Abstract
Background
It is reasonable to think that cancer patients undergoing chemotherapy, targeted therapy or immunotherapy could have a more aggressive course if positive for Coronavirus disease CoV-2 (COVID- 19).
Methods
We conducted a literature review on https://www.ncbi.nlm.nih.gov/pubmed/, https://scholar.google.com, www.arxiv.org, www.biorxiv.org, of all articles published using the keywords COVID-19 therapy or treatment and cancer until May 2, 2020. A total of 205 articles were identified and 53 were included in this review.
Results
We describe the ongoing COVID-19 therapies that should be known by oncologists and highlight the potential interactions with antineoplastic drugs, commonly used in clinical practice. The main drug interactions were found with tocilizumab, ruxolitinib and colchicine. Conclusions. The literature provides an inconclusive picture on potential preferred treatments for COVID-19 and their interactions with antineoplastic agents. Future clinical trials are needed to better understand the interactions between different drugs in the context of COVID-19 pandemic.
Keywords: Coronavirus disease SARS-CoV-2 (COVID-19), Cancer, Antiviral therapy, Management, Drug-interactions
1. Introduction
In our recent history there have been three epidemics related to coronavirus infections: the SARS-CoV (severe acute respiratory syndrome), 2002-03; the MERS-CoV (Middle-East-Respiratory-Syndrome), 2012; and currently the SARS-CoV-2, (2019-2020) (Author 1, 2020). Until May 2, 2020, there were approximately 209000 confirmed cases, with 28 000 deaths due to coronavirus disease CoV-2 (COVID-19) in Italy, according to the Italian Civil Protection bulletin (Author 2, 2020). According to the World Health Organization (WHO) as of May 2 2020 due to COVID-19, in Spain there were 215000 confirmed cases and 25000 deaths; in the United States, 1 milion confirmed cases and 57000 deaths; France has 128000 confirmed cases with 24000 deaths while United Kingdom has 177000 cases and 27000 deaths (Author 3, 2020).
From the available scientific literature it is evident that about 19.4% of the deaths with the coranavirus had an oncological pathology as comorbidity (Remuzzi et al., 2020; Landmann et al., 2020). Thus, during this COVID-19 crisis, cancer patients are regarded as a highly vulnerable group. It was found that within 14 days, anti-cancer treatments were significantly associated with occurrence of severe clinical events in COVID-19 infection (Zhang et al., 2000). The cancer population subjected to chemotherapy and/or radiotherapy is more exposed to infections in general and, therefore, also to that from Coronavirus primarily due to the effect of the cytotoxic action on the hematopoietic and immune systems with a reduction in the number of neutrophils, the first bulwark of infections, and decreased immune capacity (Remuzzi et al., 2020). Although there is no data yet on the risks of contracting coronavirus infection or on the clinical course of the infection during immunotherapy and/or immunosuppressive treatment with chemotherapy. It is reasonable to think, by analogy of what happens in the case of seasonal flu, due to the presence of immunosuppression, that in treated cancer patients, there may be a greater number of complications and the clinical course to be more serious (Author 1, 2020).
Therefore there remains an urgent need to answer whether COVID-19-positive cancer patients will have worse outcomes, such as death, from the coronavirus-induced pneumonia for example, and whether cancer patients should receive anti-cancer treatments. Additionally, oncologists are required to know the toxic effects of the drugs used in the experimental therapy of COVID-19 and the possible interactions of these drugs with the commonly used antineoplastic drugs.
