Abstract
Background
As the incidence of primary total joint arthroplasty rises in the United States, it is important to investigate how this will impact rates of revision arthroplasty. The purpose of this study was to analyze the incidence and future projections of revision total hip arthroplasty (rTHA) and revision total knee arthroplasty (rTKA) to 2030. Anticipating surgical volume will aid surgeons in designing protocols to efficiently and effectively perform rTHA/rTKA.
Methods
The national inpatient sample was queried from 2002 to 2014 for all rTHA/rTKA. Using previously validated measures, Poisson and linear regression analyses were performed to project annual incidence of rTHA/rTKA to 2030, with subgroup analyses on modes of failure and age.
Results
In 2014, there were 50,220 rTHAs and 72,100 rTKAs. From 2014 to 2030, rTHA incidence is projected to increase by between 43% and 70%, whereas rTKA incidence is projected to increase by between 78% and 182%. The 55–64 and 65–74 age groups increased in revision incidence during the study period, whereas 75–84 age group decreased in incidence. For rTKA, infection and aseptic loosening are the 2 most common modes of failure, whereas periprosthetic fracture and infection are most common for rTHA.
Conclusion
The incidence of rTHA/rTKA is projected to increase, particularly in young patients and for infection. Given the known risk factor profiles and advanced costs associated with revision arthroplasty, our projections should encourage institutions to generate revision-specific protocols to promote safe pathways for cost-effective care that is commensurate with current value-based health care trends.
Level of Evidence
IV.
Keywords: revision hip arthroplasty, revision knee arthroplasty, incidence, projections, infection
Primary hip and knee arthroplasty ranks among the top 5 most common procedures performed and among the top 5 fastest growing procedures each year, across all surgical disciplines in the United States [1]. Within orthopedics, hip and knee arthroplasty incidence far outpaces all other surgical procedures and thus routinely sits at the forefront of cost [2], value [3], outcome durability [4], and indications, [5] discussions, particularly in the eras of bundled payments [6], patient-reported outcomes [7], and quality-incentivized reimbursement [8,9]. The cost-effectiveness and quality of life benefits of total joint arthroplasty (TJA) are well documented and favor appropriately indicated surgery over nonoperative management [9–11].
Given the baseline high rate of success of modern TJA [12] and technological advances designed to extend the lifetime of primary implants [13–15], joint reconstruction that was once reserved for the low-demand population has been increasingly used in younger cohorts [16,17]. Varying reports of implant survivorship in higher demand patients range from nondifferent vs older patients [18,19] to significantly truncated [16,20]. Pragmatically, even modern implant bearings and well-fixed components have a finite life span that is more likely to be exceeded in the younger active patient.
As the more institutions adopt standardized processes for patient selection, preparation, surgical throughput, and expeditious discharge for primary total joint arthroplasty (pTJA), it is worth noting the dissimilarities between revision and primary arthroplasty. Relative to primary arthroplasty, revision surgeries have a higher rate of sepsis, prosthetic joint infection (PJI), medical complications, prolonged surgical time and length of inpatient stay, more blood loss and transfusion, nonhome discharge, and increased cost of care [21–24]. The advanced complexity of care for revision TJA (rTJA) patients calls into question the application of pTJA protocols to rTJA patients. As the incidence of rTJA continues to anecdotally rise, the importance of developing revision-specific protocols is self-evident in the efficacy of pTJA protocols [25]. Such protocols should be durable with time and thus should incorporate the projected rTJA demand to facilitate appropriate throughput capabilities. With the modern growth of primary arthroplasty, we expect an increase in the incidence of rTJA despite emphasis on pTJA survivorship. To examine this, we use statistical modeling to project long-term trends for rTJA to the year 2030. In addition, we analyze modern trends of indications and the epidemiology of revision hip and knee arthroplasty to the year 2030, based on the most recent, consecutive, and complete nationwide data, from 2002 to 2014.
Methods
Database Description
This study was exempt from institutional review board approval. Data were obtained from the national inpatient sample (NIS) database, which is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project. This sample represents an approximation of 20% of all nonfederal hospitals in the United States. Weighting variables are assigned to the data to provide estimates, which can be applied to the entire country. Given the size and nationwide breadth of the NIS database, it is ideal for use in procedural epidemiologic investigations.
Inclusion Criteria and Variables
International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes 00.80, 00.81, 00.82, 00.83, 00.84, 80.06, and 81.55 were used to identify all patients undergoing revision total knee arthroplasty (rTKA) and 00.70, 00.71, 00.72, 00.73, 70.05, and 81.53 for all revision total hip arthroplasty (rTHA) from 2002 to 2014 (Table 1). International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes were used to identify the indication for each procedure (Table 1). The date range was chosen to account for the most recent complete data set, as the transition to International Classification of Diseases, Tenth Revision coding in the fourth quarter of 2015 introduces uncertainty into the data set and has the potential to confound the results. For subgroup analysis on the modes of failure of primary arthroplasty (indications for the studied revision), these data were first available in 2006, and so the study period was adjusted to 2006–2014 for this secondary analysis, and all patients with available diagnoses other than “not otherwise specified” were included for a more granular analysis.
Table 1.
