Abstract
Everyday prosociality includes helping behaviors such as holding doors or giving directions that are spontaneous and low-cost and are performed frequently by the average person. Such behaviors promote a wide array of positive outcomes that include increased well-being, trust, and social capital, but the cognitive and neural mechanisms that support these behaviors are not yet well understood. Whereas costly altruistic responding to others’ distress is associated with elevated reactivity in the amygdala, we hypothesized that everyday prosociality would be more closely associated with activation in the bed nucleus of the stria terminalis (BNST), a region of the extended amygdala known for its roles in maintaining vigilance for relevant socio-affective environmental cues and in supporting parental care. One previous study of the neural correlates of everyday prosociality highlighted a functional cluster identified as the septal area but which overlapped with established coordinates of BNST. We used an anatomical mask of BNST (Torrisi et al., 2015) to evaluate the association of BNST activation and daily helping in a sample of 25 adults recruited from the community as well as 23 adults who had engaged in acts of extraordinary altruism. Results found that activation in left BNST during an empathy task predicted everyday helping over a subsequent 14-day period in both samples. BNST activation most strongly predicted helping strangers and proactive helping. We conclude that beyond facilitating care for offspring, activation in BNST may provide a basis for the motivation to engage in a broad array of everyday helping behaviors.
Everyday prosociality is a mainstay of human social life. Helping behaviors that comprise everyday prosociality, such as holding a door, providing directions, or delaying an elevator are immediate, unplanned, and low-cost and are performed multiple times by the typical person every day (Morelli, Rameson, & Lieberman, 2014). Despite their low cost, everyday helping behaviors promote a wide array of positive outcomes, from increased well-being at the individual level (Raposa, Laws, & Ansell, 2016) to higher levels of social trust (Helliwell, Aknin, Shiplett, Huang, & Wang, 2017) and social capital at the level of the community (Nettle, Colléony, & Cockerill, 2011). And yet little is known about the mechanisms that drive everyday helping behaviors and how they overlap with the mechanisms that support rarer acts of costly altruism (FeldmanHall, Dalgleish, Evans, & Mobbs, 2015; Marsh et al., 2014). In this study, we evaluated the behavioral and neural correlates of everyday helping to better understand the basis of this form of prosocial behavior. Participants included both typical adults recruited from the community as well as a sample of extraordinarily altruistic adults who had donated a kidney to a stranger, enabling us to assess the correspondence of everyday prosociality and extreme altruism.
Everyday prosociality remains poorly understood in part because of the notorious challenge of studying prosocial behavior experimentally. When participants’ behavior is under observation in the laboratory, they are subject to elevated social desirability and demand biases (Brethel-Haurwitz, Stoycos, Cardinale, Huebner, & Marsh, 2016; Eisenberg & Fabes, 1990). And self-report measures of prosociality can be inaccurate due to retrospection errors and self-presentation biases, concerns that are enhanced by the non-specific and transparent questions used in many prosociality measures, as well as the highly socially desirable nature of prosocial traits (Eisenberg & Lennon, 1983). As a result, laboratory-based self-report measures of prosociality often fail to predict actual helping behavior (Eisenberg & Fabes, 1990; Marsh et al., 2014). Fortunately, recent advances in data collection have provided an array of new options. Experience sampling methods, for example, now enable research participants to report on specific everyday helping behaviors outside of the lab. These surveys are typically completed at home on a computer or smartphone, providing participants a quicker and more accurate way to recount daily behaviors.
Experience sampling mitigates concerns about social desirability and retrospection bias (Iida, Shrout, Laurenceau, & Bolger, 2016) and results in rich datasets that can be used to model behavior both cross-sectionally and over time. This methodology has been used to assess real-world behaviors across a variety of populations (Aan het Rot, Hogenelst, & Schoevers, 2012; Thewissen et al., 2011) and across the lifespan (Carstensen et al., 2011). It has also been successfully used to understand correlates of everyday prosociality. For example, daily diary research finds that everyday prosocial behaviors buffer the negative effects of stress on affect and mental health in healthy adults (Raposa et al., 2016). Experience sampling approaches have now clearly established the positive outcomes of various prosocial behaviors, such as reciprocity for reactive prosociality (response to others’ need) and increased well-being for proactive (voluntary) prosociality (Aknin, Van de Vondervoort, & Hamlin, 2018; Dunn, Aknin, & Norton, 2014; Harbaugh, Mayr, & Burghart, 2007; Spitzmuller & Van Dyne, 2013). However, the neurocognitive mechanisms that support everyday helping—particularly for socially distant others, like acquaintances and strangers—are not yet well understood.
It is clear that individuals vary significantly in the degree to which they engage in prosocial behaviors in daily life (Kelly & Dunbar, 2001; Sze, Gyurak, Goodkind, & Levenson, 2012). Situational and cultural variables account for much of this variation, but some variation can also be accounted for by individual differences in neural structure and function. For example, our research has identified structural and functional correlates of engaging in extraordinary acts of altruism (such as donating a kidney to a stranger), that include increased size and responsiveness of the amygdala (Marsh et al., 2014) and stronger structural and functional connections between the amygdala and periaqueductal gray (PAG) in the midbrain (Brethel-Haurwitz et al., 2017). These findings are noteworthy in part because these structures are critical elements of the brains’ mammalian parental care network. Their association with altruism is consistent with the idea that altruistic motivation, particularly when it is driven by caring emotions like empathic concern, emerges from networks that support parental care in mammals (Batson, 2010; Marsh, 2016; Preston, 2013). Over time, it is thought that the subcortical circuits that originally evolved to support parental and alloparental caregiving have come to support care not only for offspring, but for vulnerable and distressed targets more generally (de Waal, 2008; Marsh, 2018).
Considering the evolution of altruism in this context may shed light on the circuits most likely to support everyday prosociality. The amygdala plays a critical role in caregiving particularly in response to acute distress (Decety & Lamm, 2006; Zald, 2003). This structure is involved in calculating prospective threats to offspring (and others) and communicates with other subcortical structures, including PAG and the striatum, to coordinate appropriate protective responses (Rickenbacher, Perry, Sullivan, & Moita, 2017). Consistent with this, the amygdala is active in response to the sight or sound of others’ distress, including infant cries (Newman, 2007), and nonverbal expressions conveying fear (Marsh et al., 2014). But this structure may be less relevant to everyday helping than other structures in the parental care network, such as the bed nucleus of the stria terminalis (BNST). The stria terminalis is a major efferent pathway of the amygdala and terminates primarily in the hypothalamus (Alves et al., 2013). Embedded within this pathway, the BNST, which contains up to 18 sub-nuclei, plays a critical role in a variety of social and affective processes, including parental care (Lebow & Chen, 2016). The distinct functions served by the amygdala versus BNST are best understood in the context of defensive threat responding. Whereas the amygdala is involved primarily in coordinating avoidance and escape in response to conditioned cues signaling acute threats (Adolphs, 2008) (the basolateral region responds to reward cues as well (Baxter & Murray, 2002)), BNST is primarily involved in maintaining vigilance for potential threat (Davis, Walker, Miles, & Grillon, 2010; Sokolowski & Corbin, 2012), as evidenced by findings that this structure is differentially activated in the presence of environmental cues that indicate the potential for threat (Mobbs et al., 2010; Somerville, Whalen, & Kelley, 2010). It is sometimes described as playing a “valence surveillance” role that integrates sensory affective information and contextual cues relevant to mood, energy, and motivation (Lebow & Chen, 2016). Like the amygdala, the BNST is densely populated with receptors for the neurotransmitter oxytocin, which is involved in translating the perception of relevant environmental cues encoded here into caregiving behaviors (Leng, Meddle, & Douglas, 2008; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Together, these findings suggest that the BNST may play a greater role than the amygdala in coordinating everyday helping, which requires low-level vigilance and surveillance of the environment and responding prosocially following relevant environmental cues.
