Abstract
Introduction:
Metabolic syndrome is a disorder characterized by a constellation of findings including truncal obesity, elevated blood pressure, abnormal cholesterol levels, and high blood glucose. Recent evidence suggests that metabolic syndrome may be associated with increased risk of age-related macular degeneration (AMD) and other eye diseases. Recently, C57BL/6J wild-type mice fed with a “fast food” diet consisting of high fat, cholesterol, and fructose-supplemented water showed unique systemic pathology consistent with metabolic syndrome and nonalcoholic steatohepatitis. Additionally, these mice showed higher levels of fibrosis, inflammation, endoplasmic reticulum stress, and mitochondrial dysfunction compared to mice fed with only a high fat diet alone. Since similar pathways are activated in AMD, we sought to determine whether mice fed a “fast food” diet exhibited retinal changes.
Methods:
3-month old wild-type mice were randomized to a standard chow (n=11) or a “fast food” (n=18) diet and fed for 9 months. At 1 year of age, tissues were collected and retinas were analyzed using transmission electron microscopy. Quantitative measures of Bruch’s membrane thickness and retinal pigment epithelium (RPE) cell counts were performed.
Results:
“Fast food” fed mice showed ocular pathology relevant to various stages of AMD including basal laminar deposits, focal thickening of Bruch’s membrane, and a significant loss of RPE cells.
Discussion/conclusion:
A wild-type mouse model of metabolic syndrome fed a “fast food” diet developed changes to the retina similar to some of the pathologic features seen in AMD. Further investigations into this and similar animal models as well as further epidemiological studies are needed to more clearly define the association between metabolic syndrome and AMD.
Keywords: Drusen, basal laminar deposits, macular degeneration, metabolic syndrome
Introduction
Increasing evidence is mounting that multiple aging eye diseases including age-related macular degeneration (AMD) are associated with metabolic syndrome 1–3. Metabolic syndrome represents a constellation of findings that includes enlarged waist circumference, dyslipidemia, systemic hypertension, and hyperglycemia 4, 5. With the increase in obesity rates, 1 metabolic syndrome is thought to affect nearly 1/3 of adults in the United States 4 with increased rates of diagnosis among adolescents 6. While the underlying mechanisms associated with metabolic syndrome are not fully understood, altered cellular signaling in pathways of inflammation 7 and oxidative stress 8 may contribute to cellular stress, increased senescence and end organ damage 8. Additionally, nonalcoholic steatohepatitis (NASH) shares significant commonalities with metabolic syndrome particularly with similar molecular pathway involvement, clinicopathologic features and patients affected 5.
Recently, wild-type mice fed with a “fast food” diet as a model of metabolic syndrome/NASH consisting of high fat, cholesterol, and sugar 9 showed similar obesity and insulin resistance to mice containing genetically altered inflammatory systems fed with a high fat diet alone. However, the “fast food” fed mice had more significant liver pathology consistent with steatohepatitis, including pronounced ballooning and progressive fibrosis. The liver morphology was consistent with NASH and was attributed to the presence of cholesterol and sugar along with high fat. Assessment of the hepatic transcriptome showed an increase in genes involved in inflammation, fibrosis, endoplasmic reticulum stress 9 and mitochondrial dysfunction 10.
Mice with genetic modifications in pathways associated with inflammation and oxidative stress, when fed a high fat diet, showed loss of retinal pigment epithelium (RPE), thickening of Bruch’s membrane, and sub-RPE deposits including basal laminar deposits (BlamD) 11–17 which are pathologic features relevant to AMD 18. In contrast, wild-type mice fed a high fat diet alone and used as controls in these studies were reported to have either normal RPE and Bruch’s membrane morphology 12–14, 17 or developed subtle changes such as focal inclusions, condensation of Bruch’s membrane 16 or a rare cytoplasmic vacuole 15. These studies suggest that dietary high fat alone has minimal impact on retinal ultrastructure in normal mice. However, in mice with altered genetic mechanisms that affect cellular health such as impaired metabolism or augmented inflammatory response, the high fat diet makes them vulnerable to retinal pathology.
Since “fast food” fed mice exhibited a different systemic pathology than high fat fed mice, and since metabolic syndrome and inflammation are associated with ocular diseases such as AMD 19–22, we examined the retinal morphology in 1 year old C57BL/6J wild-type mice that were fed a “fast food” diet.