International Classifications of Disease, Ninth Revision, Clinical Modification Codes for Revision Joint Arthroplasty.
| Diagnosis Code | Description | ||
| 996.41 | Mechanical loosening of prosthetic joint | ||
| 996.42 | Dislocation of prosthetic joint | ||
| 996.43 | Prosthetic join implant failure/breakage | ||
| 996.44 | Periprosthetic fracture of prosthetic joint | ||
| 996.45 | Periprosthetic osteolysis of prosthetic joint | ||
| 996.46 | Articular bearing surface wear of prosthetic joint | ||
| 996.47 | Other mechanical complication of prosthetic joint | ||
| 996.49 | Other mechanical complication of internal orthopedic device, implant, and graft | ||
| 996.66 | Infection and inflammatory reaction because of internal joint prosthesis | ||
| 84.56 | Insertion of antibiotic cement spacer | ||
| 84.57 | Removal of antibiotic cement spacer | ||
| rTKA Procedure Codes | Description | rTHA Procedure Codes | Description |
| 00.80 | Revision of tibial, patellar, and femoral components | 00.70 | Revision of all components (both acetabular and femoral) |
| 00.81 | Revision of tibial component | 00.71 | Revision of acetabular component (includes femoral head) |
| 00.82 | Revision of femoral component | 00.72 | Revision of femoral component (includes acetabular liner) |
| 00.83 | Revision of patellar component | 00.73 | Isolated revision of head/liner |
| 00.84 | Isolated revision of tibial insert | 80.05 | Arthrotomy/removal of prosthesis |
| 80.06 | Arthrotomy/removal of prosthesis | 81.53 | Revision of knee, NOS |
| 81.55 | Revision of knee, NOS | ||
rTKA, revision total knee arthroplasty; rTHA, revision total hip arthroplasty; NOS, not otherwise specified.
Statistical Analysis
The annual incidence of rTJA procedures was obtained and subgrouped by age group (in decade increments: younger than 55 years, 55–64, 65–74, 75–84, and older than 85 years) and indications for revision, when available. Independent Poisson and linear regression models were used to project future incidence for rTHA and rTKAto2030, as done previously [26]. Furthermore, Poisson and linear models were also used to predict the future incidence of PJI for both rTHA and rTKA. Adjusted R2 and pseudo-R2 values were calculated for the linear and Poisson regression model to determine model fit. The linear model was chosen as a conservative reference to quantify the rate of growth within the study period. Poisson and linear regression analysis was performed using IBM SPSS (version 25.0; IBM Corp, Armonk, NY) and RStudio (version 1.2.133; RStudio, Inc., Vienna, Austria).
Results
Epidemiology
There were a total of 50,220 rTHAs performed in 2014, a 36% increase from the 36,898 performed in 2002. The age group with the greatest magnitude of change was 55–64 years, which grew by 184%; the 65–74 age group had the second largest growth with a 52% increase in the study period. The relative percentage of all revisions accounted for by the 55–64 age group increased by 9.1%, with 15.9% of all revisions in 2002 and 25% of revisions in 2014. The relative percentage increase for 65–74 age group was 2.9%, from 25.9% in 2002 to 28.8% in 2014. The younger than 55 and 75–84 age groups declined, whereas the older than 85 age group remained most unchanged (Table 2).
Table 2.
Revision by Age Group: Total Number and Percent of Total Accounted for.
| Year | rTHA |
rTKA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Revisions |
||||||||||
| <55 | 55–64 | 65–74 | 75–84 | 85+ | <55 | 55–64 | 65–74 | 75–84 | 85+ | |
| 2002 | 8010 | 5881 | 9547 | 10,539 | 2917 | 5937 | 7870 | 11,060 | 9337 | 1402 |
| 2003 | 6902 | 5872 | 9137 | 10,081 | 2838 | 6258 | 8314 | 11,907 | 9273 | 1625 |
| 2004 | 7452 | 6588 | 9358 | 10,605 | 3258 | 7185 | 9546 | 12,784 | 9797 | 1687 |
| 2005 | 7306 | 6657 | 9125 | 11,094 | 3156 | 8649 | 11,293 | 14,074 | 10,663 | 1841 |
| 2006 | 7085 | 7184 | 8849 | 9611 | 3080 | 8233 | 12,418 | 14,073 | 10,250 | 1892 |
| 2007 | 7148 | 7241 | 9440 | 10,434 | 3319 | 8411 | 13,673 | 15,218 | 11,311 | 1998 |
| 2008 | 7832 | 8499 | 9614 | 10,996 | 4021 | 10,899 | 17,095 | 17,640 | 11,961 | 2395 |
| 2009 | 7039 | 8340 | 10,084 | 10,267 | 3922 | 9906 | 16,551 | 17,855 | 11,125 | 2258 |
| 2010 | 8382 | 9722 | 11,228 | 10,580 | 3923 | 11,709 | 19,154 | 19,190 | 12,272 | 2441 |
| 2011 | 9351 | 11,741 | 12,402 | 11,164 | 3993 | 12,268 | 21,473 | 20,803 | 12,405 | 2470 |
| 2012 | 8805 | 11,160 | 13,000 | 10,895 | 3880 | 12,090 | 20,460 | 20,980 | 12,315 | 2215 |
| 2013 | 8555 | 12,680 | 14,160 | 10,755 | 4015 | 11,305 | 21,450 | 22,640 | 12,070 | 2375 |
| 2014 | 8345 | 12,520 | 14,485 | 10,575 | 4285 | 11,570 | 22,045 | 23,960 | 11,995 | 2530 |
| Percentage (%) of Total Revisions Accounted for by Age Group | ||||||||||
| 2002 | 21.7 | 15.9 | 25.9 | 28.6 | 7.9 | 16.6 | 22.0 | 31.1 | 26.4 | 4.0 |
| 2014 | 16.7 | 25.0 | 28.8 | 21.0 | 8.5 | 16.0 | 30.6 | 33.3 | 16.6 | 3.5 |
| Change | −5.0 | +9.1 | +2.9 | −7.6 | +0.6 | −0.6 | +8.6 | +2.2 | −7.8 | −0.5 |
rTHA, revision total hip arthroplasty; rTKA, revision total knee arthroplasty. Bold values denotes growth in incidence; italicized values denotes decrease in incidence.