2. Methods
2.1. Data sources and literature search strategy
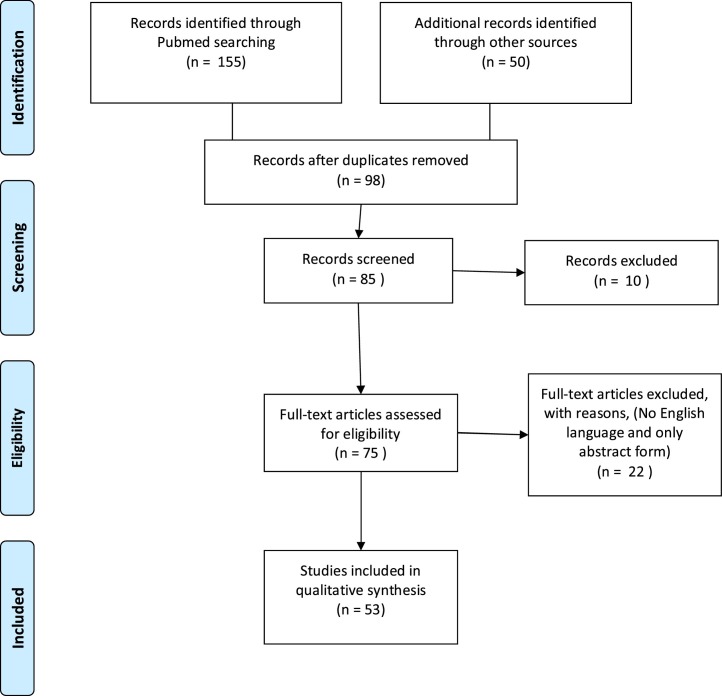
The systematic review followed the PRISMA guidelines (Fig. 1 ) ( Stewart et al., 2015). Two investigators (EL and RDT) independently conducted literature search using as combined keywords COVID-19 therapy or treatment and cancer on https://www.ncbi.nlm.nih.gov/pubmed/, www.arxiv.org (Author 4, 2020), www.biorxiv.org (Author 5, 2020) and https://scholar.google.com (Author 6, 2020). The database search was run of all the published articles from database inception until May 2, 2020. In Pubmed the following strategy was used: (COVID-19 OR Novel Coronavirus-Infected Pneumonia OR 2019 novel coronavirus OR 2019-nCoV or SARS-CoV-2 therapy or treatment) AND cancer.
Fig. 1.
Flow-chart of articles selection.
The strategy was then adapted for the other databases, including website of Italian Medicines Agency (AIFA) for ongoing trials (https://www.aifa.gov.it/emergenza-covid-19) (Author 7, 2020).
2.2. Study selection and data synthesis
All studies reporting information on both COVID-19 therapy/treatment and cancer were included. 205 articles were identified and reviewed independently by two authors (EL AND RDT) and 53 articles were considered relevant to the scope of the current review, as described in Fig. 1 (Zhang et al., 2000; Mohile et al., 2020; AminJafari and Ghasemi, 2020; Salako et al., 2020; Russell et al., 2020; De Felice et al., 2020, Akladios et al. 2020, Banna et al., 2020; Al-Shamsi et al., 2020; Zhang et al., 2020; Hanna et al., 2020; Ying et al., 2015; Coles et al., 2020; Debureaux et al., 2020; Di Saverio et al., 2020; Givi et al., 2020; Jin et al., 2020; Bersanelli, 2020; Ueda et al., 2020; Deng et al., 2020; Liang et al., 2020; Combs et al., 2020; Wang et al., 2020a; Zaorsky et al., 2020; Lim et al., 2020; Kalil, 2020; Wang et al., 2020b; Stebbin et al., 2020; Yang et al., 2020; Yu et al., 2020; Williams et al., 2020; Sheahan et al., 2020; Gautret et al., 2020; Chatre et al., 2018; Nhean et al., 2018; Gerson et al., 2018; Naqash et al., 2018; La Regina et al., 2019; Zheng et al., 2020; Author 8, 2020; Author 9, 2020; Author 10, 2020; Author 11, 2020; Author 12, 2020; Author 13, 2020; Deisseroth et al., 2015; Author 14, 2020; Author 15, 2020; JAK Inhibitors: Prospects in Connective Tissue Diseases et al., 2020; Iacobellis, 2020; Thachil, 2020; Author 16, 2020; de Bono et al. 2011; Author 17, 2020). Any inconsistencies were resolved by consensus with a third author (GDL). All health outcomes were included, due to the anticipated scarcity of data.
3. Results
The lists of the drugs being tested and their side-effects are listed in Table 1 . They are presented below in more detail.