Materials and Methods
C57BL/6J wild-type mice (Jackson Laboratories, Bar Harbor, ME) were fed with standard rodent chow (13% energy from milk fat, 0.9% saturated; PicoLab Rodent Diet 20, Lab Diet) and drinking water for the first 3 months of life. At 3 months, the mice were randomly selected to receive either a “fast food” diet (n=18) or maintained on standard rodent chow (n=11). The “fast food” diet provided 40% energy from milk fat (12% saturated) with 0.2% cholesterol (AIN-76 Western Diet, Test Diet), and drinking water supplemented with high-fructose/glucose (23.1 g/L fructose, 18.9 g/L glucose). For the current study, the same diet, time-points, and strain of mice were used as previously described 9. Animals were maintained on the “fast food” diet or continued on standard rodent chow for 9 additional months (± 2 weeks). All experiments with animals were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approval of the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic.
At 1 year of age (± 2 weeks), “fast food” and standard rodent chow fed mice were euthanized and whole eyes were enucleated, fixed in 4% paraformaldehyde/0.1 M phosphate buffer, and prepared for transmission electron microscopy imaging as previously described 23. Briefly, fixed whole eyes were post-fixed in osmium tetroxide, dehydrated in ascending ethanol concentrations, immersed in propylene oxide, and embedded in epoxy resin. Tissue blocks were sectioned at 100 nm, placed on copper grids, and stained with uranyl acetate and lead nitrate. Sequential micrographs from the posterior pole at similar distances from the optic nerve in all specimens were imaged using a JEOL 1400 transmission electron microscope (JEOL USA Inc., Peabody, MA). Images centered on the RPE were taken at 5,000x and images centered over Bruch’s membrane were taken at 25,000x.
Images were compiled in a montage, de-identified, and reviewed in a masked manner for pathologic findings as well as cell counts and thickness measurements. To determine whether there was loss of RPE, RPE nuclei were counted. Number of RPE nuclei was expressed as cells/100 μm in each montage. To determine whether there were global changes in Bruch’s membrane thickness, montages were analyzed using Image Pro Premier which allowed Bruch’s membrane length to be measured and the area of Bruch’s membrane to be traced and computed for each specimen. Bruch’s membrane has been defined either as a 3-layered structure 24 encompassing an inner and outer collagenous layer and a central elastic layer or as a 5-layered structure 25 that includes the adjacent basal lamina of the RPE anteriorly and the choriocapillaris posteriorly 26. For the purpose of measuring Bruch’s membrane, we used the 5-layered structure nomenclature and measured from the RPE basal lamina to the choriocapillaris basal lamina. Of note neither BlamD nor intercapillary pillars were included when determining Bruch’s membrane thickness.
A student’s t test was used for statistical analysis and values were considered significant at P<0.05.
Results
“Fast food” mice exhibit changes to RPE
Histologic examination of C57BL/6J wild-type mice fed with standard rodent chow revealed intact RPE nuclei and no cytoplasmic vacuolation (Fig 1A, C). In contrast, mice fed with a “fast food” diet had cytoplasmic vacuolation (Fig 1B, D), loss of normal RPE architecture (Fig 1D, E), and loss of RPE nuclei (Fig 1B, D). The RPE of some “fast food” fed mice showed nuclear and cytoplasmic condensation with early nuclear fragmentation consistent with ultrastructural features of apoptosis (Fig 1F). Compared to mice fed a standard chow diet, mice fed a “fast food” diet had 32% fewer RPE nuclei (Fig 1G, 3.9 ± 1.0 vs 2.7 ± 1.4 per 100 μm, P=0.01). Of the 11 wild-type mice fed a standard chow diet, none showed large vacuolated spaces, pyknotic RPE nuclei, or areas of RPE loss/attenuation. However, of the 18 mice fed a fast food diet, 2 showed large vacuolated spaces, 4 showed pyknotic RPE nuclei, and 3 showed RPE loss/attenuation.
Figure 1. Retinal pigment epithelium ultrastructure in standard rodent and “fast food” fed mice.