There were 72,100 rTKAs performed in 2014, a 102% increase from the 35,612 performed in 2002. The age groups with the largest change were, again, those aged 55–64 years (+195% change) and those aged 65–74 years (+119% change). The increase in the relative percentage of all revisions accounted for by the 55–64 age group was 8.6%, from 22.0% to 30.6%. For the 65–74 age group, the relative increase was 2.2%, from 31.1% to 33.3%. The 75–84 age group decreased, whereas younger than 55 and older than 85 age groups were relatively unchanged (Table 2).
Modes of Failure
From 2006 to 2014, the largest growth in causes of failure of primary total hip arthroplasty (pTHA) and indications for revision were periprosthetic fracture and PJI, with 74.7% and 65.0% increases, respectively. The relative percentage of indication for rTHA accounted for by periprosthetic fracture and PJI increased by 5.3% and 4.6%, respectively. Although aseptic loosening and prosthetic instability have remained the most common indications for revision at every time point of the study period, there was little change in overall incidence during the study period. Similarly, rarer indications for revision, osteolysis, and wear remained relatively stable in overall indigence. Implant failure declined in its incidence (Table 3).
Table 3.
Revision by Age Mode of Failure of Index Procedure: Total Number and Percent of Total Accounted for.
| Year | rTHA |
rTKA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Revisions |
||||||||||||||
| PJI | PPFx | IB | AS | O | I | BW | PJI | PPFx | IB | AS | O | I | BW | |
| 2006 | 3545 | 3322 | 3507 | 6816 | 1516 | 7225 | 1112 | 12,946 | 685 | 4307 | 7012 | 771 | 2928 | 1641 |
| 2007 | 3764 | 3662 | 4105 | 7423 | 1716 | 7450 | 1311 | 13,699 | 877 | 4936 | 8179 | 866 | 3278 | 1705 |
| 2008 | 4112 | 4144 | 6210 | 6996 | 1623 | 8412 | 1033 | 16,334 | 1010 | 9051 | 9004 | 725 | 3977 | 1282 |
| 2009 | 4034 | 3795 | 4793 | 7405 | 1737 | 7556 | 1199 | 15,043 | 828 | 6546 | 10,279 | 738 | 3844 | 1547 |
| 2010 | 4443 | 4210 | 874 | 8953 | 2153 | 8179 | 1266 | 16,625 | 947 | 919 | 13,236 | 708 | 4211 | 1388 |
| 2011 | 5109 | 4801 | 971 | 8948 | 2209 | 8785 | 1331 | 18,359 | 895 | 995 | 13,304 | 906 | 5009 | 1529 |
| 2012 | 5310 | 4555 | 1045 | 8335 | 2330 | 8525 | 1675 | 17,925 | 930 | 830 | 13,270 | 820 | 5410 | 1360 |
| 2013 | 5460 | 5420 | 945 | 7975 | 2070 | 8920 | 1560 | 18,970 | 1095 | 865 | 13,560 | 690 | 5110 | 1285 |
| 2014 | 5850 | 5805 | 800 | 7925 | 2085 | 9220 | 1395 | 19,855 | 1070 | 795 | 13,815 | 625 | 5615 | 1190 |
| Percentage (%) of Total Revisions Accounted by Each Mode of Failure | ||||||||||||||
| 2006 | 13.1 | 12.3 | 13.0 | 25.2 | 5.6 | 26.7 | 4.1 | 42.7 | 2.3 | 14.2 | 23.2 | 2.6 | 9.7 | 5.4 |
| 2014 | 17.7 | 17.6 | 2.4 | 24.0 | 6.3 | 27.9 | 4.2 | 46.2 | 2.5 | 1.9 | 32.2 | 1.5 | 13.1 | 2.8 |
| Change | +4.6 | +5.3 | −10.6 | −1.2 | +0.7 | +1.2 | +0.1 | +3.5 | +0.2 | −12.3 | +10.0 | −1.1 | +3.4 | −2.6 |
rTHA, revision total hip arthroplasty; rTKA, revision total knee arthroplasty; PJI, prosthetic joint infection; PPFx, periprosthetic fracture; IB, implant breakage; AS, aseptic loosening; O, osteolysis; I, instability; BW, bearing wear. Bold values denotes growth in incidence; italicized values denotes decrease in incidence.
From 2006 to 2014, the most common indications for revision of pTKA were aseptic loosening (+97.0% growth) and PJI (+53.4% growth). The relative percentage of indication for rTKA accounted for by loosening and PJI was 10.0% and 3.5%, respectively. Prosthetic instability also underwent significant growth, with a 91.8% increase in indications for revision but was less common in overall incidence than PJI or aseptic loosening at all time points. Again, implant breakage decreased substantially in incidence, whereas periprosthetic fracture, osteolysis, and bearing wear all underwent smaller decreases in incidence (Table 3).
Projections
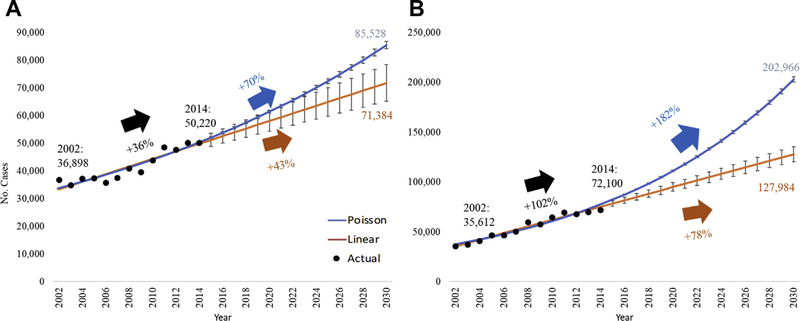
The Poisson regression model predicted a 70% increase from 2014 in the incidence of rTHA to 85,528 (95% confidence interval [95% CI], 84,201–86,876; pseudo-R2 = 0.865) procedures in 2030. Likewise, the linear model predicted a 43% increase from 2014 to 2030, to 71,384 procedures (95% CI, 65,241–78,426; R2 = 0.852) (Fig. 1A). For rTKA, the Poisson model predicted an increase to 202,966 (95% CI, 200,251–205,717; pseudo-R2 = 0.939) procedures from 2014 to 2030, a 182% increase. The linear model predicted a 78% increase to 127,984 (95% CI, 120,369–135,599; R2 = 0.962) procedures from 2014 to 2030 (Fig. 1B).