Two existing studies exploring the neural correlates of everyday prosociality were conducted by Morelli and colleagues (Morelli et al., 2014; Rameson, Morelli, & Lieberman, 2012) who measured neural responses to empathy-eliciting cues in the laboratory and their relation to everyday helping behavior toward acquaintances and strangers. Of particular interest, Morelli and colleagues (2014) identified a functional cluster in which activation observed in response to a variety of affective states in others—including pain, anxiety, and happiness—predicted participants’ average daily helping over 14 days. The authors describe the functional cluster in this study as located in the septal area. However, the coordinates of this cluster (xyz = 6,2,7) (Morelli, 2013; Morelli et al., 2014) are located superior and posterior to typical anatomical definitions of the septal area (Bramati et al., 2012; Butler et al., 2014), at the intersection of caudate, lateral ventricle and BNST. The anatomical definition in Morelli, et al. (2014) was based on a 3D box that did not include the anterior and posterior portions of the septal area (Mai, Paxinos, & Assheuer, 2004), and it extends too far laterally, encompassing various other anatomical features, including other subcortical regions and large portions of white matter. The identified region of activation appears to be more consistent with BNST (Avery et al., 2014; Torrisi et al., 2015). It is possible that this study in fact identified a role for BNST in daily helping, although the authors did not specifically test this hypothesis.
Conclusions that can be drawn from this study are also somewhat limited by the fact that it only calculated helping as an average, although data were collected as counts. Count data are typically non-normally distributed and thus ideally analyzed using a Poisson or negative binomial regression (Gardner, Mulvey, & Shaw, 1995), as well as longitudinally, for maximum interpretative power. Finally, because in this study helping only toward distant others was assessed, no comparison of everyday prosociality toward targets of varying social distances has yet been conducted.
In the present study, we aimed to more directly consider the role of BNST in everyday helping toward targets of varying social distances. We hypothesized that BNST responses to empathic cues during fMRI brain scanning would be associated with subsequent everyday helping across groups. We also assessed the relationship between everyday helping and responses in bilateral amygdala, following evidence of its involvement in supporting costly altruism (Marsh et al., 2014); and bilateral anterior insula, given multiple lines of evidence for its role in prosocial motivation (Brethel-Haurwitz et al., 2018; Hein, Silani, Preuschoff, Batson, & Singer, 2010; Morelli et al., 2014). We used the experience sampling method originally developed by Morelli and colleagues (2014) in a sample that included both extraordinary altruists (altruistic kidney donors) and matched controls recruited from the community. Based on prior work showing no differences in everyday helping in extraordinary altruists and controls (Brethel-Haurwitz et al., 2016) and indicating that altruists primarily differ from controls in their responses to acute distress (Marsh et al., 2014) we predicted that altruists and controls would not differ in their overall everyday helping. We analyzed rates of everyday helping using an approach optimized for count data as well as changes over time, in part due to suggestions that experience sampling can serve as an intervention in that asking repeatedly about specific behaviors can yield increases in those behaviors across time (Napa Scollon, Prieto, & Diener, 2009). Using this approach, we aimed to develop a more refined account of the cognitive and neural correlates of everyday prosociality.
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
Methods
Participants
101 healthy adults aged 22 to 67 years (M = 40.86, SD = 9.88) completed daily experience sampling as part of a larger study protocol; of this total, a subset of eligible participants (N = 48) completed fMRI brain scanning prior to completing the experience sampling protocol. The sample who underwent fMRI scanning was composed of 23 highly altruistic adults who had donated a kidney to a stranger as well as 25 demographically matched adults recruited from the local community (Table 1). Sample size was determined using fMRIPower based on pilot data from a previous study of altruistic kidney donors (Marsh et al., 2014). Altruists were recruited from across North America via local and national transplant organizations. Each altruist’s kidney donation status was verified through an independent source (e.g., letter of confirmation from the transplant hospital). Six altruists had donated to a specified stranger (in response to, for example, a flier or an internet post); the remaining altruists had donated a kidney via non-directed donations in which the recipient was anonymous at the time of donation. The remaining participants were recruited from the Washington, D.C. area via fliers and online advertisements. They were excluded if they had ever volunteered to donate an organ to any individual, or were interested in receiving more information about signing up to become an organ donor.
Table 1.
Participant demographics
| Total sample | Neuroimaging subset sample | |||||
|---|---|---|---|---|---|---|
| Altruists (N = 49) | Controls (N = 52) | p | Altruists (N = 23) | Controls (N = 25) | p | |
| Gender (Male/Female, % Male) | 15/34 (30.61%) | 19/33 (36.54%) | .523 | 8/15 (34.78%) | 10/15 (40.00%) | .709 |
| Race (White/Non-White, % White) | 44/5 (89.80%) | 46/6 (88.46%) | .830 | 21/2 (91.30%) | 23/2 (92.00%) | .931 |
| Age, M (SD) | 43.18 (10.63) | 38.67 (8.68) | .021* | 41.61 (9.75) | 38.84 (8.03) | .287 |
| Education ≥ Four-year degree | 36/13 (73.47%) | 47/5 (90.38%) | .026* | 15/8 (65.21%) | 18/7 (72.00%) | .123 |
| Population (tens of thousands), M (SD) | 2.54 (143) | 2.79 (141) | .373 | 3.03 (145) | 2.72 (139) | .434 |
All participants completed a preliminary online screening assessing donation status, relevant demographics (age, sex, ethnicity, education, and zip code) (Table 1), and behavioral measures. All analyses incorporated demographic covariates previously linked to prosociality, including gender, which was coded as a 0 for female and 1 for male; age, which was entered as a continuous variable; and residential population size, an index of urbanicity calculated from census estimates of the population in the participant’s ZIP code of residence and entered as tens of thousands. For altruist status, 0 indicated a typical participant and 1 an altruistic kidney donor. Participants were compensated for their participation. The study was approved by the Institutional Review Board at Georgetown University and all participants provided informed written consent prior to participation. No parts of the study procedures or analyses were pre-registered prior to the research being conducted.