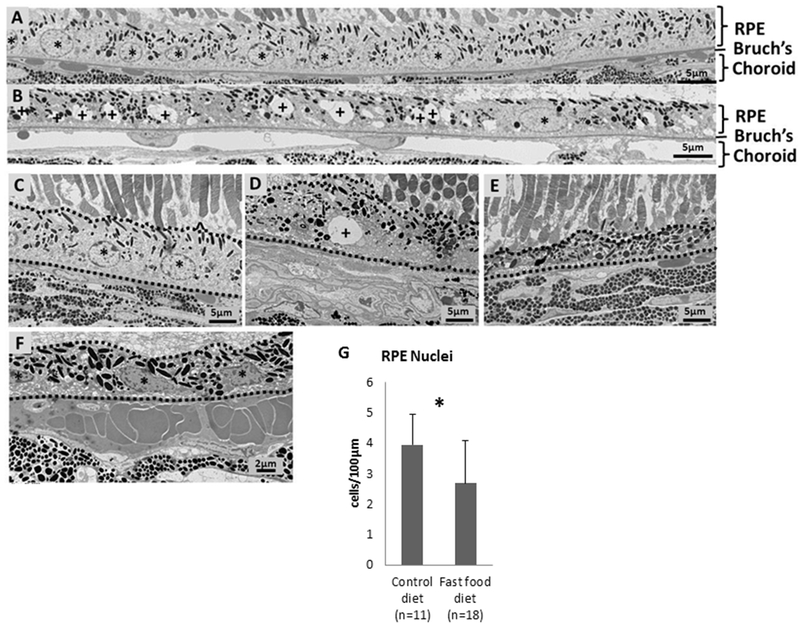
(A) Montage of four adjacent transmission electron microscopy images (5,000x) showing the RPE of a 1 year old C57BL/6J wild-type mouse fed with a standard rodent chow diet. These mice have intact RPE nuclei (asterisk) and show no signs of vacuolation. (B) Montage of four adjacent transmission electron microscopy images (5,000x) showing the RPE of a 1 year old C57BL/6J mouse fed with a standard rodent chow for the first 3 months of life followed by a “fast food” diet for the next 9 months. These “fast food” fed mice have RPE vacuolation (denoted with a plus sign) and only a few normal appearing nuclei (denoted with asterisks) compared to littermate controls (A) fed a standard rodent chow diet. (C) Mouse fed with a standard rodent chow diet reveals intact RPE nuclei (denoted with asterisk). RPE is bordered by a broken line. (D, E) Mice fed with a “fast food” diet reveal vacuolation (D, denoted with a plus sign), disorganization (D, E), and attenuation (E). (F) The RPE (outlined with a broken line) of mice fed a “fast food” diet exhibited ultrastructural features of apoptosis (denoted with asterisk), including nuclear and cytoplasmic condensation and nuclear fragmentation. (G) Compared to mice fed a standard chow diet, mice fed a “fast food” diet had significantly fewer RPE nuclei (3.9 ± 1.0 vs 2.7 ± 1.4 per 100 μm, P=0.01).]
“Fast food” mice exhibit changes in Bruch’s membrane
A histological montage showing Bruch’s membrane of 1 year old C57BL/6J wild-type mice fed standard rodent chow revealed an intact Bruch’s membrane with an age-appropriate thickness 27 (Fig 1F, H) and no BlamD deposits. In contrast, Bruch’s membrane in C57BL/6J wild-type mice fed with a “fast food” diet appeared visually thicker in some areas in most animals with the presence of nearly confluent BlamD deposits (Fig 2B, G). Additional ultrastructural changes in mice fed a “fast food” diet included disruption of the RPE basal infoldings (Fig 2F) and accumulation of basement membrane-like material with nodular extensions into the region of the basal infoldings of the RPE (Fig 2G) and into the inner choroid (Fig 2H). Since mice fed a “fast food” diet had areas of thickening of Bruch’s membrane we sought to determine whether these changes were significantly different if quantified globally. When analyzed globally across the entire montage and while there was a trend towards slightly increased Bruch’s membrane thickness, the measurements were not statistically significant (Fig 2I, 0.37 ± 0.07 vs 0.40 ± 0.13 μm2/μm, P=0.4).
Figure 2. Bruch’s membrane ultrastructure in standard rodent and “fast food” fed mice.