Fig. 1.
(A) Projections of revision total hip arthroplasty to 2030. (B) Projections of revision total knee arthroplasty to 2030. Error bars are 95% confidence interval.
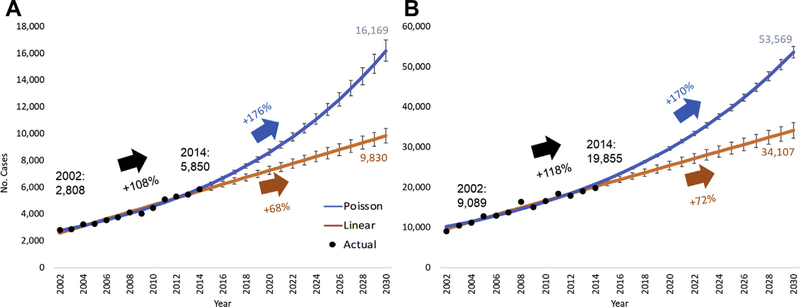
Prediction models were also used to predict the rates of revisions secondary to PJI given their commonality of growth between both procedures. The Poisson model for rTHA predicts a 176% increase from 2014 to 2030, estimating 16,169 (95% CI, 15,392–16,985; pseudo-R2 = 0.943) procedures. Likewise, the linear model saw an increase to 9830 (95% CI, 9291–10,370; R2 = 0.969) procedures by 2030, a 68% increase from 2014 (Fig. 2A). PJI diagnoses for rTKA were projected by the Poisson model to increase 170% over 2014, to 53,569 (95% CI, 2,195–54,980; pseudo-R2 = 0.933) procedures by 2030. The linear model predicted a 72% increase, 34,107 (95% CI, 32,206–36,009; R2 = 0.966) procedures in 2030 over 2014 (Fig. 2B).
Fig 2.
(A) Projections of revision total hip arthroplasty for prosthetic joint infection to 2030. (B) Projections of revision total knee arthroplasty for prosthetic joint infection to 2030. Error bars are 95% confidence interval.
Discussion
Both rTHA and rTKA procedural volumes in the United States experienced substantial growth between 2002 and 2014, although the rate of growth for rTKA was nearly 3 times that of rTHA. Although our linear model represents conservative predictions, and the Poisson model incorporates exponential growth and signifies a more aggressive forecast, both models projected year-over-year growth in nationwide rTJA volume, based on a per-annum increase from 2002 to 2014, which raises the fear of uncontrolled growth of rTJA. These trends likely represent the summation of multiple forces. First, there is well-documented growth in the primary arthroplasty sector, which simply injects more potential revision candidates into the US population [27]. Furthermore, many of the problems that have plagued pTJA in the past have been addressed, as represented by our modes of failure subanalyses. For example, implant breakage has rapidly declined in incidence. Other modes of failure, such as bearing wear, osteolysis, and pTHA instability, remained relatively stable during our study period, despite the aforementioned growth in the pTJA sector [27], which is supported by their relative decrease in revisions accounted for during our study period. However, some problems remain unsolved despite extensive financial and academic investments. The growth of PJI during our study period reflects similar trends noted in other investigations [28–30]. Aseptic pTKA loosening also grew in incidence during our study period, as constraint and fixation method are continuously debated. Finally, as more pTJA are being performed, we have yet to make marked improvements in the long-term postoperative prevention of geriatric falls after pTJA; this inherent fact disproportionately affects THA in our study, as algorithmic approaches more commonly favor rTHA over open reduction and internal fixation, whereas fracture fixation is more commonly favored in fractured pTKA [31]. Our epidemiologic investigation and modeling offers the most modern insight into current trends of revision, which simultaneously identifies areas of need for further improvement of survivorship of pTJA and enables institutions to anticipate revision demand.
Although the overarching trends in the growth of rTHA and rTKA are noteworthy, subgroup analysis on modes of failure of pTJA reveal even more troublesome predictions. Despite perpetually growing interest in PJI prevention [32,33], even at a societal level [34], the 13 years of most recent data do not suggest any slowing of the incidence of revision for PJI. Rather, our models predict further growth by as much as 176% and 170% for THA and TKA, respectively, by 2030, supporting prior literature that suggests a losing battle against this devastating postoperative outcome [28,35]. However, the etiology of the continued increase of PJI as a mode of failure of pTJA also may be partially attributable to more standardized, sophisticated, and reliable means of diagnosis [36–41], whereas the gold standard for treatment remains under active investigation and debate [42]. Although unrestrained growth of all-cause rTJA is of concern, focal increase in revision for PJI poses major risk of accelerated growth in cost of care [43,44] and even patient morbidity, when compared with aseptic revisions [45–47]. Furthermore, the rTHA cohorts had a growth in revisions driven by periprosthetic fracture, which has a similarly disproportionate cost and morbidity detriment to the health care system and the patient [48,49]. Periprosthetic fractures are one of the most prominent drivers of readmission after index pTJA, which contributes to both the morbidity and resource utilization of the plague revisions for periprosthetic fracture [50].
