Abstract
Multiple lines of evidence suggest that illness development in schizophrenia and other psychotic disorders predates the first psychotic episode by many years. In this study, we examined a sample of 15 pre-adolescent children, ages 7 through 12 years, who are at familial high-risk (FHR) because they have a parent or sibling with a history of schizophrenia or related psychotic disorder. Using multi-voxel pattern analysis (MVPA), a data-driven fMRI analysis, we assessed whole-brain differences in functional connectivity in the FHR sample as compared to an age- and sex-matched control (CON) group of 15 children without a family history of psychosis. MVPA analysis yielded a single cluster in right posterior superior temporal gyrus (pSTG/BA 22) showing significant group-differences in functional connectivity. Post-hoc characterization of this cluster through seed-to-voxel analysis revealed mostly reduced functional connectivity of the pSTG seed to a set of language and default mode network (DMN) associated brain regions including Heschl’s gyrus, inferior temporal gyrus extending into fusiform gyrus, (para)hippocampus, thalamus, and a cerebellar cluster encompassing mainly Crus I/II. A height-threshold of whole-brain p < .001 (two-sided), and FDR-corrected cluster-threshold of p < .05 (non-parametric statistics) was used for post-hoc characterization. These findings suggest that abnormalities in functional communication in a network encompassing right STG and associated brain regions are present before adolescence in at-risk children and may be a risk marker for psychosis. Subsequent changes in this functional network across development may contribute to either disease manifestation or resilience in children with a familial vulnerability for psychosis.
Keywords: Schizophrenia, offspring, familial high-risk, resting-state functional connectivity, MVPA, STG
Introduction
The first psychotic episode that marks the formal onset of schizophrenia typically occurs in adolescence or early adulthood. However, several lines of evidence suggest that the first episode is actually a rather late stage in the development of the illness (Insel, 2010; Keshavan et al., 2011). A decline in neurocognitive functioning precedes the onset of psychosis by almost a decade (Kahn and Keefe, 2013), and established neuroimaging findings in schizophrenia are consistent with a pathophysiological process that predates the onset of psychosis by many years. Reduced intracranial volume (ICV), for example, is a frequent finding in schizophrenia patients (Woods et al., 2005). As ICV is driven by brain growth and the brain reaches its maximum size around 12 years (Giedd et al., 1996), reductions in ICV are suggestive of abnormalities in brain development before adolescence. Converging evidence thus suggest that the manifestation of schizophrenia in adolescence or early adulthood is the end result of abnormal developmental processes that date back to childhood and before.
Indeed, family and birth cohort studies indicate developmental impairments in children and infants with a familial risk for schizophrenia, as well as children who will go on to develop the illness later in life (Keshavan et al., 2005; Liu et al., 2015). These impairments include early motor delays, speech and receptive language delays (Liu et al., 2015; Welham et al., 2009), and other impairments such as depressed mood and anxiety, social maladjustment, inattention, lower IQ, and scholastic underperformance (Fuller et al., 2002; Liu et al., 2015; Reichenberg et al., 2010; Seidman et al., 2013; van Oel et al., 2002; Welham et al., 2009). The neurobiological substrate for these developmental impairments remains to be clarified. As offspring and siblings of schizophrenia patients have approximately a 1 in 10 chance of developing schizophrenia and a 1 in 3 chance of developing any severe mental illness (Rasic et al., 2014), studying children with a family history of schizophrenia offers the opportunity to elucidate abnormal patterns of brain development that may lead up to the manifestation of mental illness.
Neuroimaging studies in nonpsychotic children with a familial vulnerability for schizophrenia have reported abnormalities in brain volume (Rajarethinam et al., 2004; Sugranyes et al., 2015), task-related brain activation (Rajarethinam et al., 2011), and functional and structural brain connectivity and organization (Collin et al., 2017) in at-risk children. These findings suggest that abnormalities in brain structure and function precede the manifestation of overt psychosis. However, it is unclear whether premorbid brain abnormalities arise during adolescence or before, as the average age of previous FHR cohorts ranges from 11 (Sugranyes et al., 2015) to 16 years (Rajarethinam et al., 2011). Indeed, in the youngest cohort (Sugranyes et al., 2015), supplemental analyses performed in a subgroup of children up to 12 years of age failed to show significant brain-volume differences in the younger at-risk children relative to controls.
In the current study, we examined a familial high-risk (FHR) group of pre-pubertal children, ages 7 to 12 years, with a first-degree relative with a psychotic disorder, and compared them to a group of control (CON) children without a family history of psychosis. Using an agnostic, data-driven resting-state fMRI analysis, we investigated whole-brain differences in functional connectivity between FHR and CON children. Our aim was to assess whether premorbid brain differences could be detected in at-risk children prior to adolescence, in order to increase our understanding of the trajectory of brain abnormalities leading up to psychosis.
Materials and methods
Participants
This study involved 30 children aged 7 to 12 years, including 15 FHR children and 15 age- and sex-matched CON children. The FHR children originated from a total of 10 families affected by psychotic illness (i.e., schizophrenia, schizoaffective disorder, bipolar disorder with psychotic features, or major depression with psychotic features) and included three sibling pairs and one set of three siblings. CON children originated from a total of 12 families and included 3 sibling pairs. Participants were recruited between October 2012 and June 2016 at the Department of Psychiatry of Beth Israel Deaconess Medical Center (BIDMC) in Boston. BIDMC’s Institutional Review Board (IRB) approved the study. All parents provided informed consent and the minor participants provided assent for participation in the study.