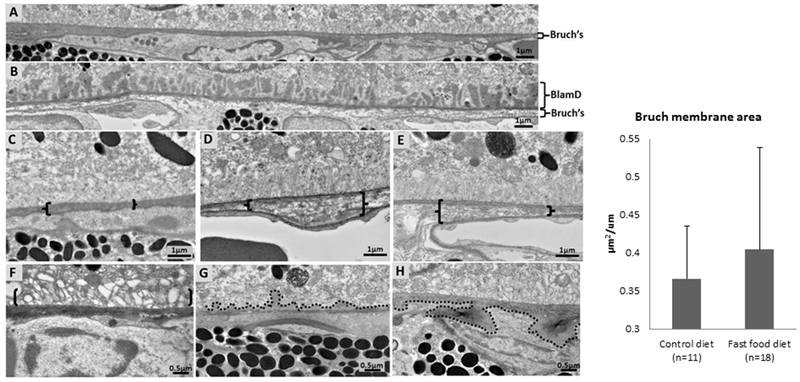
(A) Montage of six adjacent transmission electron microscopy images (25,000x) showing Bruch’s membrane of a 1 year old C57BL/6J wild-type mouse fed with standard rodent chow. (B) Montage of six adjacent transmission electron microscopy images (25,000x) showing Bruch’s membrane of a 1 year old C57BL/6J mouse fed with a standard rodent chow for the first 3 months of life followed by a “fast food” diet for the next 9 months. The “fast food” fed mice have basal laminar deposits (BlamD) and thickening of Bruch’s membrane. (C) Mouse fed standard rodent chow reveals intact Bruch’s membrane (in brackets). (D, E) Thickening and disorganization of Bruch’s membrane (brackets) was found in C57BL/6J wild-type mice fed a “fast food” diet. (F) Mice fed a “fast food” diet showed loss of RPE basal infoldings with apparent vacuolation (region denoted by brackets). (G) Mice fed a “fast food” diet also had accumulation of basement membrane-like material with nodular extension (denoted by broken line) into the region of the basal infoldings of the RPE. (H) Nodular extensions of basement membrane-like material (denoted by broken line) into the inner choroid of the RPE. (I) There was no significant difference in Bruch’s membrane thickness when measured across the entire montage (0.37 ± 0.07 vs 0.40 ± 0.13 μm2/μm, P=0.4).
Of the 11 wild-type mice fed a standard chow diet, one showed mild and non-confluent BlamD in one area, one showed a focal area of Bruch’s membrane thickening >1μm, and none showed loss of basal infoldings or nodular extensions of basement membrane-like material. However, in mice fed a “fast food” diet, 4 had nearly confluent BlamD (see Fig 2B), 6 had a focal area of Bruch’s membrane thickening >1 μm (See Fig 2D), 4 had loss of basal infoldings (see Fig 2F), and 3 had nodular extensions of basement membrane like material (see Fig 2G, H).
Discussion/Conclusion
“Fast food” diets likely play a major role in the development of obesity, insulin resistance, hypertension, and atherogenic dyslipidemia 28, 29, which are key elements of metabolic syndrome. As metabolic syndrome becomes increasingly more common 30, its potential as an underlying risk factor for other diseases needs to be investigated. Epidemiologic studies related to “fast food,” metabolic syndrome and its components as well as related findings including high cholesterol are challenging due to imprecise definitions of the diets/behaviors, the spectrum and overlap of systemic conditions, and multiple confounders that are often associated with diet and systemic health including exercise 31, 32.
10Though the data are conflicting in some clinical studies, progression to AMD has been associated with metabolic syndrome 1, 3 and it’s components, including hypertension 33, 34, abdominal obesity 35, 36, BMI 33, 34, 37–39 and high total serum cholesterol 40, 41. Additionally, minimal physical activity, low serum HDL, and elevated serum triglycerides have been associated with macular drusen, a hallmark of AMD 42. In contrast, other studies have found no association with some of these metrics, including obesity 43 and diabetes 44. Another association that initially received great consideration as being associated with AMD is high cholesterol, as histopathological studies showed high cholesterol content in drusen and aging Bruch’s membrane and several cholesterol-related genes were found to be risk factors for AMD 45. However, epidemiological studies have not shown this same degree of association. One study measured plasma apolipoproteins B and A-1, the principal proteins of low density and high density lipoproteins (LDL and HDL, respectively) and found no association with age-related macular disease 46. Interestingly, more recent studies have shown that HDL, which is protective for cardiovascular disease, was actually associated with an increased risk of AMD 47, 48.