Of additional worry, younger patients (aged 55–64) underwent the greatest increase in incidence of revision arthroplasty during the study period. As the survivorship profile of rTJA is far worse than that of pTJA [51,52], there is likely to be a growing population of multiply revised patients. pTJA remains one of the most successful surgical procedures in the United States. There is a nearly 96% all-cause survivorship at 15 years in pTKA [53], and approximately 82.3% of patients remain revision free at 25 years [54]. Similarly, approximately 77.8% of pTHA prostheses are still in situ at 25 years [55]. Furthermore, modern technology, such as ultraehigh molecular weight polyethylene [56,57], newer tibial polyethylene geometries [58], components design to impart prosthetic stability [59], additives such as vitamin E [60], and advancements in bearing surfaces [61–63], introduces many new variables to the primary arthroplasty survivorship equation. Yet, in concert with technological advances, approximately 20% of patients with pTHA are younger than 60 years old [17,64], and pTKA is projected to experience the largest growth in the younger than 55-year-old age group by 2030 [17,65], a population that is, in particular, at elevated risk of early failure [66]. As indications perpetually expand to a younger more active population, our findings support the notion that more patients are requiring revision surgery earlier in their lifetime. Unfortunately, even modern technology may not be sufficient in a young revision population to yield reliable outcomes in a high-demand population [67]. For example, early data have shown a significant decline in activity level and working status before and after revision joint arthroplasty: 95% and 93% of patients worked before rTHA and rTKA; only 33% and 7%, respectively, returned to work postoperatively [68]. As such, our findings of an increasingly young rTJA population are an alarming trend in this country.
Our findings shed light on the future demand for rTJA in the United States, and use of a large nationally representative database enables an externally valid prediction but carries notable limitations. However, the use of a database carries the inherent weaknesses of unverifiable accuracy of coding and data input. Furthermore, variables that potentially contribute to the likelihood of rTJA are excluded from the NIS database: implant choice [69], index case complexity [70], pertinent comorbidities (ie, chronic kidney disease) [71], included diseases’ severity (ie, HbA1c) [72,73], and social determinants of health [74]. Such exclusions underscore the importance of a universal US arthroplasty registry that includes procedure-specific data and should aim to include contributions from high-volume tertiary specialty centers to rural community hospitals to most accurately portray current joint replacement trends. Furthermore, the distribution of revision arthroplasty incidence and data from 2002 to 2014 is heterogeneous and may not be perfectly described by classical statistical models because of relatively significant changes in care and resource utilization trends for both pTJA and rTJA [17,75–79]. Although we elected to use both a linear regression (assumes binomial distribution) and Poisson regression given its precedence in prior literature [80–82], it is plausible that neither model properly accounts for real-world variability as they do not define an upper limit of growth [83].
Conclusions
Although there are many factors that contribute to the demand for rTJA in the United States, projections of future need remain crucial. For example, our data demonstrate that by 2030, the incidence of rTKA may approach the volume of pTKA performed just 26 years ago, in 1993 [82]. In other words, revision arthroplasty, once considered a rare procedure, may grow in incidence to reach a recent nationwide procedural volume of primary replacement, one of the most commonly performed procedures across all surgical disciplines [1]. As such, our findings both advocate for the necessity of a national push toward anticipatory development of revision-specific perioperative pathways for these patients and estimate the actual throughput volumes such protocols may need to accommodate. Ignoring such trends and treating revision arthroplasty as an off-protocol procedure may miss a significant opportunity to decrease complications and improve outcomes through procedure-specific standardization of care [25,84–88]. Furthermore, early implementation of such protocols may also serve as disaster mitigation, in the event of another unexpected and unforeseen spike in revisions because of complications from newer hardware technology, akin to the development of adverse local tissue reactions and trunnion disease from metal-on-metal total hip arthroplasty [89–92], as such revisions for hardware complications have the potential for high resource and time utilization [92–94]. Furthermore, our data suggest uncontrolled growth in both revision for PJI and rTJA in a younger patient population, both of which have poorer outcomes than all-cause revision. These predictions isolate high-yield areas for further clinical investigation on limiting these morbid modes of failure, which could facilitate curtailed resource utilization and improved postrevision outcomes. Generally speaking, however, as high-volume centers, indications, and the patient pool continue to burgeon in pTJA, so will the need for all rTJAs; evidence-driven anticipation is the cornerstone of perpetual improvements in the management of arthroplasty patients in the revision setting.
Acknowledgments
No acknowledgments are necessary for the preparation, analysis, or data gathering for this manuscript.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2020.02.030.