Demographic, clinical and neuropsychological evaluation
Clinical diagnoses of affected family members were confirmed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002) and/or medical history, interviewing at least one informant, and determined by consensus in meetings attended by senior clinicians (LJS, MSK, RMG). All children were assessed for current psychiatric disorders using the SCID for Childhood Diagnoses (Kid-SCID) (Hien et al., 1994). The Hollingshead scale was used to measure parental socioeconomic status (SES) for all children (Hollingshead, 1975). To this end, information on parental education and occupation was used to obtain a single SES score for each participant, ranging from class 1 (high-SES) to class 5 (low-SES) (Hollingshead and Redlich, 2007). Overall IQ was estimated using subtests from all four domains of the Wechsler Intelligence Scale for Children-IV (WISC-IV) (Wechsler, 2003). Puberty status of children was assessed through parental interviews using the Tanner criteria (Marshall and Tanner, 1969, 1970). Table 1 summarizes demographic, clinical, and neuropsychological characteristics for both groups.
Table 1.
Demographic and clinical variables
Statistical comparison was performed using analysis of variance (ANOVA) for continuous and chi-squared tests for categorical variables. SES = Socioeconomic status; DSM = Diagnostic and Statistical Manual; IQ = Intelligence Quotient (estimated based on WISC-IV subtests); SZ = schizophrenia; SA = schizoaffective disorder, BD = bipolar disorder; PD = psychotic depression; a higher values reflect lower SES, range 1-5 (Hollingshead and Redlich, 2007); b Current diagnosis based on Kid-SCID (Hien et al., 1994) interview, including ADHD (N = 7), co-morbid ODD (N = 3), and PTSD (N = 1); c data missing for one subject.
| FHR N = 15 |
CON N = 15 |
Stats | |
|---|---|---|---|
| Age in years, mean (sd) [range] | 9.8 (2.0) [7.1 – 12.4] |
9.4 (1.6) [7.3 – 12.2] |
F(1,28) = 0.34, p = .57 |
| Sex, M/F | 8 / 7 | 7 / 8 | χ2 = 0.13, p = .72 |
| Parental SES a, mean (sd) [range] | 3.5 (1.7) [1 – 5] |
1.7 (0.9) [1 – 3] |
F(1,28) = 13.79, P < .01 |
| IQ, mean (sd) [range] | 98.5 (17.2) [62 – 132] |
106.0 (13.0) [81 – 125] |
F(1,28) = 1.83, p = .19 |
| Tanner1, Tanner 2 mean | 1.33, 2.13 | 1.57, 2.07 | p= .36, p= .87 |
| DSM diagnosis participant b, N (%) | 8 (53%) | 0 (0%) | χ = 10.9, p < .01 |
| Relationship affected proband, mother / father /sibling | 10 / 0 / 5 | N/A | |
| Diagnosis affected proband, SZ / SA / BD / PD | 6 / 8 / 0 / 1 | N/A |
Image acquisition
MRI scans were acquired on a Siemens 3T scanner, MAGNETOM Trio, a Tim System (Siemens AG, Healthcare Sector, Erlangen, Germany), using a commercially available 32-channel radio frequency brain array coil (Siemens AG, Healthcare Sector). A 3-dimensional high-resolution T1-weighted structural scan was collected with voxel size = 1.3×1×1.3 mm3; TR / TE / inversion time / FA = 2530 msec / 3.39 msec / 1100 msec / 7°. A single-shot gradient EPI sequence was used to collect resting-state functional MRI (rs-fMRI) data (eyes open with fixation cross-hair, one run with scan duration of 6 min and 24 sec). In order to account for whole-brain coverage at high spatial resolution (2-isotropic) TR was chosen to be 6 seconds. Sixty-seven interleaved slices with orientation parallel to AC-PC plane were collected with TE = 30 msec, and FA = 90°.
Image preprocessing
Image preprocessing of rs-fMRI scans was performed using the CONN toolbox (version 18a) (https://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012) and included realignment, normalization to MNI space, slice timing correction, 6mm spatial smoothing, and band pass filtering (0.008-0.09 Hz). Physiological sources of noise including signals from white matter and cerebrospinal fluid were regressed out using the aCompcor method (Behzadi et al., 2007). Spurious correlations in time series due to head motion were addressed using the Artifact Detection Tool (ART, http://www.nitrc.org/projects/artifact_detect) with outliers defined as rs-fMRI volumes showing head displacement in the x, y, or z direction greater than 1.5 mm relative to the previous frame or global mean intensity greater than three standard deviations from the mean intensity of the entire scan. ART outliers along with the six realignment parameters were added as regressors of no interest. There was no significant difference in head motion between groups.
Multivariate pattern analysis
CONN toolbox implementation of whole-brain multi-voxel pattern analysis (MVPA) (Whitfield-Gabrieli and Nieto-Castanon, 2012) was used to detect brain areas with abnormal functional connectivity patterns in FHR compared to CON participants. Features of interest were extracted at the first level using 64 PCA components and three MVPA components were taken to the group level for an omnibus test F test for the FHR > CON contrast. The number of components was set to three as this corresponds to 10% of the total number of subjects in the present study, following conventions from previous investigations (Thompson et al., 2016). A detailed description of this methodology is provided in a previous report from our group (Arnold Anteraper et al., 2018). Clusters surviving a height threshold of p < .001 and FDR cluster-level threshold of p < .05 were taken for post-hoc analysis. The same threshold was applied for post-hoc characterization of resting-state functional connectivity (RsFc) for FHR > CON, as detailed in the following section.