The “fast food” fed mouse shares features of metabolic syndrome including insulin resistance, elevated serum lipids and cholesterol 9. Since the initial report, a longitudinal analysis has been performed examining hepatic changes at earlier time-points 10. Additionally the hepatic damage observed in mice fed a fast food diet can be accelerated with thioacetamide 49 or diminished with tacrolimus and everolimus 50. Our current study provides evidence that a wild-type mouse model of metabolic syndrome fed a “fast food” diet showed retinal ultrastructural changes relevant to AMD. This in vivo data supports some previous observations that metabolic syndrome may be an underlying risk factor for age-related retinal changes relevant to AMD 1 and that additional in vivo and epidemiologic studies are needed. While previous studies have demonstrated pathologic changes relevant to AMD in mice fed a high fat diet, these mice required a genetic modification in pathways associated with inflammation and oxidative stress 11–17. In the current study, our mice did not have any underlying genetic anomalies but still developed outer retinal pathology following a diet high in fat, cholesterol, and sugar. Our findings suggest that the “fast food” diet is a causal factor associated with the ultrastructural changes in the retina. It appears that excess fructose and cholesterol in addition to high fat is necessary for manifestation of retinal changes in these mice, since previous studies in wild-type mice that were fed with a high fat diet alone did not report consistent changes in the retina 11–17. Other studies have commented on morphological changes to the retina and a decline in retinal function in mice fed a high fat diet, but no ultrastructural images were provided 30, 51. Studies of diabetic retinopathy have provided some insight into the effects of high sugar alone, though much of the work in diabetic retinopathy has utilized models of type 1 diabetes 52. Rats fed with a high fructose diet have exhibited altered retinal function and were shown to express genes involved in endoplasmic reticulum stress and mitochondrial dysfunction; however, ultrastructural changes in the retina were not evaluated 53. Some evidence does exist to implicate high cholesterol diet in the pathogenesis of age-related ocular changes. New Zealand rabbits fed with a high cholesterol diet showed retinal thinning and vacuolated spaces with cellular evidence of increased apoptosis, endoplasmic reticulum stress, and oxidative damage 54. However, given the limited data surrounding these reports, it is currently unclear as to the extent in which various dietary components (fat, cholesterol or sugar) contribute to the pathogenesis of age-related ocular disease. Of note, a recent study showed that wild-type aged mice developed RPE and photoreceptor changes as well as lipofuscin accumulation when fed a high glycemic diet for one year 55. Future studies are necessary to evaluate the importance of high fat, high cholesterol and high sugar individually and in paired combination to identify which of the three dietary components (or combinations thereof) are involved in inducing altered retinal morphology in wild-type mice.
This study was limited by a single time-point description of morphological changes (1 year of age – 9 months on a “fast food” diet). However, it is important to note that mice fed with the standard rodent chow did not show alterations to outer retinal ultrastructure beyond what would be expected for mice of this age. In contrast, most wild-type mice fed a “fast food” diet demonstrated ultrastructural changes to the retina, with variation in the degree and specific pathologic changes within the spectrum of findings seen in AMD. Clinically, AMD is observed as a spectrum with presence of subretinal and sub-RPE deposits i that are associated with RPE loss and dysfunction and eventual progression to geographic atrophy 56. It is also important to note that while the histopathologic features seen in mice fed a “fast food” diet have relevance to AMD, this diet-induced model does not exactly replicate the features seen clinically. For example, similar RPE vacuolation has been seen in mice previously 57, However, RPE vacuolation in human AMD is rare and the ultimate fate of such cells is not known 58, 59. Additionally, it should also be clarified that while BLamD are thought to be a marker of AMD progression 60, these deposits are distinct from soft drusen and basal linear deposits which are pathognomonic deposits in AMD 61, 62. Drusen and basal linear deposits are accumulations of extracellular material between the RPE basement membrane and the inner collagenous layer of Bruch’s membrane which is also known as the sub-RPE-basal lamina space as visible on spectral-domain OCT63–65. BLamD which we observed in are accumulations of basement membrane material between the plasma membrane and basement membrane of the RPE 66.
Though we only examined retina at a single time-point, we hypothesize that we are capturing animals at different stages in disease progression from sub-RPE deposits to Bruch’s membrane thickening to RPE dysfunction, vacuolation, and eventually RPE cell loss. While our study is descriptive in nature, the strong correlation between diet and retinal changes in these animals suggests that mice fed a “fast food” diet may be a model for studying pathological progression of retinal diseases and their relationship to dietary habits and metabolic syndrome. Though systemic markers of fibrosis, inflammation, endoplasmic reticulum stress, and mitochondrial dysfunction have been shown to be upregulated in non-ocular tissues isolated from mice fed a “fast food” diet, future studies will be necessary to determine whether these same mechanisms are associated with development of AMD-like features in the retina. 9, 10.
Acknowledgments
Funding sources:
National Eye Institute grants EY 21727 and EY26490; Mayo Foundation, Rochester, MN; Liles Macular Degeneration Research Fund, Baylor Scott & White–Central Texas Foundation, Temple, TX.