Neuroimaging methods
The neuroimaging task investigating empathic neural responses to pain was conducted prior to experience sampling as part of a larger study protocol (Brethel-Haurwitz et al., 2018). Exclusion criteria for scanned participants included history of head injury or neurological illness, pain disorders, hearing difficulties, IQ < 80 (as assessed using the Kaufman Brief Intelligence Test–Second Edition; (Kaufman & Kaufman, 2004)), current use of psychotropic medication, clinically significant psychopathology, and contraindications to safe MRI scanning. Three altruists and one control subject were excluded for excessive motion during the MRI scan (greater than 15% TRs with greater than 0.5 mm head-movement).
Before scanning commenced, participants underwent pressure pain calibration, which was achieved by applying pressure to the right thumbnail using a computerized device that maintained constant pressure for 6 seconds. Prior to the task, pneumatic pressure per square inch (PSI) was titrated to a pain level that was reported to be “slightly intense” for each subject (rated 13.5 on a 21-point Gracely Box Scale; Gracely, 1990). Thus, all participants were familiar with the experience of pressure pain before beginning the empathic component of the task. Then, upon entering the console room outside of the scanner, participants were briefly introduced to, but did not speak with, a female stranger (their study partner) whose pain they would be viewing in real time. Participants then completed three runs of an empathic pain task (12 minutes 18 seconds each). In the first run, they passively observed the study partner receiving pressure pain stimulation on her thumbnail via live video feed. In the second run, they observed the study partner receiving pressure pain stimulation after receiving instructions to empathize with her. In the third run, they watched a live video feed of their own hand as they received the pain on their own thumbnail. Each run consisted of 30 trials, with each trial made up of a variable anticipation period (6, 9, 12, or 15 seconds) the administration (or omission) of painful pressure stimulation (6 seconds), and a variable rest period (3, 6, 9, or 12 seconds). Within each block, half of the trials were safe trials, in which participants knew that there would be no thumb pressure; the other half of the trials were threat trials, in which participants knew there was a potential for thumb pressure. Pressure was omitted on one third of these potentially painful trials to keep the administration of pain probabilistic rather than deterministic. Auditory cues indicated the trial structure.
Task stimuli and results for self/other neural pain signatures are reported in previous studies (Brethel-Haurwitz et al., 2018; O’Connell et al., 2019). The present analyses focus only on the second run of the scan, during which participants were instructed to empathize with their study partner as they watched her experience pressure pain. This block was chosen to maximize consistency with Morelli and colleagues (2014), who assessed neural responses in participants explicitly instructed to empathize with the stimuli presented during scanning. Participants heard this instructional prompt prior to the beginning of the run: “Please watch your partner during the following session of the task closely. As you watch and listen, please imagine how your partner is feeling during the task. Really try to understand her thoughts and emotions during each trial of the task.”
MR images were acquired with a 3T Siemens Tim Trio scanner (Siemens Medical Solutions) and a 12-channel phased-array head coil. Functional data were collected using a T2*-weighted echo-planar imaging sequence (46 3.0 mm transversal slices; 64 × 64 matrix; repetition time, 2,500 ms; echo time, 30 ms; field of view, 192 mm2; 3.0 × 3.0 × 3.0 mm voxels). High-resolution T1-weighted anatomical images were also acquired (3D Magnetization Prepared Rapid Acquisition Gradient Echo; 176 1.0-mm axial slices; field of view, 250 mm2; repetition time, 1,900 ms; echo time, 2.52 ms; 246 × 256 matrix). Preprocessing of functional images was completed in AFNI (Cox, 1996). The first 4 volumes of each run were removed and the remaining images were despiked, slice-time corrected, and motion-corrected. Functional images were aligned to the anatomical grid and non-linearly warped to MNI space using AFNI’s 3dQwarp and the ICBM 2009c nonlinear symmetric template (Fonov et al., 2011). Images were subsequently spatially smoothed using a 6 mm FWHM Gaussian filter. Motion artifacts were modeled using six rigid-body motion parameters that were included in the regression model for each subject. (Whole-brain motion per TR was estimated by taking the Euclidean Norm of the 6 directional motion shifts relative to the previous volume.) Volumes with motion estimates exceeding 0.5 mm were censored. Volumes with high motion were censored by setting a matrix column in the regression model to 0 and were therefore “scrubbed” from analysis. Low frequency signal drifts (>100 s) were removed.
Ordinary least squares linear regression (via AFNI’s 3dDeconvolve) was used to obtain parameter estimates for conditions of interest. Contrast images were created at the single-subject level (empathy for pain > no-pain control). Parameter estimates during the empathic induction task were obtained for the six a priori regions of interest (bilateral amygdala, BNST, and anterior insula). We used the automated anatomical labeling (AAL) atlas for the right and left amygdala and anterior insula ROIs (Rolls, Huang, Lin, Feng, & Joliot, 2019; Rolls, Joliot, & Tzourio-Mazoyer, 2015; Tzourio-Mazoyer et al., 2002). Right and left BNST were defined using masks derived from manual tracings of BNST in 36 adults at ultra-high field 7 Tesla MRI (Torrisi et al., 2015). We used a conservative probabilistic threshold of 50% to ensure anatomical specificity of BNST. This is in contrast to the functionally defined region of interest used by Morelli and colleagues that appears to span white matter, gray matter and cerebrospinal fluid.
Post-hoc whole brain analyses applied a permutation approach to determine cluster-size thresholding via the -Clustsim flag in AFNI’s 3dttest++, which randomizes and permutes input datasets using 10,000 Monte Carlo simulations. This approach was developed to reduce the false positive rate (Cox, Chen, Glen, Reynolds, & Taylor, 2017; Eklund, Nichols, & Knutsson, 2016). Cluster significance was determined using an underlying voxel height threshold of p<.001 and a cluster forming threshold to control the false positive rate at p < .05. A mask based on 50% overlap of the epi mask of all subjects, transformed to standard space, was used.
Daily Experience Sampling
All participants completed daily experience sampling to measure everyday helping (Iida et al., 2016). After indicating their interest in the study, participants completed a consent form that described the study in detail and also included questions about their preferred start date for the study and their time zone (most altruists resided out of state). Once this was complete, participants received an email with a link to an online survey, via Qualtrics, at 5pm each day (in their indicated time zone) for 14 consecutive days. Participants were instructed to complete the survey immediately before going to sleep each evening.
Each survey consisted of 11 previously validated items adapted from the Self-Report Altruism Scale (Rushton et al., 1981) measuring daily helping behaviors separately towards family members, friends, acquaintances, and strangers (Morelli et al., 2014). These 11 items were presented in a random order each time the survey was completed. The items were subdivided into those that were reactive (R) or proactive (P) helping behaviors. Reactive helping was operationally defined as helping most likely to be a reaction to a request or need (Spitzmuller & Van Dyne, 2013), with relevant items including: gave directions, made change, helped a disabled or elderly person, picked up a fallen object for someone, lent or gave money, and lent an item of value (tool, clothes, car, etc.). Proactive helping was defined as a behavior that is more likely to be spontaneously initiated (Spitzmuller & Van Dyne, 2013), with relevant items including: delayed elevator, held open a door, let someone go ahead of you in line, asked someone if they needed help. The final item (helped with schoolwork) was not classified as either.