Post-hoc characterization of MVPA-derived cluster
For within and between-group RsFc post-hoc characterization, the MVPA-derived cluster of interest was used for a whole-brain seed-to-voxel analysis. Pearson’s correlation coefficients between the MVPA seed-time course and the time course of all other voxels in the brain were computed and then converted to normally distributed z-scores using Fisher transformation in order to carry out second-level general linear model analyses. A height threshold of whole-brain p < .001 (two-sided) and FDR-corrected cluster threshold of p < .05 were used. Additionally, a non-parametric permutation analysis (1000 iterations) was used for between-group comparison. This method has been shown to be effective in curbing false positives, thereby adding validity to the cluster size inferences (Eklund et al., 2016).
Results
Participants
Group-comparisons of basic demographic and clinical characteristics confirmed that FHR and CON groups were well-matched for age, sex, and pubertal (Table 1). Parental socioeconomic status was lower in FHR children than in CON children (p = .001). Mean IQ was 8 points lower in the FHR than the CON group, but this difference was not statistically significant (p = .19). FHR children had significantly more DSM diagnoses as based on the Kid-SCID interview (p < .001), including mainly ADHD with or without co-morbid ODD. The average Tanner 1 and Tanner 2 scores across all participants were 1.5 and 2.1 respectively. The CON and FHR groups did not significantly differ on Tanner 1 (CON: mean = 1.57; FHR: mean = 1.33) or Tanner 2 scores (CON: mean = 2.07; FHR: mean = 2.13), suggesting that the groups were matched on puberty status. The average number of outlier time-points that were eliminated did not differ between the CON (6.00±4.96) and FHR (4.93±3.24) groups (p = 0.49).
MVPA results
A cluster in the right posterior superior temporal gyrus (pSTG/BA 22) was the only cluster to survive whole-brain MVPA between-group comparison (peak cluster MNI coordinates = 70, −14, 0) at whole-brain height-threshold of p<.001, and FDR-corrected cluster-threshold of p < .05). This region corresponds to auditory association cortex, situated close to primary auditory cortex at the intersection of auditory unimodal association areas A4 and A5 (Glasser et al., 2016) (Figure 1).
Figure 1.
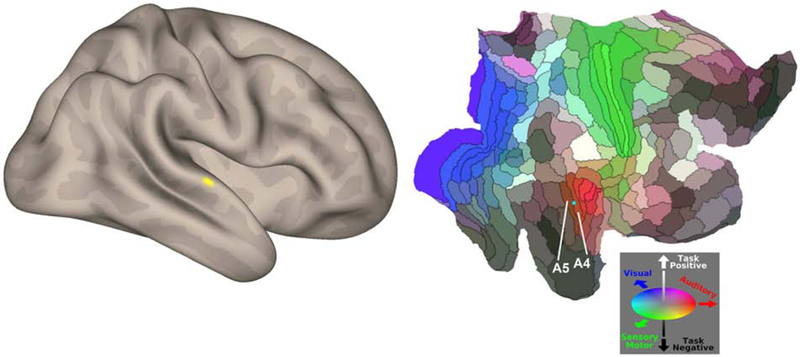
MVPA between-group results (whole-brain p<.001 for height-threshold, and FDR-corrected cluster-threshold of p < .05).
Left panel shows results from whole-brain analysis revealing abnormal connectivity in right posterior superior temporal gyrus. Right panel depicts Glasser’s multimodal brain parcellation, shown here on a flatmap of the right cerebral cortex, indicating that the peak of the right posterior STG cluster (indicated by a cyan sphere) corresponds to auditory association cortex, situated close to primary auditory cortex, at the intersection of auditory unimodal association areas A4 and A5 (Glasser et al., 2016).
Post-hoc characterization of MVPA cluster
Between-group characterization of the MVPA cluster as a region of interest for whole-brain seed- to-voxel analysis yielded ten clusters showing mostly reduced functional connectivity (whole-brain p<.001 for height-threshold, and FDR-corrected cluster-threshold of p < .05), with the MVPA-derived pSTG seed (Table II). There was significantly reduced functional connectivity with nine of these ten clusters, which were located at bilateral inferior temporal gyrus (ITG) (peak location in fusiform gyrii), bilateral transverse temporal gyrus of Heschl (HG), left parahippocampal gyrus, right hippocampus, right thalamus, bilateral cerebellar Crus I/II, and right posterior ITG (the only connection showing increased RsFc with the pSTG seed).
Table 2.
Post-hoc characterization of right STG cluster
Results from second-level seed-to-voxel RsFc analysis using MVPA-derived right STG cluster as a seed for FHR versus CON contrast. A height-threshold of whole-brain p < .001 (two-sided), and FDR-corrected cluster-threshold of p < .05 (non-parametric statistics) was used for post-hoc characterization.
| Brain Regions | Peak cluster coordinates (MNI) |
Voxels per cluster |
Tmax |
|---|---|---|---|
| BA 37/right fusiform gyrus (a) | 64 −46 −22 | 4239 | 9.36 |
| Cerebellar Crus I/II (b) | 2 −86 −36 | 1344 | 5.26 |
| BA 37/left fusiform gyrus (c) | −66 −52 −12 | 723 | 5.93 |
| BA 41/left Heschl’s gyrus (d) | −34 −30 2 | 240 | 5.97 |
| BA 36/left parahippocampal gyrus (e) | −28 −20 −28 | 211 | 4.72 |
| BA 41/right Heschl’s gyrus (f) | 50 −14 4 | 186 | 5.72 |
| Right Hippocampus (g) | 18 −38 0 | 172 | 7.62 |
| Right thalamus (h) | 8 4 2 | 165 | 7.36 |
| BA 20/Posterior inferior temporal gyrus (i) | 42 −30 −16 | 144 | −5.78 |
| Cerebellar lobule I-IV (j) | −4 −48 −6 | 138 | 7.05 |
Cerebellar clusters surviving between-group comparison were plotted onto cerebellar resting-state parcellations developed by Buckner and colleagues (Buckner et al., 2011) and along cerebellar functional gradients as developed by Guell and colleagues (Guell et al., 2018; Guell et al., 2019) for visualization purposes (Figure 2B, C). Figure 2D shows box plot representations of the clusters for the purpose of showing distributions of RsFc strengths.