References
- [1].Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003–2012: Statistical Brief #186, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- [2].Palsis JA, Brehmer TS, Pellegrini VD, Drew JM, Sachs BL. The cost of joint replacement: comparing two approaches to evaluating costs of total hip and knee arthroplasty. J Bone Joint Surg Am 2018;100:326–33. [DOI] [PubMed] [Google Scholar]
- [3].Gabriel L, Casey J, Gee M, Palmer C, Sinha J, Moxham J, et al. Value-based healthcare analysis of joint replacement surgery for patients with primary hip osteoarthritis. BMJ Open Qual 2019;8:e000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Castagnini F, Sudanese A, Bordini B, Tassinari E, Stea S, Toni A. Total knee replacement in young patients: survival and causes of revision in a registry population. J Arthroplasty 2017;32:3368–72. [DOI] [PubMed] [Google Scholar]
- [5].Gademan MG, Hofstede SN, Vliet Vlieland TP, Nelissen RG, Marang-van de Mheen PJ. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord 2016;17: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med 2017;10:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Luna IE, Kehlet H, Peterson B, Wede HR, Hoevsgaard SJ, Aasvang EK. Early patient-reported outcomes versus objective function after total hip and knee arthroplasty: a prospective cohort study. Bone Joint J 2017;99-B:1167–75. [DOI] [PubMed] [Google Scholar]
- [8].Springer BD, Odum SM, Vegari DN, Mokris JG, Beaver WB Jr. Impact of inpatient versus outpatient total joint arthroplasty on 30-day hospital readmission rates and unplanned episodes of care. Orthop Clin North Am 2017;48:15–23. [DOI] [PubMed] [Google Scholar]
- [9].Amanatullah DF, McQuillan T, Kamal RN. Quality measures in total hip and total knee arthroplasty. J Am Acad Orthop Surg 2019;27:219–26. [DOI] [PubMed] [Google Scholar]
- [10].Kamaruzaman H, Kinghorn P, Oppong R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2017;18:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schwartz AJ, Bozic KJ, Etzioni DA. Value-based total hip and knee arthroplasty: a framework for understanding the literature. J Am Acad Orthop Surg 2019;27:1–11. [DOI] [PubMed] [Google Scholar]
- [12].Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med 2008;168:1576–84. [DOI] [PubMed] [Google Scholar]
- [13].Sobieraj M, Marwin S. Ultra-high-molecular-weight polyethylene (UHMWPE) in total joint arthroplasty. Bull Hosp Jt Dis (2013) 2018;76:38–46. [PubMed] [Google Scholar]
- [14].Lim SJ, Jang SP, Kim DW, Moon YW, Park YS. Primary ceramic-on-ceramic total hip arthroplasty using a 32-mm ceramic head with a titanium-alloy sleeve. Clin Orthop Relat Res 2015;473:3781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Delaunay CP, Putman S, Puliero B, Begin M, Migaud H, Bonnomet F. Cementless total hip arthroplasty with metasul bearings provides good results in active young patients: a concise followup. Clin Orthop Relat Res 2016;474: 2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017;389:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol 2012;26: 637–47. [DOI] [PubMed] [Google Scholar]
- [18].Sedrakyan A, Romero L, Graves S, Davidson D, de Steiger R, Lewis P, et al. Survivorship of hip and knee implants in pediatric and young adult populations: analysis of registry and published data. J Bone Joint Surg Am 2014;96(Suppl. 1):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martin JR, Sutak AK, Milbrandt TA, Martin VA, Trousdale RT. Adolescent total knee arthroplasty. Arthroplast Today 2017;3:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hannouche D, Devriese F, Delambre J, Zadegan F, Tourabaly I, Sedel L, et al. Ceramic-on-ceramic THA implants in patients younger than 20 years. Clin Orthop Relat Res 2016;474:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bohl DD, Samuel AM, Basques BA, Della Valle CJ, Levine BR, Grauer JN. How much do adverse event rates differ between primary and revision total joint arthroplasty? J Arthroplasty 2016;31:596–602. [DOI] [PubMed] [Google Scholar]
- [22].Isaacson MJ, Bunn KJ, Noble PC, Ismaily SK, Incavo SJ. Quantifying and predicting surgeon work input in primary vs revision total hip arthroplasty. J Arthroplasty 2016;31:1188–93. [DOI] [PubMed] [Google Scholar]
- [23].Bunn KJ, Isaacson MJ, Ismaily SK, Noble PC, Incavo SJ. Quantifying and predicting surgeon work effort for primary and revision total knee arthroplasty. J Arthroplasty 2016;31(9 Suppl):59–62. [DOI] [PubMed] [Google Scholar]
- [24].Nichols CI, Vose JG. Clinical outcomes and costs within 90 days of primary or revision total joint arthroplasty. J Arthroplasty 2016;31:1400–1406.e3. [DOI] [PubMed] [Google Scholar]
- [25].Tessier JE, Rupp G, Gera JT, DeHart ML, Kowalik TD, Duwelius PJ. Physicians with defined clear care pathways have better discharge disposition and lower cost. J Arthroplasty 2016;31(9 Suppl):54–8. [DOI] [PubMed] [Google Scholar]
- [26].Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100: 1455–60. [DOI] [PubMed] [Google Scholar]
- [27].Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [28].Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the medicare population. J Arthroplasty 2018;33:3238–45. [DOI] [PubMed] [Google Scholar]
- [29].Henderson RA, Austin MS. Management of periprosthetic joint infection: the more we learn, the less we know. J Arthroplasty 2017;32:2056–9. [DOI] [PubMed] [Google Scholar]
- [30].Kokko MA, Abdel MP, Berry DJ, Butler RD, Van Citters DW. A retrieval analysis perspective on revision for infection. Arthroplast Today 2019;5:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haidukewych GJ, Langford J, Liporace FA. Revision for periprosthetic fractures of the hip and knee. J Bone Joint Surg Am 2013;95:368–76. [PubMed] [Google Scholar]
- [32].Frank RM, Cross MB, Della Valle CJ. Periprosthetic joint infection: modern aspects of prevention, diagnosis, and treatment. J Knee Surg 2015;28:105–12. [DOI] [PubMed] [Google Scholar]