Data were compiled into daily counts to enable assessment of changes over time and divided into subgroups for analysis. To address our hypotheses, overall daily helping counts were measured, as were helping for family and friends (sum family/friend helping), helping for acquaintances and strangers (sum acquaintance/stranger helping), reactive helping, and proactive helping. To examine counts across surveys, an exposure variable was also created in order to account for the effect of the number of surveys each participant completed (Hutchinson & Holtman, 2005). This changes the interpretation of the outcome variable (in this case, helping) to rate per unit of exposure, or in this case rate of helping per day.
To assess the appropriate regression modeling strategy for the helping data, experience sampling count data were inspected for over-dispersion. For all relevant dependent variables, the variance was substantially larger than the mean, suggesting a negative binomial regression model should be used (Gardner, Mulvey, & Shaw, 1995). As expected, all alpha coefficients in the negative binomial regressions presented were greater than 1, such that robust standard error procedures were used for these regressions. For ease of interpretation, IRR (incident rate ratios) are reported. The IRR values for each dependent variable represent the estimated rate ratio for a one-unit increase in that variable, holding all other variables constant. To examine changes in helping over time, time-series negative binomial regression models were conducted after transforming the dataset from WIDE to LONG format and including a “day” variable to denote time. To standardize the day variable and interpret beta coefficients, day 1 was recoded as day 0 and day 14 was recoded as day 13.
Behavioral Measures
Following hypotheses about the importance of empathic concern for promoting prosocial behavior (FeldmanHall et al., 2015; Mobbs et al., 2010; Rameson et al., 2012; Vekaria et al., 2017), all participants completed a measure of empathic traits, the Interpersonal Reactivity Index (IRI, Davis, 1983), as part of online screening. The IRI is a 28-item measure composed of four subscales: Perspective Taking, Fantasy, Empathic Concern, and Personal Distress. Each item is rated on a five-point scale. Average scores from the Interpersonal Reactivity Index were compiled. The Empathic Concern scale of the IRI was selected for inclusion in subsequent regression models. As in previous studies (Marsh et al., 2014; Brethel-Haurwitz et al., 2018) altruists and controls did not significantly differ on any IRI subscales or total scores (all ps > .05).
Results
Participants completed 1,315 total everyday helping surveys (M completion = 13.02, SD = 1.88). 93.0% of sent surveys were completed. No group difference in completion was observed, t(99) = .31, p = .754. (2 participants were excluded from the analytic sample for completing 2 surveys or less.) Number of surveys was included as an exposure variable in all regression models to account for variable survey completion rates and mitigate bias through weighting of observations. Total counts of everyday helping were calculated (M = 54.86, SD = 33.57), as were counts of helping for family and friends (M = 23.67, SD = 20.06), and acquaintances and strangers (M = 30.15, SD = 20.20). Separately, total counts of reactive helping (M = 15.65, SD = 13.70) and proactive helping (M = 37.01, SD = 21.21) were calculated. Results of an independent samples Mann-Whitney U-test found that altruists and typical adults did not differ in total everyday helping (U = 1.68, p = .195) or in any sub-categories.
We first explored the correspondence between total daily helping and patterns of neural activation when participants empathized with a target as they watched her undergo pressure-pain stimulation. Using bivariate rank-order (rs = Spearman’s rho) correlations, we found that empathic activation in left BNST predicted subsequent total daily helping counts (Figure 1), rs(48) = .384, p = .007, whereas the same was not true for right BNST rs(48) = .131, p = .375. Activation in the remaining four a priori ROIs also were not associated with helping following Benjamini-Hochberg FDR correction for six multiple comparisons utilizing q = .05 threshold (see Supplemental Table 1), indicating < 5% chance that the left BNST finding was a false positive. No group difference in left BNST activation was observed during the task, t(46) = −1.62, p = .113.
Figure 1.
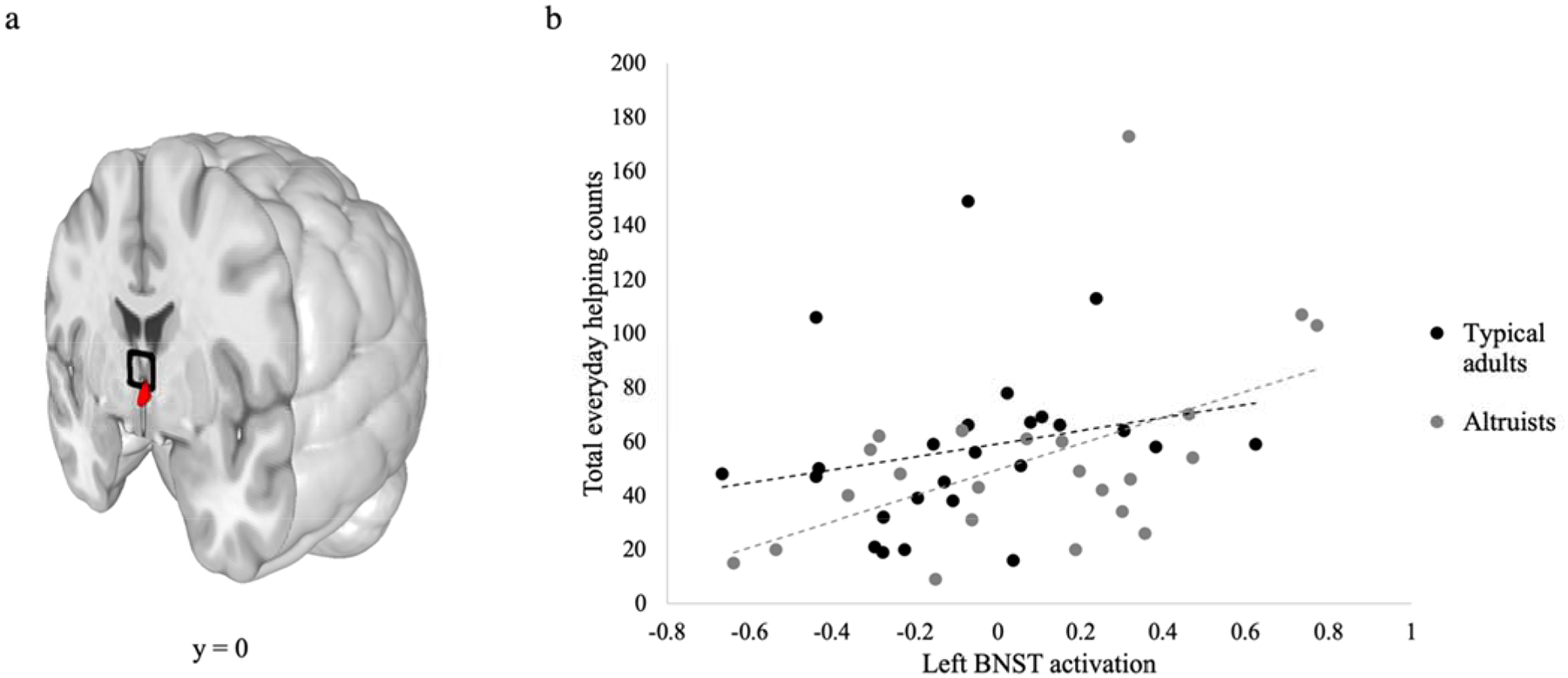
a) Images presents left BNST ROI (red) (Torrisi et al., 2015) alongside the septal area ROI (black) used by Morelli, et al. (2014) b) Scatter plot of activation in left BNST in empathy for pain task (pain > no pain) parameter estimates in relation to total counts of daily helping, with linear correlation estimates.