Figure 2.

Post-hoc characterization of MVPA cluster
Results from post-hoc seed-to-voxel group comparison using the MVPA cluster (shown in Figure 1) as a seed. (A) Cerebral cortical results including FHR group main effect, CON group main effect, and FHR vs CON group comparison. (B) Left: Cerebellar results for the between-group contrast presented on a cerebellar flatmap (Diedrichsen and Zotow, 2015). Right: Cerebellar cluster shown in B represented in functional gradient space as developed by (Guell et al., 2019; Guell et al., 2018). (C) Cerebellar cluster shown in (B) overlaid on cerebellar representations of cerebral cortical networks (Buckner et al., 2011): visual in dark purple; somatomotor in blue; dorsal attention in green; ventral attention in violet; limbic in cream; frontoparietal in orange; default mode network in red. (D) Boxplots of FHR and CON connectivity data extracted from clusters shown in (A) and (B). Cluster labels in (A), (B), and (D) correspond to Table 2. HC=Healthy CON group. A height-threshold of whole-brain p < .001 (two-sided), and FDR-corrected cluster-threshold of p < .05 (non-parametric statistics) was used for post-hoc characterization.
Discussion
It remains to be determined whether schizophrenia-associated brain differences emerge in adolescence just prior to the typical timing of (prodromal) psychotic symptoms, or already present in early childhood. This study is the first evidence for abnormalities in functional brain connectivity in young children with a familial history of schizophrenia or a related psychotic disorder prior to adolescence, and thus many years before some of these children are likely to first develop psychotic symptoms. The differences in functional connectivity found in at-risk children as compared to a group of age- and sex-matched controls emerged from a data-driven and unbiased connectome-wide approach to identify functional brain abnormalities. Nonpsychotic children with a family history of schizophrenia showed disruptions in functional connectivity of the right posterior superior temporal gyrus (STG). Post-hoc characterization of this STG cluster indicated that its connections within a network of neighboring temporo-occipital, limbic, and cerebellar regions were most affected in children with a parental history of schizophrenia. These findings suggest that schizophrenia-related abnormalities in functional connectivity develop well before, perhaps a decade or more before, the typical age of onset of the disorder.
The childhood difference in STG functional connectivity is noteworthy because STG abnormalities in schizophrenia have been well-established (Shenton et al., 2001). Reductions in STG volume and density are among the most consistently reported structural brain abnormalities in patients with schizophrenia (Honea et al., 2005; Shenton et al., 2001). In addition, studies have shown abnormalities in structural (Burns et al., 2003; Kawashimaa et al., 2009; Kubicki et al., 2002) and functional (Oertel-Knöchel et al., 2013; Shinn et al., 2013) connectivity of the STG, and its activation during language-related tasks (Li et al., 2007; Woodruff et al., 1997). Given the STG’s role in auditory perception and language, a long-standing hypothesis in schizophrenia is that abnormalities of this region may underlie symptoms such as auditory hallucinations and thought disorder. Indeed, studies in patients undergoing epilepsy surgery have shown that electrical stimulation of the STG elicits complex auditory hallucinations (Nakai et al., 2017; Penfield and Roberts, 1959). Moreover, event-related fMRI analysis in actively hallucinating schizophrenia patients has shown STG activation just prior to and during auditory verbal hallucinations (Dierks et al., 1999) and other studies in schizophrenia have linked structural and functional STG abnormalities to auditory verbal hallucinations (Alderson-Day et al., 2015; Modinos et al., 2013; Mørch-Johnsen et al., 2017) and formal thought disorder (Horn et al., 2010, 2009; Sans-Sansa et al., 2013; Subotnik et al., 2003; Weinstein et al., 2007). In all, there is considerable evidence to suggest that STG abnormalities are part of the neuropathology of schizophrenia and contribute to some of its cardinal symptoms.
The present finding of abnormal functional connectivity of the STG in nonpsychotic children with a familial history of schizophrenia is consistent with the findings of several previous studies. Task-based fMRI studies have shown abnormal STG functioning during language processing in individuals with a familial vulnerability for schizophrenia (Rajarethinam et al., 2011; Thermenos et al., 2013). Specifically, abnormal activation of the right STG within a network encompassing inferior frontal gyrus, middle temporal gyrus, and cerebellum has been demonstrated in young FHR adults performing a semantic association task (Thermenos et al., 2013) and reduced activation of bilateral STG during auditory comprehension was shown in nonpsychotic adolescent offspring of schizophrenia patients (Rajarethinam et al., 2011). The same group also reported significant volume reductions of bilateral STG in FHR adolescents (Rajarethinam et al., 2004). Moreover, increased activation of the STG has been found in young adults with a familial susceptibility for schizophrenia during a working-memory task, possibly reflecting a failure to deactivate the STG during working memory performance (Choi et al., 2012). Further, studies of individuals in the prodromal or Clinical High-Risk (CHR) stage of schizophrenia suggest that structural and functional STG changes are predictive of conversion to psychosis (Collin et al., 2018; Sabb et al., 2010). The present findings thus converge with prior findings suggesting that STG abnormalities may be a premorbid marker of schizophrenia risk. Such abnormalities may worsen over time and contribute to the eventual manifestation of psychosis, or may normalize with development in nonaffected individuals (Mattai et al., 2011; Zalesky et al., 2015).