References
- 1.Poh S, Mohamed Abdul RB, Lamoureux EL, Wong TY, Sabanayagam C. Metabolic syndrome and eye diseases. Diabetes Res Clin Pr 2016:113(86–100. [DOI] [PubMed] [Google Scholar]
- 2.Sabanayagam C, Wang JJ, Mitchell P, Tan AG, Tai ES, Aung T, Saw SM, Wong TY. Metabolic syndrome components and age-related cataract: The singapore malay eye study. Invest Ophth Vis Sci 2011:52(5): 2397–2404. [DOI] [PubMed] [Google Scholar]
- 3.Maralani HG, Tai BC, Wong TY, Tai ES, Li J, Wang JJ, Mitchell P. Metabolic syndrome and risk of age-related macular degeneration. Retina 2015:35(3): 459–466. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the united states, 2003–2012. JAMA 2015:313(19): 1973–1974. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Touros A, Kim WR. Nonalcoholic fatty liver disease and metabolic syndrome. Clin Liver Dis 2018:22(1): 133–140. [DOI] [PubMed] [Google Scholar]
- 6.Gaston SA, Tulve NS, Ferguson TF. Abdominal obesity, metabolic dysfunction, and metabolic syndrome in u.S. Adolescents: National health and nutrition examination survey 2011-2016. Ann Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2013:2(4): 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging and dis 2015:6(2): 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: Novel small animal model of nash with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastr L 2011:301(5): G825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan A, Abdullah TS, Mounajjed T, Hartono S, McConico A, White T, LeBrasseur N, Lanza I, Nair S, Gores G et al. A longitudinal study of whole body, tissue, and cellular physiology in a mouse model of fibrosing nash with high fidelity to the human condition. Am J Physiol Gastr L 2017:312(6): G666–G680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Z, Li J, Leng Y, Sun X, Hu H, He Y, Tan Z, Ge J. Cyclic intensive light exposure induces retinal lesions similar to age-related macular degeneration in appswe/ps1 bigenic mice. BMC neuroscience 2012:13(34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot SJ, Catanuto P, Espinosa-Heidmann DG, Fernandez P, Hernandez E, Saloupis P, Korach K, Karl M, Cousins SW. Estrogen receptor beta protects against in vivo injury in rpe cells. Exp eye res 2010:90(1): 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provost AC, Vede L, Bigot K, Keller N, Tailleux A, Jais JP, Savoldelli M, Ameqrane I, Lacassagne E, Legeais JM et al. Morphologic and electroretinographic phenotype of sr-bi knockout mice after a long-term atherogenic diet. Invest Ophth Vis Sci 2009:50(8): 3931–3942. [DOI] [PubMed] [Google Scholar]
- 14.Sallo FB, Bereczki E, Csont T, Luthert PJ, Munro P, Ferdinandy P, Santha M, Lengyel I. Bruch’s membrane changes in transgenic mice overexpressing the human biglycan and apolipoprotein b-100 genes. Exp eye res 2009:89(2): 178–186. [DOI] [PubMed] [Google Scholar]
- 15.Fujihara M, Bartels E, Nielsen LB, Handa JT. A human apob100 transgenic mouse expresses human apob100 in the rpe and develops features of early amd. Exp eye res 2009:88(6): 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Erfurth U, Rudolf M, Funk M, Hofmann-Rummelt C, Franz-Haas NS, Aherrahrou Z, Schlotzer-Schrehardt U. Ultrastructural changes in a murine model of graded bruch membrane lipoidal degeneration and corresponding vegf164 detection. Invest Ophth Vis Sci 2008:49(1): 390–398. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa-Heidmann DG, Sall J, Hernandez EP, Cousins SW. Basal laminar deposit formation in apo b100 transgenic mice: Complex interactions between dietary fat, blue light, and vitamin e. Invest Ophth Vis Sci 2004:45(1): 260–266. [DOI] [PubMed] [Google Scholar]
- 18.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 lorenz e. Zimmerman lecture. Ophthalmology 1993:100(10): 1519–1535. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. Jof leukocyte biol 2015:98(5): 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ardeljan CP, Ardeljan D, Abu-Asab M, Chan CC. Inflammation and cell death in age-related macular degeneration: An immunopathological and ultrastructural model. J clinmedi 2014:3(4): 1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmeggiani F, Sorrentino FS, Romano MR, Costagliola C, Semeraro F, Incorvaia C, D’Angelo S, Perri P, De Nadai K, Bonomo Roversi E et al. Mechanism of inflammation in age-related macular degeneration: An up-to-date on genetic landmarks. Mediat of inflamm 2013:2013(435607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussenblatt RB, Lee RW, Chew E, Wei L, Liu B, Sen HN, Dick AD, Ferris FL. Immune responses in age-related macular degeneration and a possible long-term therapeutic strategy for prevention. AmJ of ophthalmol 2014:158(1): 5–11 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury UR, Holman BH, Fautsch MP. Atp-sensitive potassium (k(atp)) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophth Vis Sci 2013:54(7): 4892–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gass J Stereoscopic atlas of macular diseases: Diagnosis and treatment. 4th ed ed. Louis St: Mosby; 1997. [Google Scholar]