Activation in left BNST was next entered into a negative binomial regression model, to identify whether this activation could predict total helping scores above and beyond the effects of group (altruist, control), self-reported empathic concern, the interaction between these variables, and demographic covariates (Table 2). In the first block, holding covariates constant, altruist status was not associated with total helping. However, gender was associated with total helping, with females helping 12% more than males. Including empathic concern and the interaction between empathic concern and altruist status in the second block revealed a main effect of altruist status once empathic concern was held constant (with altruists helping less than controls) and an empathic concern by altruist status interaction such that for altruists relative to controls, a one unit increase in empathic concern scores led to an increase in estimated rates of total helping by 45%. After empathic concern and its interaction were entered into the model, no further effect of gender on helping was observed. After entering left BNST activation in the model in the third block, results revealed that even after holding the remaining variables constant, a one-unit increase in left BNST activation during empathy for pain predicted increased estimated rates of helping by 86% for total everyday helping during a subsequent 14 day period (Table 2).
Table 2.
Negative binomial regression analyses predicting total everyday helping counts from altruist status (Blocks 1–3), empathic concern (EC) (Blocks 2–3); and left BNST activation during empathy for pain (Block 3)
| Block 1 | Block 2 | Block 3 | ||||
|---|---|---|---|---|---|---|
| IRR | SE | IRR | SE | IRR | SE | |
| Altruist status | 0.84 | 0.11 | 0.23* | 0.14 | 0.19** | 0.09 |
| Empathic concern (EC) | 1.15 | 0.15 | 1.04 | 0.12 | ||
| Altruist status × EC | 1.45* | 0.26 | 1.55** | 0.23 | ||
| Left BNST activation | 1.86** | 0.39 | ||||
| Demographics | ||||||
| Gender | 0.88* | 0.11 | 0.88 | 0.10 | 1.07 | 0.16 |
| Age | 0.99 | 0.01 | 1.00 | 0.01 | 1.01 | 0.01 |
| Population size | 1.04 | 0.05 | 1.03 | 0.04 | 1.00 | 0.05 |
| Education | 0.95 | 0.14 | 0.81 | 0.13 | 0.89 | 0.13 |
| Race | 0.89 | 0.10 | 0.89 | 0.09 | 1.21 | 0.22 |
Note: Standard errors are robust standard errors.
p < .05,
p < .01
In follow-up analyses, we considered whether observed relationships were specific to specific types of everyday helping (Table 3), including family and friend helping versus acquaintance and stranger helping, and reactive versus proactive helping. No effects of demographic variables on any observed outcome were observed. However, the effects of BNST on helping appeared strongest for helping more socially distant others (acquaintances and strangers) and more proactive helping. This activation also predicted rates of helping increased by 98% for acquaintance and stranger helping, and 107% for proactive helping, holding other variables constant. Observed effects of altruist status were similarly stronger for helping more socially distant others (acquaintances and strangers) and more proactive helping.
Table 3.
Negative binomial regression analyses predicting daily helping for categories of everyday helping from left BNST activation
| Family & Friend | Acquaintance & Stranger | Reactive | Proactive | |||||
|---|---|---|---|---|---|---|---|---|
| IRR | SE | IRR | SE | IRR | SE | IRR | SE | |
| Altruist status | 0.20 | 0.20 | 017** | 0.11 | 0.27 | 0.21 | 0.18** | 0.08 |
| Empathic concern (EC) | 1.08 | 0.26 | 0.95 | 0.16 | 1.22 | 0.21 | 0.96 | 0.11 |
| Altruist status × EC | 1.54 | 0.47 | 1.58* | 0.32 | 1.35 | 0.33 | 1.61** | 0.24 |
| Left BNST activation | 1.99 | 0.79 | 1.98* | 0.58 | 1.42 | 0.51 | 2.07** | 0.43 |
| Demographics | ||||||||
| Gender | 0.73 | 0.20 | 1.30 | 1.30 | 1.04 | 0.27 | 1.11 | 0.17 |
| Age | 1.00 | 0.02 | 1.00 | 1.00 | 1.01 | 0.01 | 1.00 | 0.01 |
| Population size | 0.85 | 0.08 | 1.09 | 1.09 | 0.97 | 0.08 | 0.98 | 0.05 |
| Education | 0.85 | 0.19 | 0.80 | 0.80 | 0.90 | 0.21 | 0.87 | 0.13 |
| Race | 1.15 | 0.43 | 1.13 | 1.13 | 1.31 | 0.48 | 1.13 | 0.19 |
Note: Standard errors are robust standard errors.
p < .05,
p < .01
Post-hoc neuroimaging analyses also examined the relationship of total daily helping with whole brain activation in the empathy for pain task with helping data residuals (after covarying out demographics variables and number of surveys completed). Using this approach, no regions of activation that predicted total daily helping survived cluster correction for multiple comparisons. Cluster significance was determined using an underlying voxel height threshold of p<.001 and a cluster forming threshold to control the false positive rate at p < .05. Post-hoc exploratory neuroimaging analyses also considered how activity in other cortical regions might correspond to daily helping, and, like Morelli and colleagues (2014) we found minimal associations between helping and patterns of activation in cortical regions that included anterior cingulate cortex (ACC), temporoparietal junction (TPJ), and medial prefrontal cortex (mPFC) (Supplemental Table 1). These findings also held under the Benjamini–Hochberg FDR procedure (Supplemental Material) in order to correct for multiple comparisons, using q = .05. We additionally ran a subsequent analysis utilizing the coordinates of the original Morelli et al. (2014) region of interest described as septal area and did not find that activation in this region correlated with daily prosocial behavior (Supplemental Material), strengthening our interpretation of the findings.
In order to assess the effects of time, and test the degree to which participants were affected by the potential “intervention effect” of completing daily surveys about helping behavior, the same daily helping variables were examined across time using time series negative binomial regression models with day included as a key variable (see Supplemental Material).