We note that neuroimaging studies in schizophrenia typically show more pronounced abnormalities in the left as compared to the right STG (for review see (Honea et al., 2005; Shenton et al., 2001). Reports of STG abnormalities in young high-risk subjects, however, tend to suggest bilateral (Rajarethinam et al., 2011, 2004) or right-hemisphere (Choi et al., 2012; Thermenos et al., 2013) differences. Moreover, a study comparing adolescents with early-onset schizophrenia to adult-onset patients showed significant reductions in right STG volume in the adolescent as compared to the adult patients, with more extensive volume reductions correlating with younger age at onset and greater severity of hallucinations and conceptual disorganization (Matsumoto et al., 2001). There is thus some evidence to suggest that the right STG may be particularly affected in younger individuals and in the premorbid phase of the illness.
Post-hoc FHR versus CON characterization using the right posterior STG cluster as a seed revealed reduced connectivity from STG to bilateral HG, fusiform gyrus (FG), thalamus, (para)hippocampus, and cerebellar territories in bilateral Crus I/II and parts of lobules I-IV, and increased connectivity between STG and posterior inferior temporal gyrus (ITG). As the STG is a known hub in a network of brain regions subserving speech and language (Pearlson, 1997), the regions identified in the post-hoc analysis may reflect an extended language-related network. HG contains primary auditory cortex, FG has been shown to be involved in semantic processing, particularly in children (Balsamo et al., 2006), IFG contributes to semantic and phonological processing and inner-speech generation (Liakakis et al., 2011), cerebellum modulates multiple aspects of language processing (Guell et al., 2015), with certain language tasks specifically activating Crus I/II (X. Guell et al., 2018), and there is evidence that the thalamus (Klostermann et al., 2013) and hippocampus (Covington and Duff, 2016) also support language processing. Abnormal functional connectivity within a language-related circuit in FHR children would be consistent with previous findings of language network abnormalities in young at-risk individuals (Colibazzi et al., 2017; Thermenos et al., 2013).
Additionally, several regions exhibiting atypical functional connectivity with the right STG in the FHR children are associated with the default-mode network (DMN). Specifically, STG, ITG, FG, and parahippocampal gyrus have all been reported as part of the DMN (Hu et al., 2017; Joshi et al., 2017; Long et al., 2008; Uddin et al., 2009). Moreover, the regions of the cerebellum exhibiting atypical functional connectivity with the right STG have been linked to the DMN in typical adults using both discrete (Buckner et al., 2011) and gradient-based (Guell et al., 2018) atlases of cerebellar functional neuroanatomy (Figure 2B, C). These findings tie in with previous structural investigations of schizophrenia showing specific abnormalities in cerebellar DMN territories in Crus I/II (see figure 4 in Moberget et al. 2018) and preliminary evidence suggesting that TMS stimulation of cerebellar Crus I/II may improve symptoms in the disorder (Brady et al., 2019; Demirtas-Tatlidede et al., 2010). Previous studies have highlighted DMN dysfunction in schizophrenia (Hu et al., 2017; Whitfield-Gabrieli et al., 2009), and the present findings suggest that atypical functional relations between language and DMN networks may occur early in the development of risk for schizophrenia.
There are a number of limitations to this study that should be considered when interpreting these findings. The first and principal limitation is the small size of our sample. Children with a parent or sibling diagnosed with schizophrenia are difficult to recruit, particularly in the young age range of our sample, due to issues such as reduced fecundity in patients and the disorganization of families as a result of the illness (Laursen and Munk-Olsen, 2010; Power et al., 2013). Given our small sample size, we suggest that our results are taken as preliminary evidence of abnormal STG functional connectivity in pre-adolescent children with a familial vulnerability for schizophrenia. Future studies are needed to replicate our results and determine how these putative abnormalities in functional connectivity change as the children develop. Second, approximately half the FHR children had a DSM diagnosis (mainly ADHD with or without co-morbid ODD) while there were no diagnoses in the CON group. Childhood developmental disorders including ADHD are common in schizophrenia offspring (Keshavan et al., 2003; Öner and Munir, 2005) and it is difficult to disentangle the influence of these disorders from the vulnerability for schizophrenia. As at-risk offspring with ADHD-like symptoms may be particularly prone to psychosis (Keshavan et al., 2003), excluding FHR children with these symptoms is not appropriate as this may effectively exclude the children with the highest schizophrenia risk. Future studies directly comparing FHR children with ADHD (-like) symptoms to children with ADHD but without a familial history of schizophrenia may help elucidate whether brain abnormalities as found in the current study are part of the neurobiology of schizophrenia or ADHD. Moreover, while ADHD is common in the general community (i.e., without familial risk for psychotic illness), DSM-diagnoses were an exclusion criterion for CON children in our current study. Future studies comparing FHR children to a community control group may be better equipped to assess the relative frequency of childhood DSM diagnoses in FHR children. Similarly, SES was lower in FHR compared to controls. Future studies with higher statistical power will be needed to dissociate brain correlates of socioeconomic conditions from risk of schizophrenia. SES-controlled studies may detect brain correlates that can be more confidently attributed to macro-scale abnormalities directly linked to psychosis. However, as in the case of ADHD symptomatology, these correlates would arguably be more distant from the naturalistic neurophysiological reality of risk of schizophrenia that is influenced by additional psychiatric phenomena and socioeconomical difficulties, and exclude those individuals at highest risk. Third, the right posterior STG cluster yielded by the MVPA analysis is relatively small in size, which may relate to our stringent primary cluster threshold (p < .001). Our choice for this threshold is in accordance with current recommendations on cluster-based thresholding in fMRI analyses (Woo et al., 2014) and while our analysis was entirely data-driven, our findings fit well in existing literature on schizophrenia and at-risk subjects. Fourth, we used a combination of 32 channel receive coil and (long) 6s TR for data acquisition. Our goal in using this combination was to acquire higher spatial resolution without compromising whole-brain coverage. Studies have shown a substantial improvement in temporal signal to noise ratio at high-resolution acquisition compared to lower resolutions (Robinson et al., 2008). Moreover, 32 channel receive coils provide the biggest increases in tSNR at high spatial resolution, because thermal noise contributes significantly to resting state time-series’ standard deviation in the high-resolution regime as opposed to physiological noise, which dominates in bigger voxel volumes (Triantafyllou et al., 2011). In addition to capturing less physiological fluctuation, moving towards lower voxel volumes is also desirable for improving spatial specificity, to avoid partial volume effects and achieve precise localization of neuronal activity. With respect to our long TR, we note that a study comparing TRs of 2.5 and 5 seconds found no significant difference in functional connectivity correlations between resting state networks (Dijk et al., 2010).
