].Beck AT, Weissman A, Lester D, Trexler L, The measurement of pessimism: the hopelessness scale, J. Consult. Clin. Psychol 42 (1974) 861–865, 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- [42].Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA, Relationship between hopelessness and ultimate suicide: a replication with psychiatric outpatients, Am. J. Psychiatry 147 (1990) 190–195, 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- [43].Beck AT, Steer RA, Beck JS, Newman CF, Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression, Suicide Life, Threat. Behav 23 (1993) 139–145, 10.1111/j.1943-278X.1993.tb00378.x. [DOI] [PubMed] [Google Scholar]
- [44].Dozois DJA, Covin R, Brinker JK, Normative data on cognitive measures of depression, J. Consult. Clin. Psychol. 71 (2003) 71–80, 10.1037/0022-006X.71.1.71. [DOI] [PubMed] [Google Scholar]
- [45].Spitzer RL, Kroenke K, Williams JB, Lowe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Arch. Intern. Med. 166 (2006) 1092–1097, 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- [46].Weiss DS, The impact of event scale: revised, Cross-Cult. Assess. Psychol. Trauma PTSD, Springer Science + Business Media, New York, NY, US, 2007, pp. 219–238, 10.1007/978-0-387-70990-1_10. [DOI] [Google Scholar]
- [47].Mystakidou K, Tsilika E, Parpa E, Galanos A, Vlahos L, Psychometric properties of the Impact of Event Scale in Greek cancer patients, J. Pain Symptom Manage. 33 (2007) 454–461, 10.1016/j.jpainsymman.2006.09.023. [DOI] [PubMed] [Google Scholar]
- [48].Broadbent E, Petrie KJ, Main J, Weinman J, The brief illness perception questionnaire, J. Psychosom. Res. 60 (2006) 631–637, 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- [49].Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M, The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials, Eur. J. Cancer 30 (1994) 635–642, 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- [50].Roller M, Warncke S, Hjermstad MJ, Arraras J, Pompili C, Harle A, Johnson CD, Chie W-C, Schulz C, Zeman F, van Meerbeeck JP, Kuliś D, Bottomley A, Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: a systematic review of the literature 20 years after its development, Cancer 121 (2015) 4300–4323, 10.1002/cncr.29682. [DOI] [PubMed] [Google Scholar]
- [51].Salvo N, Hadi S, Napolskikh J, Goh P, Sinclair E, Chow E, Quality of life measurement in cancer patients receiving palliative radiotherapy for symptomatic lung cancer: a literature review, Curr. Oncol 16 (2009) 16–28, 10.3747/co.v16i2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X, Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L), Qual. Life Res 20 (2011) 1727–1736, 10.1007/S11136-011-9903-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Janssen B, Szende A, Population norms for the EQ-5D, in: Szende A, Janssen B, Cabases J (Eds.), Self-Rep. Popul. Health Int. Perspect. Based EQ-5D, Springer, Dordrecht, 2014(accessed April 7, 2019), http://www.ncbi.nlm.nih.gov/books/NBK500364/. [Google Scholar]
- [54].Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM, Social ties and susceptibility to the common cold, JAMA 277 (1997) 1940–1944, 10.1001/jama.1997.03540480040036. [DOI] [PubMed] [Google Scholar]
- [55].Tukey JW, Comparing individual means in the analysis of variance, Biometrics 5 (1949) 99–114, 10.2307/3001913. [DOI] [PubMed] [Google Scholar]
- [56].Holm S, A simple sequentially rejective multiple test procedure, Scand. Stat. Theory Appl. 6 (1979) 65–70, 10.2307/4615733. [DOI] [Google Scholar]
- [57].Meraner V, Gamper E-M, Grahmann A, Giesinger JM, Wiesbauer P, Sztankay M, Zeimet AG, Sperner-Unterweger B, Holzner B, Monitoring physical and psychosocial symptom trajectories in ovarian cancer patients receiving chemotherapy, BMC Cancer 12 (2012) 77, 10.1186/1471-2407-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schneider A, Kotronoulas G, Papadopoulou C, McCann L, Miller M, McBride J, Polly Z, Bettles S, Whitehouse A, Kearney N, Maguire R, Trajectories and predictors of state and trait anxiety in patients receiving chemotherapy for breast and colorectal cancer: results from a longitudinal study, Eur. J. Oncol. Nurs 24 (2016) 1–7, 10.1016/j.ejon.2016.07.001. [DOI] [PubMed] [Google Scholar]