Discussion
The current study explored neural and behavioral predictors of everyday prosocial behavior in both a sample of typical adults (recruited from the community) and a sample of extraordinary adults (altruistic kidney donors). Using experience sampling methods and a targeted anatomical mask of BNST, we found that activation in left BNST while empathizing with another person’s pain predicted rates of everyday helping over an ensuing period of 14 days in both ordinary adults and altruists. We observed no interaction with group, suggesting that this mechanism operates similarly for promoting everyday helping across both of the groups we evaluated. Even after accounting for gender, age, education, race, the population of their place of residence, and self-reported empathic concern, BNST activation was a robust predictor of multiple forms of everyday helping, particularly helping for more socially distant others (strangers and acquaintances) and proactive helping, although effect sizes for both proactive and reactive helping were generally similar. This relationship was evident when examining both total daily helping as well as time series models. These finding suggest an important role for BNST in maintaining vigilance not only for cues relevant to personal safety in the environment (Somerville et al., 2010) but for cues suggesting that others in the vicinity may need help.
Although we considered potential relationships between everyday helping in bilateral BNST, amygdala, and anterior insula (as well as, in supplementary analyses, additional cortical regions) only left BNST emerged as a predictor of everyday helping. These results are in line with the known role of BNST in facilitating caregiving for offspring, which may provide a basis for extending care to distant others (Decety, Norman, Berntson, & Cacioppo, 2012; Leng et al., 2008; Marsh, 2018; Preston, 2013). Across vertebrate species, BNST is a key component of the oxytocin-modulated neural pathways that support parental care (Grinevich, Knobloch-Bollmann, Eliava, Busnelli, & Chini, 2016) and has in animal models, been shown to play an essential role in parent-initiated (proactive) caregiving responses, such as infant retrieval, whereas it is less involved in passive care behaviors, like feeding (Numan, 2006). In rodents and humans, BNST is also implicated in the neurobiology of social recognition and motivation for forming attachment bonds, with deficits in this region being associated with symptoms of social anxiety (Lebow & Chen, 2016). Conclusions about the importance of lateralization in this structure remain speculative; in our sample, right BNST was also positively associated with total helping counts, rs(48) =.313, p =.375, however this relationship was not statistically significant. In prior studies, left (but not right) BNST has previously been implicated in hypervigilant threat monitoring in humans (Somerville et al., 2010). Our findings suggest that empathic activation in this structure may also play a role in vigilance and monitoring relevant to the needs of others in the social environment.
Behaviorally, when examining the relationship between altruist status and daily helping using negative binomial regression models for count data, we found no significant difference between altruists’ and controls’ daily helping. However, including trait empathic concern as an independent variable of interest allowed for a more nuanced explanation of daily prosocial behaviors. We observed a positive interaction between altruist status and trait empathic concern, such that for altruists, increased empathic concern increased estimated daily helping counts at a higher rate for close and distant targets. When examining daily helping over the 14 days of surveys, including time as a variable of interest did not predict daily helping--that is, across all subjects helping did not significantly increase over time (Supplemental Material). However, when examining controls relative to altruists through the inclusion of an interaction variable (altruist status by time), we observed an interaction such that controls’ helping increased more over time relative to altruists.
As predicted, we observed no significant difference in overall daily helping when initially comparing altruists and typical adults. These results are consistent with past examinations of extreme altruists’ responses on the Self-Reported Altruism scale, from which the everyday helping questions in this study were drawn (Brethel-Haurwitz et al., 2016; Morelli et al., 2014; Rushton et al., 1981). However, when subsequent models controlled for other individual-level covariates, we found that typical adults report slightly more everyday helping across all categories of helping and over time. Given altruists’ previous non-normative helping behavior, as well as the anonymous nature of such donations (with altruists’ identity remaining anonymous in many cases), altruists may be less focused on reputational benefits of helping and may therefore be less susceptible to the intervention bias associated with experience sampling. The tendency to increase behaviors over time as a function of being reminded about them may be associated with social desirability concerns (Napa Scollon et al., 2009; Spook, Paulussen, Kok, & Van Empelen, 2013). One consideration is that prosocial individuals in the present study may be those who are more responsive to the intervention we provided. An alternative interpretation of our time-series data, however, is that this method may serve as an effective intervention for increasing everyday prosocial behaviors in typical adults by prompting them to reflect on how often they engage in these behaviors. However, whether this interpretation is correct will require more targeted studies. In addition, it should be noted that the overall effects of time were small relative to other independent variables, like trait empathic concern.
That self-reported empathic concern was more strongly associated with increases in everyday helping for altruists relative to controls was a particularly interesting finding, given that the two groups did not differ in trait empathic concern or any other IRI subscales, which is generally consistent with prior studies using similar sample sizes (Marsh et al., 2014). Note however, that we have previously identified group differences in coldheartedness, which is the conceptual inverse of empathic concern, with altruists reporting lower levels of coldheartedness (Vekaria et al., 2017). And laboratory experiments have also found self-reported empathic concern to predict greater costly helping in the laboratory (FeldmanHall et al., 2015) with the caveat that costly helping in the laboratory is unavoidably subject to elevated social desirability concerns. In our current sample, it appears the combination of individual variation in the propensity for costly altruism for strangers (reflected in past acts of extraordinary altruism) coupled with increased trait empathic concern can predict increased rates of everyday helping across socially close and distant recipients. Interestingly, everyday helping in our study was largely unrelated to a variety of other demographic variables sometimes linked to altruism, including sex, age, education, and the population of participants’ geographic regions.
Limitations of this study must be considered as well. Our experience sampling paradigm was limited to one daily survey for 14 days, a technique likely to be less precise than randomized ecological momentary sampling assessments (EMA) multiple times a day across longer periods of time, made possible by recent technological advancements and which may assess daily behaviors with greater precision (Aan het Rot et al., 2012; Spook et al., 2013). In addition to measuring everyday prosocial behaviors, future work should also collect daily measures of mood and affect to identify the influence of these variables on helping behavior over time (Grühn, Rebucal, Diehl, Lumley, & Labouvie-Vief, 2008; Raposa et al., 2016). Additionally, although experience sampling mitigates many of the shortcomings of laboratory-based self-assessments of prosociality (Eisenberg & Fabes, 1990), it remains a possibility that the group and group-by-time differences we identified reflect self-report biases rather than genuinely higher levels of everyday helping in controls, and collecting measures of social desirability would help mitigate these concerns. Finally, like prior work in extraordinary altruists, the data we could collect, including our sample size was constrained in various ways by the extreme rarity of this population (Marsh et al., 2014; Organ Procurement and Transplantation Network, 2016). The fact that altruists were geographically dispersed and many were flown to Georgetown University for testing limited the scope of our neuroimaging testing, such that our empathy for pain task (Brethel-Haurwitz et al., 2018) was conducted to explore only empathic responses to distress and using only one social distance target (a stranger). Future work would ideally assess targets who vary in social distance and empathic responses to various affective states in order to generalize the interpretations of our findings more broadly. It should be noted, however, that the region we identified, which partially overlaps with the cluster identified by Morelli and colleagues (2014) was found in that study to exhibit overlapping responses to multiple affective states.