In all, this data-driven study in a small sample of children aged 7 to 12 years with a parent or sibling with a psychotic disorder provides preliminary evidence that children with a familial vulnerability for psychosis show abnormal functional connectivity of the right STG within a network of language- and DMN-associated brain regions. This finding suggests that abnormal functional communication between brain networks implicated in adult schizophrenia predates the manifestation of the illness and may be considered a premorbid risk marker for psychosis detectable many years before the potential onset of symptoms. Longitudinal FHR studies are needed to elucidate how developmental changes in such premorbid risk markers contribute to either disease manifestation or resilience in children and youth at risk for psychosis.
Acknowledgements
This study was supported by NIMH grant R21 MH092840 (L.J. Seidman, M.S. Keshavan and J.D. Gabrieli MPIs); G. Collin was supported by a EU-Marie Curie Global Fellowship (Grant no. 749201); X. Guell was supported by the MGH Tosteson & Fund for Medical Discovery Award. The authors also acknowledge the Athinoula A. Martinos Imaging Center and Poitras Center for Psychiatric Disorders Research at the McGovern Institute for Brain Research (MIT).
Role of Funding Source
NIMH grant R21 MH092840 (L.J. Seidman, M.S. Keshavan and J.D. Gabrieli MPIs) was instrumental in funding for the MRI data collection for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest.
References
- Alderson-Day B, McCarthy-Jones S, Fernyhough C, 2015. Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev 55, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Anteraper S, Guell X, D’Mello A, Joshi N, Whitfield-Gabrieli S, Joshi G, 2019. Disrupted Cerebro-cerebellar Intrinsic Functional Connectivity in Young Adults with High-functioning Autism Spectrum Disorder: A Data-driven, Whole-brain, High Temporal Resolution Functional Magnetic Resonance Imaging Study. Brain Connect 9, 48–59. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD, 2006. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage 31, 1306–1314. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Gonsalvez I, Lee I, Öngür D, Seidman LJ, 2019. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am J Psychiatry [Epub ahead of print]. 10.1176/appi.ajp.2018.18040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Ay TM, 2003. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 182, 439–443. [PubMed] [Google Scholar]
- Choi JS, Park JY, Jung MH, Jang JH, Kang DH, Jung WH, Han JY, Choi CH, Hong KS, Kwon JS, 2012. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophr Bull 38, 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi T, Yang Z, Horga G, Yan CG, Corcoran CM, Klahr K, Brucato G, Girgis RR, Abi-Dargham A, Milham MP, Peterson BS, 2017. Aberrant Temporal Connectivity in Persons at Clinical High Risk for Psychosis. Biol Psychiatry 2, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Scholtens LH, Kahn RS, Hillegers MHJ, van den Heuvel MP, 2017. Affected Anatomical Rich Club and Structural-Functional Coupling in Young Offspring of Schizophrenia and Bipolar Disorder Patients. Biol. Psychiatry 1–10. [DOI] [PubMed] [Google Scholar]
- Collin G, Seidman LJ, Keshavan MS, Stone WS, Qi Z, Zhang T, Tang Y, Li H, Anteraper SA, Niznikiewicz MA, McCarley RW, Shenton ME, Wang J, Whitfield-Gabrieli S, 2018. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry [Epub ahead of print]. 10.1038/s41380-018-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington NV, Duff MC, 2016. Expanding the Language Network: Direct Contributions from the Hippocampus. Trends Cogn Sci 20, 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A, 2010. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res 124, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Zotow E, 2015. Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS One 10, e0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T, J Linden DE, Jandl M, Formisano E, Goebel R, 1999. Activation of Heschl’s Gyrus during Auditory Hallucinations of these studies could directly differentiate between the hallucinatory and nonhallucinatory states within one scanning session. Because of this restriction, they did. Neuron 22, 615–621. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, 2010. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory , Properties , and Optimization. J Neurophysiol 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho B, Andreasen N, 2002. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry 159, 1183–1189. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL, 1996. Quantitative magnetic resonance imaging of human brain development: Cereb Cortex 6, 551–560. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Gabrieli JDE, Schmahmann JD, 2018. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Goncalves M, Kaczmarzyk JR, Gabrieli JDE, Schmahmann JD, Ghosh SS, 2019. LittleBrain: A gradient-based tool for the topographical interpretation of cerebellar neuroimaging findings. PLoS One 14, e0210028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Hoche F, Schmahmann JD, 2015. Metalinguistic Deficits in Patients with Cerebellar Dysfunction: Empirical Support for the Dysmetria of Thought Theory. Cerebellum 14, 50–58. [DOI] [PubMed] [Google Scholar]
- Guell X, Schmahmann J, Gabrieli J, Ghosh S, 2018. Functional gradients of the cerebellum. Elife 7, e36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien D, Matzner FJ, First MB, Spitzer RL, Gibbon M, Williams JBW, 1994. Structured clinical interview for DSM-IV-child edition (Version 1.0). New York: Colombia University. Columbia University, New York. [Google Scholar]