- [59].American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., (1994) Washington, DC. [Google Scholar]
- [60].Dozois DJA, Covin R, Brinker JK, Normative data on cognitive measures of depression, J. Consult. Clin. Psychol 71 (2003) 71–80, 10.1037/0022-006X.71.1.71. [DOI] [PubMed] [Google Scholar]
- [61].Overholser J, Fisher L, Braden A, Peak N, Despair beyond repair? Severity of hopelessness in depressed psychiatric inpatients, J. Depress. Ther 1 (2015) 1–10, 10.14302/issn.2476-1710.jdt-14-567. [DOI] [Google Scholar]
- [62].Fisher LB, Overholser JC, Ridley J, Braden A, Rosoff C, From the outside looking in: sense of belonging, depression, and suicide risk, Psychiatry 78 (2015) 29–41, 10.1080/00332747.2015.1015867. [DOI] [PubMed] [Google Scholar]
- [63].Lo C, Zimmermann C, Rydall A, Walsh A, Jones JM, Moore MJ, Shepherd DA, Gagliese L, Rodin G, Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer, J. Clin. Oncol 28 (2010) 3084–3089, 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 64].Chochinov HM, Wilson KG, Enns M, Lander S, Depression, hopelessness, and suicidal ideation in the terminally ill, Psychosomatics 39 (1998) 366–370, 10.1016/S0033-3182(98)71325-8. [DOI] [PubMed] [Google Scholar]
- [65].Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, Whiteford HA, The epidemiological modelling of major depressive disorder: application for the Global Burden of Disease Study 2010, PLoS One 8 (2013) e69637, , 10.1371/journal.pone.0069637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spijker J, de Graaf R, Bijl RV, Beekman ATF, Ormel J, Nolen WA, Duration of major depressive episodes in the general population: results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS), Br. J. Psychiatry J. Ment. Sci 181 (2002) 208–213, 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- [67].Eaton WW, Shao H, Nestadt G, Lee BH, Bienvenu OJ, Zandi P, Population-based study of first onset and chronicity in major depressive disorder, Arch. Gen. Psychiatry 65 (2008) 513–520, 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Frank E, Cassano GB, Rucci P, Thompson WK, Kraemer HC, Fagiolini A, Maggi L, Kupfer DJ, Shear MK, Houck PR, Calugi S, Grochocinski VJ, Scocco P, Buttenfield J, Forgione RN, Predictors and moderators of time to remission of major depression with interpersonal psychotherapy and SSRI pharmacotherapy, Psychol. Med 41 (2011) 151–162, 10.1017/S0033291710000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National comorbidity survey replication, the epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R), JAMA 289 (2003) 3095–3105, 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- [70].Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J, The frequency and correlates of dyspnea in patients with advanced cancer, J. Pain Symptom Manage 19 (2000) 357–362, 10.1016/S0885-3924(00)00126-3. [DOI] [PubMed] [Google Scholar]
- [71].Dudgeon DJ, Lertzman M, Askew GR, Physiological changes and clinical correlations of dyspnea in cancer outpatients, J. Pain Symptom Manage 21 (2001) 373–379, 10.1016/S0885-3924(01)00278-0. [DOI] [PubMed] [Google Scholar]
- [72].Shin JA, Kosiba JD, Traeger L, Greer JA, Temel JS, Pirl WF, Dyspnea and panic among patients with newly diagnosed non-small cell lung cancer, J. Pain Symptom Manage 48 (2014) 465–470, 10.1016/j.jpainsymman.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS, Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer, J. Psychosom. Res 65 (2008) 549–552, 10.1016/j.jpsychores.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Asukai N, Kato H, Kawamura N, Kim Y, Yamamoto K, Kishimoto J, Miyake Y, Nishizono-Maher A, Reliability and validity of the Japanese-language version of the Impact of Event Scale-Revised (IES-R-J): four studies of different traumatic events, J. Nerv. Ment. Dis. 190 (2002) 175–182, 10.1097/00005053-200203000-00006. [DOI] [PubMed] [Google Scholar]