This study provides the first exploration of the neural correlates of everyday helping behavior across samples of both extraordinary altruists and controls. Hearteningly, our results indicate that most individuals help both close and distant others every day (helping on average over 4 times per day on the measures we assessed). Our neural findings (that individual differences in activation in BNST during the empathic pain paradigm track with everyday helping) suggest that the mechanisms thought to be engaged in surveying the daily environment for signs of potential threat may also support vigilance for signs of others’ need, and this translates to greater helping behavior in real life. BNST is a brain region consistently associated with vigilance for socio-affective cues and with parental care, and empathic activation in this region predicted everyday helping both overall and across time, particularly for distant social others. The relationship between BNST activity and everyday helping was similar across both extraordinary altruists and controls, suggesting that the mechanisms underlying everyday helping and extreme altruism are dissociable. Controls showed slightly increased rates of helping over time relative to altruists, suggesting a potential intervention target to increase helping behavior over time. Whereas extraordinary forms of altruism are costly and often risky and may not be attainable for everyone, our findings suggest that frequent engagement in lower-cost prosocial behaviors toward both close and distant others are well within reach for most people.
Supplementary Material
Acknowledgments
This project was supported by John Templeton Foundation Grant 47861 to A. A. Marsh and National Institutes of Health National Center for Advancing Translational Sciences Grant 1KL2RR031974-01 to J. W. VanMeter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data and Code Availability: Data and code for the study presented in this manuscript are available at the Open Science Framework: https://osf.io/uvtsm/
References
- Aan het Rot M, Hogenelst K, & Schoevers RA (2012). Mood disorders in everyday life: A systematic review of experience sampling and ecological momentary assessment studies. Clinical Psychology Review, 32(6), 510–523. 10.1016/j.cpr.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Adolphs R (2008). Fear, faces, and the human amygdala. Current Opinion in Neurobiology, 18(2), 166–172. 10.1016/j.conb.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aknin LB, Van de Vondervoort JW, & Hamlin JK (2018). Positive feelings reward and promote prosocial behavior. Current Opinion in Psychology, 20, 55–59. 10.1016/j.copsyc.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Alves F, Resstel L, Gomes F, Correa F, Crestani C, & Herman J (2013). Mechanisms in the Bed Nucleus of the Stria Terminalis Involved in Control of Autonomic and Neuroendocrine Functions: A Review. Current Neuropharmacology, 11(2), 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD (2010). the Naked Emperor: Seeking a More Plausible Genetic Basis for Psychological Altruism. Economics and Philosophy, 26(02), 149–164. 10.1017/S0266267110000179 [DOI] [Google Scholar]
- Baxter MG, & Murray EA (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563–573. 10.1038/nrn875 [DOI] [PubMed] [Google Scholar]
- Bramati IE, Paiva FF, Tovar-Moll F, Moll J, Zahn R, Sato JR, … Lima DO (2012). A Neural Signature of Affiliative Emotion in the Human Septohypothalamic Area. Journal of Neuroscience, 32(36), 12499–12505. 10.1523/jneurosci.6508-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brethel-Haurwitz KM, Cardinale EM, Vekaria KM, Robertson EL, Walitt B, VanMeter JW, & Marsh AA (2018). Extraordinary Altruists Exhibit Enhanced Self– Other Overlap in Neural Responses to Distress. Psychological Science, 095679761877959. 10.1177/0956797618779590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brethel-Haurwitz KM, O’Connell K, Cardinale EM, Stoycos SA, Lozier LM, Vanmeter JW, & Marsh AA (2017). Amygdala-midbrain connectivity indicates a role for the mammalian parental care system in extraordinary altruism. [DOI] [PMC free article] [PubMed]
- Brethel-Haurwitz KM, Stoycos SA, Cardinale EM, Huebner B, & Marsh AA (2016). Is costly punishment altruistic? Exploring rejection of unfair offers in the Ultimatum Game in real-world altruists. Scientific Reports, 6(January), 18974 10.1038/srep18974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Zaborszky L, Pirraglia E, Li J, Wang XH, Li Y, … Thesen T (2014). Comparison of human septal nuclei MRI measurements using automated segmentation and a new manual protocol based on histology. NeuroImage, 97, 245–251. 10.1016/j.neuroimage.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, … Nesselroade JR (2011). Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychology and Aging, 26(1), 21–33. 10.1037/a0021285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI Clustering in AFNI: False Positive Rates Redux. Brain Connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH (1983). A Multidimensional Approach to Individual Differences in Empathy. Journal of Personality and Social Psychology, 44(1), 113–126. 10.1037/0022-3514.44.1.113 [DOI] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Vega A, Chang LJ, Banich MT, Wager TD, & Yarkoni T (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience. 10.1523/JNEUROSCI.4402-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM (2008). Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology, 59, 279–300. 10.1146/annurev.psych.59.103006.093625 [DOI] [PubMed] [Google Scholar]
- Decety J, & Lamm C (2006). Human empathy through the lens of social neuroscience. TheScientificWorldJournal, 6, 1146–1163. 10.1100/tsw.2006.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Norman GJ, Berntson GG, & Cacioppo JT (2012). A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Progress in Neurobiology, 98(1), 38–48. 10.1016/j.pneurobio.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Dunn EW, Aknin LB, & Norton MI (2014). Prosocial Spending and Happiness. Current Directions in Psychological Science, 23(1), 41–47. 10.1177/0963721413512503 [DOI] [Google Scholar]
- Eisenberg N, & Fabes RA (1990). Empathy: Conceptualization, measurement, and relation to prosocial behavior. Motivation and Emotion, 14(2), 131–149. 10.1007/BF00991640 [DOI] [Google Scholar]
- Eisenberg N, & Lennon R (1983). Sex differences in empathy and related capacities. Psychological Bulletin, 94(1), 100–131. 10.1037/0033-2909.94.1.100 [DOI] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeldmanHall O, Dalgleish T, Evans D, & Mobbs D (2015). Empathic concern drives costly altruism. NeuroImage, 105, 347–356. 10.1016/j.neuroimage.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, & Collins DL (2011). Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 10.1016/j.neuroimage.2010.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W, Mulvey EP, & Shaw EC (1995). Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychological Bulletin, 118(3), 392–404. 10.1111/j.1399-6576.2011.02589.x [DOI] [PubMed] [Google Scholar]
- Gracely RH (1990). Measuring pain in the clinic. Anesthesia Progress, 37(2–3), 88–92. [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, & Chini B (2016). Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biological Psychiatry, 79(3), 155–164. 10.1016/j.biopsych.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Grühn D, Rebucal K, Diehl M, Lumley M, & Labouvie-Vief G (2008). Empathy across the adult lifespan: Longitudinal and experience-sampling findings. Emotion, 8(6), 753–765. 10.1037/a0014123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, & Burghart DR (2007). Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science, 316(5831), 1622–1625. 10.1126/science.1140738 [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, & Singer T (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68(1), 149–160. 10.1016/j.neuron.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Helliwell J, Aknin L, Shiplett H, Huang H, & Wang S (2017). Social Capital and Prosocial Behaviour as Sources of Well-Being. Cambridge, MA: 10.3386/w23761 [DOI] [Google Scholar]