- Hollingshead AB, 1975. Four factor index of social status, Unpublished manuscript. New Haven, CT. [Google Scholar]
- Hollingshead AB, Redlich FC, 2007. Social class and mental illness: A community study. Am J Public Heal. 97, 1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Sc B, Crow TJ, Ph D, Passingham D, Ph D, Mackay CE, Ph D, 2005. Regional Deficits in Brain Volume in Schizophrenia : A Meta-Analysis of Voxel-Based Morphometry Studies. Am J Psychiatry 162, 2233–2245. [DOI] [PubMed] [Google Scholar]
- Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Walther S, Strik W, 2010. Gray matter volume differences specific to formal thought disorder in schizophrenia. Psychiatry Res 182, 183–186. [DOI] [PubMed] [Google Scholar]
- Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Wang JJ, Strik W, 2009. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry 194, 130–138. [DOI] [PubMed] [Google Scholar]
- Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, He Y, Chen XG, Tang JS, 2017. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci Bull 33, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, 2010. Rethinking schizophrenia. Nature 468, 187–193. [DOI] [PubMed] [Google Scholar]
- Joshi G, Arnold Anteraper S, Patil K, Semwal M, Goldin R, Furtak S, Chai XJ, Saygin Z, Gabrieli JD, Biederman J, Whitfield-Gabrieli S, 2017. Integration and Segregation of Default Mode Network Resting-state Functional Connectivity in Transition-age Males with High-functioning Autism Spectrum Disorder: A Proof of Concept Study. Brain Connect 7, 558–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Keefe RSE, 2013. Schizophrenia is a cognitive illness: Time for a change in focus. JAMA psychiatry 70, 1107–12. [DOI] [PubMed] [Google Scholar]
- Kawashimaa T, Nakamuraa M, Bouix S, Kubickia M, Salisbury DF, Westin C-F, McCarley RW, Shenton ME, 2009. Uncinate Fasciculus Abnormalities in Recent Onset Schizophrenia and Affective Psychosis: A Diffusion Tensor Imaging Study. Schizophr Res 110, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Delisi LE, Seidman LJ, 2011. Early and broadly defined psychosis risk mental states. Schizophr Res 126, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA, 2005. Premorbid indicators and risk for schizophrenia: A selective review and update. Schizophr Res 79, 45–57. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Sujata M, Mehra A, Montrose DM, Sweeney JA, 2003. Psychosis proneness and ADHD in young relatives of schizophrenia patients. Schizophr Res 59, 85–92. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Krugel LK, Ehlen F, 2013. Functional roles of the thalamus for language capacities. Front Syst Neurosci 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C-F, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME, 2002. Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study. Am J Psychiatry 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen T, Munk-Olsen T, 2010. Reproductive patterns in psychotic patients. Schizophr Res 121, 234–240. [DOI] [PubMed] [Google Scholar]
- Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE, 2007. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophr Res 96, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ, 2011. Diversity of the inferior frontal gyrus-A meta-analysis of neuroimaging studies. Behav Brain Res 225, 341–347. [DOI] [PubMed] [Google Scholar]
- Liu CH, Keshavan MS, Tronick E, Seidman LJ, 2015. Perinatal risks and childhood premorbid indicators of later psychosis: Next steps for early psychosocial interventions. Schizophr Bull 41, 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long XY, Zuo XN, Kiviniemi V, Yang Y, Zou QH, Zhu CZ, Jiang TZ, Yang H, Gong QY, Wang L, Li KC, Xie S, Zang YF, 2008. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Methods 171, 349–355. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM (1969). Variations in the pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Simmons A, Williams S, Hadjulis M, Pipe R, Murray R, Frangou S, 2001. Superior temporal gyrus abnormalities in early-onset schizophrenia: Similarities and differences with adult-onset schizophrenia. Am J Psychiatry 158, 1299–1304. [DOI] [PubMed] [Google Scholar]
- Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, Tossell JW, Rapoport JL, Gogtay N, 2011. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry 50, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Doan NT, Alns D, Kaufmann T, Córdova-Palomera A, Lagerberg TV, Diedrichsen J, Schwarz E, Zink M, Eisenacher S, Kirsch P, Jönsson EG, Fatouros-Bergman H, Flyckt L, Pergola G, Quarto T, Bertolino A, Barch D, Meyer-Lindenberg A, Agartz I, Andreassen OA, Westlye LT, 2018. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry 23, 1512–1520. [DOI] [PubMed] [Google Scholar]
- Modinos G, Costafreda SG, Van Tol MJ, McGuire PK, Aleman A, Allen P, 2013. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex 49, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Mørch-Johnsen L, Nesvåg R, Jørgensen KN, Lange EH, Hartberg CB, Haukvik UK, Kompus K, Westerhausen R, Osnes K, Andreassen OA, Melle I, Hugdahl K, Agartz I, 2017. Auditory cortex characteristics in schizophrenia: Associations with auditory hallucinations. Schizophr Bull 43, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Jeong J, Brown EC, Rothermel R, Kojima K, Kambara T, Shah A, Mittal S, Sood S, Asano E, 2017. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain 140, 1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Knochel C, Matura S, Prvulovic D, Linden DEJ, van de Ven V, 2013. Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr. Res 147, 331–338. [DOI] [PubMed] [Google Scholar]