Table 1.
Undesirable effects of the drugs tested in COVID19.
| Drugs tested in COVID-19 | Side effects |
|---|---|
| Favipiravir | Most common: nausea, vomiting, diarrhea, abdominal pain, anorexia, headache, anemia, muscle and joint pain. |
| Remdesivir | Common: leukopenia and thrombocytopenia, abnormal liver function; confusion, aggressive behaviour, agitation, migraine, tremors, paresthesia, dysgeusia. |
| Lopinavir | Uncommon: muscle weakness, pancreas dysfunction, lethargy, convulsions, coma, hallucinations, ataxia, kidney failure |
| Ritonavir | |
| Ribavirin | |
| Umifenovir | |
| Chloroquine / hydroxychloroquine | Common: anorexia, weight loss, nausea, abdominal pain, diarrhea, vomiting, corneal changes; uncommon: affective lability, nervousness, headache, dizziness, accommodation disorders |
| Rare: retinopathy with changes in pigmentation and visual field defects, edema and opacity dizziness, tinnitus, bundle branch block, atrioventricular block, abnormal liver function tests, hypoglycemia skin rash, graying of the hair, alopecia, rashes, tiredness, psoriasis not sensitive to light | |
| Azithromycin | Most common: diarrhea. |
| Common: nausea, vomiting, abdominal pain, headache. | |
| Uncommon: asthenia, irritability, dermatological disorders as rush skin, edema, urticaria | |
| Rare: abnormal liver function | |
| Anakinra | Most common: itching, erythema, pain, bruising, bleeding in the inoculation site, headache; common: abdominal pain, nausea, diarrhea |
| Tocilizumab | Most common: upper airway infections, hypercholesterolemia |
| Common: pneumonia, cellulite, herpes zoster, abdominal pain, neutropenia, thrombocytopenia, abnormal liver function, gastrointestinal perforation, episodes of hypertension during drug infusion, headache, skin reactions | |
| Uncommon: hypothyroidism, stomach ulcers | |
| Ivermectin | Common: dizziness, nausea, diarrhea, swelling of the joints, rapid heart beat |
| Uncommon: loss of appetite, vomiting, constipation, weakness, drowsiness, swelling of the eyes, face, arms, hands, feet | |
| Eculizumab | Most common: headache |
| Common: dizziness, nausea and diarrhea, abdominal pain, arthralgias, asthenia | |
| Uncommon: back pain, abnormal liver function, kidney failure. | |
| Emapalumab | Most common: infections, hypertension |
| Sarilumab | Most common: neutropenia; common: ALT elevation, injection site erythema |
| Ruxolitinib | Most common: Myelosuppression, anemia, urinary tract infections, bleeding and increased systolic blood pressure. |
| Common: neutropenia |
*Adverse events frequency:
Most common ≥1/10; Common <1/10 - ≥ 1/100.
Uncommon < 1/100->1/1000; Rare < 1/1000->1/10000.
3.1. Antiviral drugs
Considering the etiological therapy of SARS-CoV-2, antiviral agents, already on the market and in use for other viral pathologies, in monotherapy or in combination are currently being tested.
3.2. Favipiravir (Avigan)
It has a mechanism of action related to the selective inhibition of the Viral polymerase-RNA-dependent RNA and is used as a backup drug when other therapies do not work (Sheahan et al., 2020).
3.3. Ribavirin
Commonly used for the treatment of hepatitis C and for inflammatory lung diseases, such as bronchiolitis, this antiviral drug is always used in combination with other medicines, such as interferon alfa and peginterferon alfa and can be used both in the treatment of adult patients and in the treatment of pediatric patients (Zheng et al., 2020).
3.4. Remdesivir
It is an antiviral drug in the class of nucleotide analogues. It was developed as a treatment for Ebola virus disease and Marburg virus infections. It has also been shown to have antiviral activity against RNA viruses such as human respiratory syncytial virus and coronaviruses, including viruses that cause the MERS and SARS (Sheahan et al., 2020).