- Hutchinson MK, & Holtman MC (2005). Analysis of count data using Poisson regression. Research in Nursing and Health, 28(5), 408–418. 10.1002/nur.20093 [DOI] [PubMed] [Google Scholar]
- Iida M, Shrout PE, Laurenceau J-P, & Bolger N (2016). Using diary methods in psychological research. APA Handbook of Research Methods in Psychology, Vol 1: Foundations, Planning, Measures, and Psychometrics., (January), 277–305. 10.1037/13619-016 [DOI] [Google Scholar]
- Kaufman A, & Kaufman N (2004). Kaufman Brief Intelligence Test 2. Pearson, London. [Google Scholar]
- Kelly S, & Dunbar RIM (2001). Who dares, wins. Human Nature, 12(2), 89–105. 10.1007/s12110-001-1018-6 [DOI] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Meddle SL, & Douglas AJ (2008). Oxytocin and the maternal brain. Current Opinion in Pharmacology, 8(6), 731–734. 10.1016/j.coph.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, & Assheuer JK (2004). Atlas of the Human Brain (2nd editio). Amsterdam: Elsevier Academic Press. [Google Scholar]
- Marsh AA (2016). Neural, cognitive, and evolutionary foundations of human altruism. Wiley Interdisciplinary Reviews: Cognitive Science, 7(1), 59–71. 10.1002/wcs.1377 [DOI] [PubMed] [Google Scholar]
- Marsh AA (2018). The Caring Continuum: Evolved Hormonal and Proximal Mechanisms Explain Prosocial and Antisocial Extremes. Annual Review of Psychology, 70(1), 347–371. 10.1146/annurev-psych-010418-103010 [DOI] [PubMed] [Google Scholar]
- Marsh AA, Stoycos SA, Brethel-Haurwitz KM, Robinson P, VanMeter JW, & Cardinale EM (2014). Neural and cognitive characteristics of extraordinary altruists. Proceedings of the National Academy of Sciences of the United States of America, 111(42), 15036–15041. 10.1073/pnas.1408440111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12(9), 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, & Dalgleish T (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences, 107(47), 20582–20586. 10.1073/pnas.1009076107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli Sylvia A., Rameson LT, & Lieberman MD (2014). The neural components of empathy: Predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience, 9(1), 39–47. 10.1093/scan/nss088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli Sylvia Annette. (2013). The neural and behavioral basis of empathy for positive and negative emotions. Dissertation Abstracts International: Section B: The Sciences and Engineering, 73(10-B(E)) Retrieved from http://search.ebscohost.com.proxy-ub.rug.nl/login.aspx?direct=true&db=psyh&AN=2013-99080-361&site=ehost-live&scope=site [Google Scholar]
- Napa Scollon C, Prieto C-K, & Diener E (2009). Experience Sampling: Promises and Pitfalls, Strength and Weaknesses (Vol. 39, pp. 157–180). 10.1007/978-90-481-2354-4_8 [DOI] [Google Scholar]
- Nettle D, Colléony A, & Cockerill M (2011). Variation in Cooperative Behaviour within a Single City. PLoS ONE, 6(10), e26922 10.1371/journal.pone.0026922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JD (2007). Neural circuits underlying crying and cry responding in mammals. Behavioural Brain Research, 182(2), 155–165. 10.1016/j.bbr.2007.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M (2006). Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews, 5(4), 163–190. 10.1177/1534582306288790 [DOI] [PubMed] [Google Scholar]
- O’Connell K, Brethel-Haurwitz KM, Rhoads SA, Cardinale EM, Vekaria KM, Robertson EL, … Marsh AA (2019). Increased similarity of neural responses to experienced and empathic distress in costly altruism. Scientific Reports. 10.1038/s41598-019-47196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organ Procurement and Transplantation Network. (2016). Living Donor Transplants By Donor Relation. Retrieved from https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- Philippe Rushton J, Chrisjohn RD, & Cynthia Fekken G (1981). The altruistic personality and the self-report altruism scale. Personality and Individual Differences, 2(4), 293–302. 10.1016/0191-8869(81)90084-2 [DOI] [Google Scholar]
- Preston SD (2013). The origins of altruism in offspring care. Psychological Bulletin, 139(6), 1305–1341. 10.1037/a0031755 [DOI] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, & Lieberman MD (2012). The Neural Correlates of Empathy: Experience, Automaticity, and Prosocial Behavior. Journal of Cognitive Neuroscience, 24(1), 235–245. 10.1162/jocn_a_00130 [DOI] [PubMed] [Google Scholar]
- Raposa EB, Laws HB, & Ansell EB (2016). Prosocial behavior mitigates the negative effects of stress in everyday life. Clinical Psychological Science, 4(4), 691–698. 10.1177/2167702615611073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbacher E, Perry RE, Sullivan RM, & Moita MA (2017). Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. ELife, 6, 1–17. 10.7554/elife.24080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Huang C-C, Lin C-P, Feng J, & Joliot M (2019). Automated anatomical labelling atlas 3. NeuroImage, 116189 10.1016/j.neuroimage.2019.116189 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Joliot M, & Tzourio-Mazoyer N (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage, 122, 1–5. 10.1016/j.neuroimage.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Sokolowski K, & Corbin JG (2012). Wired for behaviors: from development to function of innate limbic system circuitry. Frontiers in Molecular Neuroscience, 5(April), 1–15. 10.3389/fnmol.2012.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, & Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–424. 10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzmuller M, & Van Dyne L (2013). Proactive and reactive helping: Contrasting the positive consequences of different forms of helping. Journal of Organizational Behavior, 34(4), 560–580. 10.1002/job.1848 [DOI] [Google Scholar]
- Spook JE, Paulussen T, Kok G, & Van Empelen P (2013). Monitoring dietary intake and physical activity electronically: Feasibility, usability, and ecological validity of a mobile-based ecological momentary assessment tool. Journal of Medical Internet Research, 15(9), 1–13. 10.2196/jmir.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Goodkind MS, & Levenson RW (2012). Greater emotional empathy and prosocial behavior in late life. Emotion, 12(5), 1129–1140. 10.1037/a0025011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen V, Bentall RP, Oorschot M, À Campo J, Van Lierop T, Van Os J, & Myin-Germeys I (2011). Emotions, self-esteem, and paranoid episodes: An experience sampling study. British Journal of Clinical Psychology, 50(2), 178–195. 10.1348/014466510X508677 [DOI] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, … Ernst M (2015). Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Human Brain Mapping, 36(10), 4076–4088. 10.1002/hbm.22899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Vekaria K, Brethel-Haurwitz K, Cardinale E et al. Social discounting and distance perceptions in costly altruism. Nat Hum Behav 1, 0100 (2017). 10.1038/s41562-017-0100 [DOI] [Google Scholar]
- Zald DH (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41(1), 88–123. 10.1016/S0165-0173(02)00248-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