- Oner O, Munir K, 2005. Attentional and neurocognitive characteristics of high-risk offspring of parents with schizophrenia compared with DSM-IV attention deficit hyperactivity disorder children. Schizophr Res 76, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, 1997. Superior temporal gyrus and planum temporale in schizophrenia: A selective review. Prog Neuro-Psychopharmacol Biol Psychiatry 21, 1203–1229. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L, 1959. Speech and brain mechanisms. Princeton University Press, Princeton, NJ. [Google Scholar]
- Power R, Kyaga S, Uher R, MacCabe J, Langstrom N, Landen M, McGuffin P, Lewis C, Lichtenstein P, Svensson A, 2013. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA psychiatry 70, 22–30. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS, 2004. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry 161, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Venkatesh BK, Peethala R, Phan KL, Keshavan M, 2011. Reduced activation of superior temporal gyrus during auditory comprehension in young offspring of patients with schizophrenia. Schizophr Res 130, 101–105. [DOI] [PubMed] [Google Scholar]
- Rasic D, Hajek T, Alda M, Uher R, 2014. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: A meta-analysis of family high-risk studies. Schizophr Bull 40, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harrington H, Houts R, Keefe RSE, Murray RM, Poulton R, Moffitt TE, 2010. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: A 30-year study. Am J Psychiatry 167, 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Pripfl J, Bauer H, 2008. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the Default Mode. Magn Reson Mater Phy 21, 279–290. [DOI] [PubMed] [Google Scholar]
- Sabb FW, van Erp TGM, Hardt ME, Dapretto M, Caplan R, Cannon TD, Bearden CE, 2010. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res 116, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans-Sansa B, McKenna PJ, Canales-Rodríguez EJ, Ortiz-Gil J, López-Araquistain L, Sarró S, Dueñas RM, Blanch J, Salvador R, Pomarol-Clotet E, 2013. Association of formal thought disorder in schizophrenia with structural brain abnormalities in language-related cortical regions. Schizophr Res 146, 308–313. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL, 2013. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychol Med 43, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW, 2001. A review of MRI findings in schizophrenia. Schizophr. Res 49, 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Cohen BM, Öngür D, 2013. Functional Connectivity of Left Heschl’s Gyrus in Vulnerability to Auditory Hallucinations in Schizophrenia. Schizophr Res 143, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subotnik KL, Bartzokis G, Green MF, Nuechterlein KH, 2003. Neuroanatomical correlates of formal thought disorder in schizophrenia. Cogn Neuropsychiatry 8, 81–88. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, De La Serna E, Romero S, Sanchez-Gistau V, Calvo A, Moreno D, Baeza I, Diaz-Caneja CM, Sanchez-Gutierrez T, Janssen J, Bargallo N, Castro-Fornieles J, 2015. Gray Matter Volume Decrease Distinguishes Schizophrenia From Bipolar Offspring During Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 54, 677–684. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Whitfield-Gabrielie S, Seidman LJ, Kuperberg G, Juelich RJ, Divatia S, Riley C, Jabbar GA, Shenton ME, Kubicki M, Manschreck T, Keshavan MS, DeLisi LE, 2013. Altered language network activity in young people at familial high-risk for schizophrenia. Schizophr Res 151, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WH, Thelin EP, Lilja A, Bellander B-M, Fransson P, 2016. Functional resting-state fMRI connectivity correlates with serum levels of the S100B protein in the acute phase of traumatic brain injury. Neuroimage Clin. 12, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou C, Polimeni JR, Wald LL, 2011. Neuroimage Physiological noise and signal-to-noise ratio in fMRI with multi-channel array coils. Neuroimage 55, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP, 2009. Functional Connectivity of Default Mode Network Components: Correlation, Anticorrelation, and Causality. Hum Brain Mapp 30, 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oel CJ, Sitskoorn MM, Cremer MPM, Kahn RS, 2002. School performance as a premorbid marker for schizophrenia: a twin study. Schizophr Bull 28, 401–414. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2003. Wechsler Intelligence Scale for Children - Fourth Edition: Technical and interpretive manual. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Weinstein S, Woodward TS, Ngan ETC, 2007. Brain activation mediates the association between structural abnormality and symptom severity in schizophrenia. Neuroimage 36, 188–193. [DOI] [PubMed] [Google Scholar]
- Welham J, Scott J, Williams G, Najman J, Bor W, O’Callaghan M, McGrath J, 2009. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med 39, 625–634. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ, 2009. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. PNAS 106, 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD, 2014. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM, 1997. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: A functional magnetic resonance imaging study. Am J Psychiatry 154, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Woods BT, Ward KE, Johnson EH, 2005. Meta-analysis of the time-course of brain volume reduction in schizophrenia: Implications for pathogenesis and early treatment. Schizophr Res 73, 221–228. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Pantelis C, Cropley V, Fornito A, Cocchi L, Mcadams H, Clasen L, Greenstein D, Rapoport JL, Gogtay N, 2015. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry 72, 900–908. [DOI] [PubMed] [Google Scholar]