On 29 April, the director of the US National Institute of Allergy and Infectious Diseases (NIAID), announced the results of a study in which patients taking remdesivir recovered in 11 days compared with 15 days for those on a placebo. The shortened recovery time was so significant that investigators decided to stop the trial (Author 8, 2020).
This news contradicts previous mixed results on the drug. Gilead Sciences announced that in a non-randomized trial, more than half of participants with severe COVID-19 had recovered from their disease within two weeks of receiving treatment. Another smaller trial in China announced that it had found no benefits from remdesivir when compared with a placebo. The NIAID did not release informations on safety data (Author 8, 2020).
3.5. Lopinavir-Ritonavir
Lopinavir and Ritonavir is indicated, in combination with other antiretroviral medicines, for the treatment of adults, and children over the age of 2 years with human immunodeficiency virus (HIV-1) infection (Sheahan et al., 2020). Lopinavir-Ritonavir in association with Ribavirin is being evaluated.
3.6. Anticytokines
3.6.1. Tocilizumab
It is a drug that counteracts the over-reaction of the immune system, at the origin of some of the most serious complications of COVID-19 and which in these days seems to offer hope to patients with coronavirus-induced pneumonia. In addition to rheumatoid arthritis therapy, the medicine is also used to treat cytokine release syndrome, a side effect of CAR-T anticancer therapy which consists of a massive release of inflammatory molecules in response to the immune cells used in the treatment (Author 9, 2020).
3.6.2. Emapalumab
It is an anti-interferon gamma. This drug keeps children with hemophagocytic lymphohistiocytosis (HLH) alive, awaiting transplantation. The administration of emapalumab is able to "turn off" the abnormal and excessive inflammatory response in patients with HLH, neutralizing the effects deriving from the excessive production of interferon-gamma (Author 10, 2020).
3.6.3. Sarilumab
Sarilumab is an interleukin-6 (IL-6) receptor antagonist indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs (DMARDs) (Author 11, 2020).
3.6.4. Eculizumab
It is a member of the drug class selective immunosuppressants and is used to treat Hemolytic Uremic Syndrome, Myasthenia Gravis, Neuromyelitis Optica and Paroxysmal Nocturnal Hemoglobinuria (Author 12, 2020).
3.6.5. Anakinra
It inhibits the activity of interleukin-1 (IL-1), and has already been approved for the treatment of a group of rare and potentially fatal auto-inflammatory diseases. The US Food and Drugs Administration (FDA) has granted a study to test the safety and effectiveness of individually or simultaneously blocking IL-6 and IL-1 versus standard of care on blood oxygenation and systemic cytokine release syndrome in patients with COVID-19 coronavirus infection and acute hypoxic respiratory failure and systemic cytokine release syndrome (Author 13, 2020).
3.6.6. Siltuximab
Siltuximab like tocilizumab, binds to interleukin-6 and is indicated for the treatment of adult patients with Castleman disease (Deisseroth et al., 2015). The SISCO study (Siltuximab In Serious COVID-19) is configured as an observational study conducted both on hospitalized patients and those already in intensive care (Author 18, 2020).
Table 2 shows Italian Medicines Agency (AIFA) approved experimental studies in COVID-19 therapy (Author 19, 2020; Author 20, 2020; Author 21, 2020; Author 22, 2020; Author 23, 2020; Author 24, 2020; Author 25, 2020; Author 26, 2020; Author 27, 2020; Author 28, 2020; Author 29, 2020).
Table 2.