- [75].Chambers SK, Zajdlewicz L, Youlden DR, Holland JC, Dunn J, The validity of the Distress Thermometer in prostate cancer populations, Psychooncology 23 (2014) 195–203, 10.1002/pon.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, Frierson GM, Jim HS, Carpenter KM, Shelby RA, Andersen BL, Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients, Health Psychol. 24 (2005) 288–296, 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kangas M, Henry JL, Bryant RA, Posttraumatic stress disorder following cancer: a conceptual and empirical review, Clin. Psychol. Rev 22 (2002) 499–524, 10.1016/S0272-7358(01)00118-0. [DOI] [PubMed] [Google Scholar]
- 78.Koopman C, Butler LD, Classen C, Giese-Davis J, Morrow GR, Westendorf J, Banerjee T, Spiegel D, Traumatic stress symptoms among women with recently diagnosed primary breast cancer, J. Trauma. Stress 15 (2002) 277–287, 10.1023/a:1016295610660. [DOI] [PubMed] [Google Scholar]
- [79].Phipps S, Long A, Hudson M, Rai SN, Symptoms of post-traumatic stress in children with cancer and their parents: effects of informant and time from diagnosis, Pediatr. Blood Cancer 45 (2005) 952–959, 10.1002/pbc.20373. [DOI] [PubMed] [Google Scholar]
- [80].Westbrook TD, Maddocks K, Andersen BL, The relation of illness perceptions to stress, depression, and fatigue in patients with chronic lymphocytic leukaemia, Psychol. Health 31 (2016) 891–902, 10.1080/08870446.2016.1158259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Miceli J, Geller D, Tsung A, Hecht CL, Wang Y, Pathak R, Cheng H, Marsh W, Antoni M, Penedo F, Burke L, Ell K, Shen S, Steel J, Illness perceptions and perceived stress in patients with advanced gastrointestinal cancer, Psychooncology 28 (2019) 1513–1519, 10.1002/pon.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Leventhal H, Brissette I, Leventhal EA, The common-sense model of self-regulation of health and illness, in: Cameron LD, Leventhal H (Eds.), Self-Regul. Health Illn. Behav. Routledge, New York, NY, US, 2003, pp. 42–65. [Google Scholar]
- [83].Richardson EM, Schüz N, Sanderson K, Scott JL, Schüz B, Illness representations, coping, and illness outcomes in people with cancer: a systematic review and meta-analysis, Psychooncology 26 (2017) 724–737, 10.1002/pon.4213. [DOI] [PubMed] [Google Scholar]
- [84].Attari SM, Ozgoli G, Solhi M, Alavi Majd H, Study of relationship between illness perception and delay in seeking help for breast cancer patients based on Leventhal’s Self-Regulation Model, Asian Pac. J. Cancer Prev. 17 (2016) 167–174, 10.7314/apjcp.2016.17.s3.167. [DOI] [PubMed] [Google Scholar]
- [85].Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, Lennes IT, Dahlin CM, Pirl WF, Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care, J. Clin. Oncol 29 (2011) 2319–2326, 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 86.Mendoza TR, Kehl KL, Bamidele O, Williams LA, Shi Q, Cleeland CS, Simon G, Assessment of baseline symptom burden in treatment-naïve patients with lung cancer: an observational study, Support. Care Cancer (2019), 10.1007/s00520-018-4632-0. [DOI] [PubMed] [Google Scholar]
- [87].Iyer S, Roughley A, Rider A, Taylor-Stokes G, The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study, Support. Care Cancer 22 (2014) 181–187, 10.1007/s00520-013-1959-4. [DOI] [PubMed] [Google Scholar]
- [88].Harle ASM, Blackhall FH, Molassiotis A, Yorke J, Dockry R, Holt KJ, Yuill D, Baker K, Smith JA, Cough in patients with lung cancer: a longitudinal observational study of characterization and clinical associations, Chest 155 (2019) 103–113, 10.1016/j.chest.2018.10.003. [DOI] [PubMed] [Google Scholar]