- 25.Hogan MJAJ, Weddell JE. Histology of the human eye An atlas and textbook. Philadelphia PA: WB Sauders; 1971. [Google Scholar]
- 26.Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of bruch’s membrane. Prog Retin Eye Res 2010:29(1): 1–18. [DOI] [PubMed] [Google Scholar]
- 27.Volland S, Esteve-Rudd J, Hoo J, Yee C, Williams DS. A comparison of some organizational characteristics of the mouse central retina and the human macula. PloS one 2015:10(4): e0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samson SL, Garber AJ. Metabolic syndrome. Endocrin and metab clin 2014:43(1): 1–23. [DOI] [PubMed] [Google Scholar]
- 29.Bahadoran Z, Mirmiran P, Azizi F. Fast food pattern and cardiometabolic disorders: A review of current studies. Health Promot Perspect 2015:5(4): 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang RC, Shi L, Huang CC, Kim AJ, Ko ML, Zhou B, Ko GY. High-fat diet-induced retinal dysfunction. Invest Ophth Vis Sci 2015:56(4): 2367–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuinness MB, Le J, Mitchell P, Gopinath B, Cerin E, Saksens NTM, Schick T, Hoyng CB, Guymer RH, Finger RP. Physical activity and age-related macular degeneration: A systematic literature review and meta-analysis. Am J Ophthalmol 2017:180(29–38. [DOI] [PubMed] [Google Scholar]
- 32.Joo J, Williamson SA, Vazquez AI, Fernandez JR, Bray MS. The influence of 15-week exercise training on dietary patterns among young adults. Int J Obes (Lond) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R, Deng Y, Klein BE, Hyman L, Seddon J, Frank RN, Wallace RB, Hendrix SL, Kuppermann BD, Langer RD et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women’s health initiative sight exam ancillary study. Am J of ophthalmol 2007:143(3): 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC ophthalmology 2010:10(31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams MK, Simpson JA, Aung KZ, Makeyeva GA, Giles GG, English DR, Hopper J, Guymer RH, Baird PN, Robman LD. Abdominal obesity and age-related macular degeneration. Am Jof epidemiol 2011:173(11): 1246–1255. [DOI] [PubMed] [Google Scholar]
- 36.Haas P, Kubista KE, Krugluger W, Huber J, Binder S. Impact of visceral fat and pro-inflammatory factors on the pathogenesis of age-related macular degeneration. Acta ophthalmol 2015:93(6): 533–538. [DOI] [PubMed] [Google Scholar]
- 37.Howard KP, Klein BE, Lee KE, Klein R. Measures of body shape and adiposity as related to incidence of age-related eye diseases: Observations from the beaver dam eye study. Invest Ophth Vis Sci 2014:55(4): 2592–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: Association with body mass index, waist circumference, and waist-hip ratio. Arch of ophthalmol 2003:121(6): 785–792. [DOI] [PubMed] [Google Scholar]
- 39.Klein BE, Klein R, Lee KE, Jensen SC. Measures of obesity and age-related eye diseases. Ophthalepidemiol 2001:8(4): 251–262. [DOI] [PubMed] [Google Scholar]
- 40.Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology 2004:111(7): 1280–1287. [DOI] [PubMed] [Google Scholar]
- 41.Ambreen F, Khan WA, Qureshi N, Qureshi IZ. Assessment of serum lipids in patients with age related macular degeneration from pakistan. JPMA. The J of the Pak Med Assoc 2014:64(6): 664–669. [PubMed] [Google Scholar]
- 42.Munch IC, Linneberg A, Larsen M. Precursors of age-related macular degeneration: Associations with physical activity, obesity, and serum lipids in the inter99 eye study. Invest Ophth Vis Sci 2013:54(6): 3932–3940. [DOI] [PubMed] [Google Scholar]
- 43.Jaisankar D, Swaminathan G, Roy R, Kulothungan V, Sharma T, Raman R. Association of obesity and age-related macular degeneration in indian population. Indian J Ophthalmol 2018:66(7): 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topouzis F, Anastasopoulos E, Augood C, Bentham GC, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L et al. Association of diabetes with age-related macular degeneration in the eureye study. Br J Ophthalmol 2009:93(8): 1037–1041. [DOI] [PubMed] [Google Scholar]