AIFA approved experimental studies in COVID-19 therapy.
| Study | Promoter | CE Single Opinion Date | Documents |
|---|---|---|---|
| A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of tocilizumab in patients with severe covid-19 pneumonia (Tocilizumab 2020-001154-22). | F.Hoffmann-La Roche Ltd | 30/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/TocilizumabDocumenti.zip |
| An open-label randomized multicenter study to evaluate the efficacy of early administration of Tocilizumab (TCZ) in patients with COVID-19 pneumonia (RCT-TCZ-COVID-19). | Local Health Unit-IRCCS of Reggio Emilia | 27/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/RCT-TCZ-COVID19documenti.zip |
| Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia (TOCIVID-19). | National Cancer Institute, IRCCS, G. Pascale Foundation | 22/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/TOCIVID-19documenti.zip |
| A randomized, double-blind, placebo-controlled adaptive phase 2/3 study evaluating the efficacy and safety of sarilumab for hospitalized patients with COVID-19 (Sarilumab COVID-19). | Sanofi-aventis Recherche & Développement | 26/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/Sarilumabdocumenti.zip |
| A phase 2/3, randomized, open-label, parallel, 3-arm, multicenter study investigating the efficacy and safety of intravenous doses of emapalumab, a monoclonal anti-interferon gamma (anti-IFNγ) and anakinra antibody, a interleukin -1 receptor antagonist (IL-1), compared to the standard of care, in reducing hyperinflammation and respiratory difficulties in patients with SARSCoV-2 infection (Sobi.IMMUNO-101). | SOBI | 25/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/Sobi.IMMUNO-101documenti.zip |
| A randomized phase 3 study to evaluate the safety and antiviral activity of Remdesivir (GS-5734 ™) in participants with moderate COVID-19 treatment compared to the standard of therapy. (Study GS-US-540-5774) | Gilead Sciences, Inc | 11/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/GS-US-540-5774documenti.zip |
| A randomized phase 3 study to evaluate the safety and antiviral activity of Remdesivir (GS-5734 ™) in participants with severe COVID-19. (Study GS-US-540-5773) | Gilead Sciences, Inc | 11/03/2020 | https://www.aifa.gov.it/documents/20142/1131319/GS-US-540-5773_documenti.zip |
| A randomized controlled two-phase phase 2 trial to evaluate the efficacy, safety and tolerability of baricitinib in addition to the usual treatment in patients with pneumonia in COVID19 | Pisan University Hospital | 23/04/2020 | https://www.aifa.gov.it/documents/20142/1131319/BARCHID_documenti.zip |
| Colchicine to counteract the inflammatory response in COVID pneumonia 19 | University Hospital of Parma | 23/04/2020 | https://www.aifa.gov.it/documents/20142/1131319/CoICoviddocumenti.zip |
| Intermediate dose enoxaparin in hospitalized patients with moderate-severe COVID19: a pilot phase II single-arm study, INHIXACOVID19 | Bologna University | 23/04/2020 | https://www.aifa.gov.it/documents/20142/1131319/INHIXACOVID_documenti.zip |
AIFA: Italian Medicines Agency.
The main currently ongoing studies are:
-
1)
TOCIVID-19, multicenter study on the efficacy and tolerability of Tocilizumab in the treatment of patients with COVID-19 pneumonia;
-
2)
Sobi study, IMMUNO-101, a randomized, open, 3 parallel, multicenter, phase 2/3 groups, that evaluates the efficacy and safety of intravenous doses of Emapalumab, anti-interferon gamma monoclonal antibody (anti-IFNγ), and Anakinra, interleukin receptor antagonist-1 (IL-1), compared to standard therapy;
-
3)
Sarilumab COVID-19 study: a randomized, double-blind, placebo-controlled phase 2/3 study evaluating the efficacy and safety of intravenous administration of Sarilumab, an interleukin receptor antagonist- 6 (IL- 6).
3.7. Other potential therapeutic agents
The required urgency for the identification of potential treatments has necessitated the further investigation of a wide-range of potential therapeutic agents. The ones identified in this review are listed below in some detail.
3.7.1. Avermectine
The pesticide Avermectina (otherwise known as Abamectina and Ivermectina) is registered by the US Environmental Protection Agency (EMA) as a pesticide. The study with the pesticide Ivermectin, approved by the FDA as a pesticide against scabies and already used against the HIV, Zika, Dengue, West Nile and Influenza viruses. This study, carried out at the Monash University's Biomedicine Discovery Institute of the Peter Doherty Institute of Infection and Immunity in Australia, has shown that a single dose of the medicine would be able to block the growth of the pathogen in culture, eliminating all the viral genetic material within 48 hours (Author 14, 2020).