- [33].Kapadia BH, Pivec R, Johnson AJ, Issa K, Naziri Q, Daley JA, et al. Infection prevention methodologies for lower extremity total joint arthroplasty. Expert Rev Med Devices 2013;10:215–24. [DOI] [PubMed] [Google Scholar]
- [34].Zalavras CG, Springer BD. Editorial comment: 2016 Musculoskeletal Infection Society Proceedings. Clin Orthop Relat Res 2017;475:1796–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SW. Periprosthetic joint infection is the main cause of failure for modern knee arthroplasty: an analysis of 11,134 knees. Clin Orthop Relat Res 2017;475:2194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am 2018;100:147–54. [DOI] [PubMed] [Google Scholar]
- [37].Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am 2012;94: 2247–54. [DOI] [PubMed] [Google Scholar]
- [38].Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018;33:1309–1314.e2. [DOI] [PubMed] [Google Scholar]
- [39].Koh IJ, Han SB, In Y, Oh KJ, Lee DH, Kim TK. The leukocyte esterase strip test has practical value for diagnosing periprosthetic joint infection after total knee arthroplasty: a multicenter study. J Arthroplasty 2017;32:3519–23. [DOI] [PubMed] [Google Scholar]
- [40].Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2016;98:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum DDimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am 2017;99:1419–27. [DOI] [PubMed] [Google Scholar]
- [42].Ottesen CS, Troelsen A, Sandholdt H, Jacobsen S, Husted H, Gromov K. Acceptable success rate in patients with periprosthetic knee joint infection treated with debridement, antibiotics, and implant retention. J Arthroplasty 2019;34:365–8. [DOI] [PubMed] [Google Scholar]
- [43].Puhto T, Puhto AP, Vielma M, Syrjala H. Infection triples the cost of a primary joint arthroplasty. Infect Dis (Lond) 2019;51:348–55. [DOI] [PubMed] [Google Scholar]
- [44].Kasch R, Assmann G, Merk S, Barz T, Melloh M, Hofer A, et al. Economic analysis of two-stage septic revision after total hip arthroplasty: what are the relevant costs for the hospital’s orthopedic department? BMC Musculoskelet Disord 2016;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty 2018;33:521–6. [DOI] [PubMed] [Google Scholar]
- [46].Boddapati V, Fu MC, Tetreault MW, Blevins JL, Richardson SS, Su EP. Shortterm complications after revision hip arthroplasty for prosthetic joint infection are increased relative to noninfectious revisions. J Arthroplasty 2018;33: 2997–3002. [DOI] [PubMed] [Google Scholar]
- [47].Fischbacher A, Borens O. Prosthetic-joint infections: mortality over the last 10 years. J Bone Jt Infect 2019;4:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reeves RA, Schairer WW, Jevsevar DS. Costs and risk factors for hospital readmission after periprosthetic knee fractures in the United States. J Arthroplasty 2018;33:324–330.e1. [DOI] [PubMed] [Google Scholar]
- [49].Reeves RA, Schairer WW, Jevsevar DS. The national burden of periprosthetic hip fractures in the US: costs and risk factors for hospital readmission. Hip Int 2019;29:550–7. [DOI] [PubMed] [Google Scholar]
- [50].Kurtz SM, Lau EC, Ong KL, Adler EM, Kolisek FR, Manley MT. Which clinical and patient factors influence the national economic burden of hospital readmissions after total joint arthroplasty? Clin Orthop Relat Res 2017;475: 2926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Khan M, Osman K, Green G, Haddad FS. The epidemiology of failure in total knee arthroplasty: avoiding your next revision. Bone Joint J 2016;98-B(1 Suppl. A):105–12. [DOI] [PubMed] [Google Scholar]
- [52].Riesgo AM, Hochfelder JP, Adler EM, Slover JD, Specht LM, Iorio R. Survivorship and complications of revision total hip arthroplasty with a mid-modular femoral stem. J Arthroplasty 2015;30:2260–3. [DOI] [PubMed] [Google Scholar]
- [53].Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res 2006;452:28–34. [DOI] [PubMed] [Google Scholar]
- [54].Evans JT, Walker RW, Evans JP, Blom AW, Sayers A, Whitehouse MR. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019;393:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up.Lancet 2019;393:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McGloughlin TM, Kavanagh AG. Wear of ultra-high molecular weight polyethylene (UHMWPE) in total knee prostheses: a review of key influences. Proc Inst Mech Eng H 2000;214:349–59. [DOI] [PubMed] [Google Scholar]
- [57].Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res 2004;429:6–16. [PubMed] [Google Scholar]
- [58].Mannan K, Scott G. The Medial Rotation total knee replacement: a clinical and radiological review at a mean follow-up of six years. J Bone Joint Surg Br 2009;91:750–6. [DOI] [PubMed] [Google Scholar]
- [59].Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. Bone Joint J 2018;100-B:11–9. [DOI] [PubMed] [Google Scholar]
- [60].Shareghi B, Johanson PE, Karrholm J. Wear of vitamin E-infused highly cross-linked polyethylene at five years. J Bone Joint Surg Am 2017;99:1447–52. [DOI] [PubMed] [Google Scholar]
- [61].Lee GC, Kim RH. Incidence of modern alumina ceramic and alumina matrix composite femoral head failures in nearly 6 million hip implants. J Arthroplasty 2017;32:546–51. [DOI] [PubMed] [Google Scholar]
- [62].Lim SJ, Kim SM, Kim DW, Moon YW, Park YS. Cementless total hip arthroplasty using Biolox(R)delta ceramic-on-ceramic bearing in patients with osteonecrosis of the femoral head. Hip Int 2016;26:144–8. [DOI] [PubMed] [Google Scholar]
- [63].Lim SJ, Ryu HG, Eun HJ, Park CW, Kwon KB, Park YS. Clinical outcomes and bearing-specific complications following fourth-generation alumina ceramic-on-ceramic total hip arthroplasty: a single-surgeon series of 749 hips at a minimum of 5-year follow-up. J Arthroplasty 2018;33:2182–2186.e1. [DOI] [PubMed] [Google Scholar]
- [64].Kumar A, Bloch BV, Esler C. Trends in total hip arthroplasty in young patients–results from a regional register. Hip Int 2017;27:443–8. [DOI] [PubMed] [Google Scholar]
- [65].Aujla RS, Esler CN. Total knee arthroplasty for osteoarthritis in patients less than fifty-five years of age: a systematic review. J Arthroplasty 2017;32: 2598–2603.e1. [DOI] [PubMed] [Google Scholar]