- 45.Pikuleva IA, Curcio CA. Cholesterol in the retina: The best is yet to come. Prog Retin Eye Res 2014:41(64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dashti N, McGwin G, Owsley C, Curcio CA. Plasma apolipoproteins and risk for age related maculopathy. Br J Ophthalmol 2006:90(8): 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colijn JM, den Hollander AI, Demirkan A, Cougnard-Gregoire A, Verzijden T, Kersten E, Meester-Smoor MA, Merle BMJ, Papageorgiou G, Ahmad S et al. Increased high-density lipoprotein levels associated with age-related macular degeneration: Evidence from the eye-risk and european eye epidemiology consortia. Ophthalmology 2019:126(3): 393–406. [DOI] [PubMed] [Google Scholar]
- 48.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology 2017:124(8): 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma L, Gupta D, Abdullah ST. Thioacetamide potentiates high cholesterol and high fat diet induced steato-hepatitic changes in livers of c57bl/6j mice: A novel eight weeks model of fibrosing nash. Toxicol Lett 2019:304(21–29. [DOI] [PubMed] [Google Scholar]
- 50.Love S, Mudasir MA, Bhardwaj SC, Singh G, Tasduq SA. Long-term administration of tacrolimus and everolimus prevents high cholesterol-high fructose-induced steatosis in c57bl/6j mice by inhibiting de-novo lipogenesis. Oncotarget 2017:8(69): 113403–113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miceli MV, Newsome DA, Tate DJ Jr., Sarphie TG. Pathologic changes in the retinal pigment epithelium and bruch’s membrane of fat-fed atherogenic mice. Curr eye res 2000:20(1): 8–16. [PubMed] [Google Scholar]
- 52.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis model mech 2012:5(4): 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thierry M, Pasquis B, Buteau B, Fourgeux C, Dembele D, Leclere L, Gambert-Nicot S, Acar N, Bron AM, Creuzot-Garcher CP et al. Early adaptive response of the retina to a pro-diabetogenic diet: Impairment of cone response and gene expression changes in high-fructose fed rats. Exp eye res 2015:135(37–46. [DOI] [PubMed] [Google Scholar]
- 54.Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC ophthalmol 2011:11(22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowan S, Jiang S, Korem T, Szymanski J, Chang ML, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A 2017:114(22): E4472–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curcio CA. Soft drusen in age-related macular degeneration: Biology and targeting via the oil spill strategies. Invest Ophth Vis Sci 2018:59(4): AMD160–AMD181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med 2008:14(2): 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanzottera EC, Messinger JD, Ach T, Smith RT, Freund KB, Curcio CA. The project macula retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Invest Ophth Vis Sci 2015:56(5): 3253–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanzottera EC, Messinger JD, Ach T, Smith RT, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophth Vis Sci 2015:56(5): 3269–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophth Vis Sci 2007:48(3): 968–977. [DOI] [PubMed] [Google Scholar]
- 61.Curcio CA. Antecedents of soft drusen, the specific deposits of age-related macular degeneration, in the biology of human macula. Invest Ophth Vis Sci 2018:59(4): AMD182–AMD194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Messinger JD, Zhang Y, Spaide RF, Freund KB, Curcio CA. Subretinal drusenoid deposit in age-related macular degeneration: Histologic insights into initiation, progression to atrophy, and imaging. Retina 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balaratnasingam C, Yannuzzi LA, Curcio CA, Morgan WH, Querques G, Capuano V, Souied E, Jung J, Freund KB. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Invest Ophth Vis Sci 2016:57(13): 5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li M, Dolz-Marco R, Messinger JD, Sloan KR, Ferrara D, Curcio CA, Freund KB. Clinicopathologic correlation of aneurysmal type 1 neovascularization in age-related macular degeneration. Ophthalmol Retina 2019:3(2): 99–111. [DOI] [PubMed] [Google Scholar]
- 65.Fragiotta S, Fernandez-Avellaneda P, Breazzano MP, Curcio CA, Leong BCS, Kato K, Yannuzzi LA, Freund KB. The fate and prognostic implications of hyperreflective crystalline deposits in nonneovascular age-related macular degeneration. Invest Ophth Vis Sci 2019:60(8): 3100–3109. [DOI] [PubMed] [Google Scholar]
- 66.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits aka pseudodrusen. Surv Ophthalmol 2018:63(6): 782–815. [DOI] [PubMed] [Google Scholar]