- [89].Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y, Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer, J. Pain Symptom Manage 23 (2002) 417–423, 10.1016/S0885-3924(02)00376-7. [DOI] [PubMed] [Google Scholar]
- [90].Landis JR, Koch GG, The measurement of observer agreement for categorical data, Biometrics 33 (1977) 159–174, 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- [91].Radzikowska E, Glaz P, Roszkowski K, Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases, Ann. Oncol 13 (2002) 1087–1093, 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- [92].Bentley R, Hussain A, Maddocks M, Wilcock A, Occupational therapy needs of patients with thoracic cancer at the time of diagnosis: findings of a dedicated rehabilitation service, Support. Care Cancer 21 (2013) 1519–1524, 10.1007/s00520-012-1687-1. [DOI] [PubMed] [Google Scholar]
- [93].Pergolotti M, Williams GR, Campbell C, Munoz LA, Muss HB, Occupational therapy for adults with cancer: why it matters, Oncologist 21 (2016) 314–319, 10.1634/theoncologist.2015-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Luszczynska A, Pawlowska I, Cieslak R, Knoll N, Scholz U, Social support and quality of life among lung cancer patients: a systematic review, Psychooncology 22 (2013) 2160–2168, 10.1002/pon.3218. [DOI] [PubMed] [Google Scholar]
- [95].Wu Y, Ai Z, Xu G, Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database, Oncotarget 8 (2017) 103518–103534, 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D, Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer, J. Clin. Oncol 34 (2016) 1732–1740, 10.1200/JCO.2015.63.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ramsey SD, Bansal A, Fedorenko CR, Blough DK, Overstreet KA, Shankaran V, Newcomb P, Financial insolvency as a risk factor for early mortality among patients with cancer, J. Clin. Oncol 34 (2016) 980–986, 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Al-Baimani K, Jonker H, Zhang T, Goss GD, Laurie SA, Nicholas G, Wheatley-Price P, Are clinical trial eligibility criteria an accurate reflection of a real-world population of advanced non-small-cell lung cancer patients? Curr. Oncol 25 (2018) e291–e297, 10.3747/co.25.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wild B, Eckl A, Herzog W, Niehoff D, Lechner S, Maatouk I, Schellberg D, Brenner H, Müller H, Löwe B, Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 scales: results of a validation study, Am. J. Geriatr. Psychiatry 22 (2014) 1029–1038, 10.1016/j.jagp.2013.01.076. [DOI] [PubMed] [Google Scholar]
- [100].Löwe B, Spitzer RL, Gräfe K, Kroenke K, Quenter A, Zipfel S, Buchholz C, Witte S, Herzog W, Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians’ diagnoses, J. Affect. Disord. 78 (2004) 131–140, 10.1016/S0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- [101].Löwe B, Kroenke K, Herzog W, Gräfe K, Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9), J. Affect. Disord 81 (2004) 61–66, 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- [102].Cook SA, Salmon P, Hayes G, Byrne A, Fisher PL, Predictors of emotional distress a year or more after diagnosis of cancer: a systematic review of the literature, Psychooncology 27 (2018) 791–801, 10.1002/pon.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Brown DJF, McMillan DC, Milroy R, The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer, Cancer 103 (2005) 377–382, 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- [104].Vodermaier A, Lucas S, Linden W, Olson R, Anxiety after diagnosis predicts lung cancer-specific and overall survival in patients with stage III non-small cell lung cancer: a population-based cohort study, J. Pain Symptom Manage 53 (2017) 1057–1065, https://doi.Org/10.1016/j.jpainsymman.2016.12.338. [DOI] [PubMed] [Google Scholar]