- [66].Charette RS, Sloan M, DeAngelis RD, Lee G-C. Higher rate of early revision following primary total knee arthroplasty in patients under age 55: a cautionary tale. J Arthroplasty 2019;34:2918–24. [DOI] [PubMed] [Google Scholar]
- [67].Postler AE, Beyer F, Wegner T, Lutzner J, Hartmann A, Ojodu I, et al. Patient-reported outcomes after revision surgery compared to primary total hip arthroplasty. Hip Int 2017;27:180–6. [DOI] [PubMed] [Google Scholar]
- [68].Scott CEH, Turnbull GS, Powell-Bowns MFR, MacDonald DJ, Breusch SJ. Activity levels and return to work after revision total hip and knee arthroplasty in patients under 65 years of age. Bone Joint J 2018;100-B:1043–53. [DOI] [PubMed] [Google Scholar]
- [69].Watts CD, Abdel MP, Lewallen DG, Berry DJ, Hanssen AD. Increased risk of periprosthetic femur fractures associated with a unique cementless stem design. Clin Orthop Relat Res 2015;473:2045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang D, Li LL, Wang HY, Pei FX, Zhou ZK. Long-term results of cementless total hip arthroplasty with subtrochanteric shortening osteotomy in crowe type IV developmental dysplasia. J Arthroplasty 2017;32:1211–9. [DOI] [PubMed] [Google Scholar]
- [71].Tan TL, Kheir MM, Tan DD, Filippone EJ, Tischler EH, Chen AF. Chronic kidney disease linearly predicts outcomes after elective total joint arthroplasty. J Arthroplasty 2016;31(9 Suppl):175–179.e2. [DOI] [PubMed] [Google Scholar]
- [72].Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR. Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total hip and knee arthroplasty. J Arthroplasty 2015;30: 439–43. [DOI] [PubMed] [Google Scholar]
- [73].Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplasty 2015;30:1197–202. [DOI] [PubMed] [Google Scholar]
- [74].Courtney PM, Huddleston JI, Iorio R, Markel DC. Socioeconomic risk adjustment models for reimbursement are necessary in primary total joint arthroplasty. J Arthroplasty 2017;32:1–5. [DOI] [PubMed] [Google Scholar]
- [75].Oh C, Slover JD, Bosco JA, Iorio R, Gold HT. Time trends in characteristics of patients undergoing primary total hip and knee arthroplasty in California, 2007–2010. J Arthroplasty 2018;33:2376–80. [DOI] [PubMed] [Google Scholar]
- [76].Kirksey M, Chiu YL, Ma Y, Della Valle AG, Poultsides L, Gerner P, et al. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998–2008. Anesth Analg 2012;115:321–7. [DOI] [PubMed] [Google Scholar]
- [77].Grosso MJ, Neuwirth AL, Boddapati V, Shah RP, Cooper HJ, Geller JA. Decreasing length of hospital stay and postoperative complications after primary total hip arthroplasty: a decade analysis from 2006 to 2016. J Arthroplasty 2019;34:422–5. [DOI] [PubMed] [Google Scholar]
- [78].Molloy IB, Martin BI, Moschetti WE, Jevsevar DS. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg Am 2017;99:402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK, et al. Trends and determinants of length of stay and hospital reimbursement following knee and hip replacement: evidence from linked primary care and NHS hospital records from 1997 to 2014. BMJ Open 2018;8:e019146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am 2015;97:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020–2040 using the national inpatient sample. J Rheumatol 2019;9:1134–40. [DOI] [PubMed] [Google Scholar]
- [82].Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United Statesdan alternative projection model. Osteoarthritis Cartilage 2017;25:1797–803. [DOI] [PubMed] [Google Scholar]
- [83].Inacio MCS, Graves SE, Pratt NL, Roughead EE, Nemes S. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: a conservative local model with international implicat ions. Clin Orthop Relat Res 2017;475: 2130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rutherford RW, Jennings JM, Dennis DA. Enhancing recovery after total knee arthroplasty. Orthop Clin North Am 2017;48:391–400. [DOI] [PubMed] [Google Scholar]
- [85].McCann-Spry L, Pelton J, Grandy G, Newell D. An interdisciplinary approach to reducing length of stay in joint replacement patients. Orthop Nurs 2016;35: 279–98. [DOI] [PubMed] [Google Scholar]
- [86].Bullock MW, Brown ML, Bracey DN, Langfitt MK, Shields JS, Lang JE. A bundle protocol to reduce the incidence of periprosthetic joint infections after total joint arthroplasty: a single-center experience. J Arthroplasty 2017;32: 1067–73. [DOI] [PubMed] [Google Scholar]
- [87].Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty 2017;32:S69–73. [DOI] [PubMed] [Google Scholar]
- [88].Dattilo J, Gittings D, Sloan M, Charette R, Hume E, Lee GC. ‘Hot Joints’ infection protocol reduces unnecessary post-operative re-admissions following total hip and knee arthroplasty. Bone Joint J 2017;99-B:1603–10. [DOI] [PubMed] [Google Scholar]
- [89].Marinier M, Edmiston TA, Kearns S, Hannon CP, Levine BR. A survey of the prevalence of and techniques to prevent trunnionosis. Orthopedics 2018;41: e557–62. [DOI] [PubMed] [Google Scholar]
- [90].Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am 2012;94: 1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am 2013;95:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bernstein DT, Meftah M, Paranilam J, Incavo SJ. Eighty-six percent failure rate of a modular-neck femoral stem design at 3 to 5 Years: lessons learned. J Bone Joint Surg Am 2016;98:e49. [DOI] [PubMed] [Google Scholar]
- [93].Waterson HB, Whitehouse MR, Greidanus NV, Garbuz DS, Masri BA, Duncan CP. Revision for adverse local tissue reaction following metal-onpolyethylene total hip arthroplasty is associated with a high risk of early major complications. Bone Joint J 2018;100-B:720–4. [DOI] [PubMed] [Google Scholar]
- [94].Dimitriou D, Liow MH, Tsai TY, Leone WA, Li G, Kwon YM. Early outcomes of revision surgery for taper corrosion of dual taper total hip arthroplasty in 187 patients. J Arthroplasty 2016;31:1549–54. [DOI] [PubMed] [Google Scholar]