3.7.2. Association of chloroquine or hydroxychloroquine in combination with a macrolide (azithromycin)
Two studies have been published in France on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 by the same research team as The Méditerranée Infection University Hospital Institute in Marseille. In summary, the two studies have documented a significant reduction in viral load and a clinical improvement compared to the natural progression of the disease triggered by SARS-CoV-2 infection. A death was recorded, while three patients were hospitalized in the Intensive Care wards (pooled data of the two studies) (Gautret et al., 2020; Author 15, 2020).
3.7.3. Ruxolitinib
Ruxolitinib is an inhibitor of the signal transmission pathway mediated by Janus Kinase (Jak), with anti-inflammatory effects related to the inhibition of the release of cytokines. Normally ruxolitinib is used in the hematology field and is indicated for the treatment of splenomegaly or disease-related symptoms in adult patients with primary myelofibrosis. It is also approved for the treatment of adult patients with polycythemia vera who are resistant or intolerant to hydroxyurea (JAK Inhibitors: Prospects in Connective Tissue Diseases et al., 2020). A compassionate use of Ruxolitinib has also been approved by AIFA in Covid-19 patients with respiratory failure who do not require invasive assisted ventilation.
3.7.4. Baricitinib
Baricitinib is another JAK inhibitor indicated for the treatment of active rheumatoid arthritis (JAK Inhibitors: Prospects in Connective Tissue Diseases et al., 2020). AIFA has licensed a randomized phase 2 trial to evaluate the efficacy, safety and tolerability of baricitinib in addition to the usual treatment in patients with pneumonia in COVID-19 (Barcivid study) (Author 27, 2020).
3.7.5. Anti-diabetes
A new line of research comes from diabetologists. According to a study, in addition to the main entrance door of virus, the angiotensin-converting enzyme 2 (ACE2) receptor, the DPP4 receptor must be evaluated. The Dipeptidyl peptidase 4 (Dpp4) receptor is present on all human cell types (bronchi and hearth) and is the same on which many medicines for diabetes work (Iacobellis, 2020).
3.7.6. Colchicine
Colchicine is a molecule, capable of interfering with the inflammatory immune response observed in subjects with COVID-19. Colchicine is a drug that has been used for a long time and is effective for the treatment of acute attack of gouty arthritis. Specifically, it manages to reduce the release of cytokines, molecules that, like IL-6, are responsible for the inflammatory (La Regina et al., 2019; Author 9, 2020). A study has recently started in Italy (Author 28, 2020).
3.7.7. Heparin
A reduction of up to 20% in mortality among COVID-19 patients with a marked increase in an indicator of the presence of blood clots, was achieve due to the use of an old drug, heparin (Thachil, 2020). This was reported by a study according to which the use of heparin in COVID-19 patients could have anticoagulant effects, as well as anti-inflammatory and potentially even antiviral ones. It has been shown that new coronavirus infections are associated with a high mortality in the presence of high value of D-dimer, a particularly important marker for coagulopathy (Thachil, 2020). However, it will be necessary to study in depth at what dosage the drug could have such potential antiviral properties. A recent study has been approved by AIFA with enoxeparin (INHIXACOVID) (Author 29, 2020).
3.8. Anti cancer drugs and anti COVID-19
Table 3 highlights the pharmacological interactions of some molecules tested against COVID-19 with the most common antineoplastic drugs. We have not found an overall high rate of interactions. The main drug-interactions reported are with Chloroquine/Hydroxychloroquine and chemotherapy or trastuzumab. Conduction disorders, ventricular hypertrophy and valvular dysfunction were the main side effect reported (Gautret et al., 2020; Author 16, 2020).
Table 3.
Pharmacological interactions of the drugs tested in COVID-19 with the most common antineoplastic drugs.
| Anti-covid19 | Interaction | Side effects |
|---|---|---|
| Azithromycin | Regorafenib | Therapeutic effect reduction |
| Azithromycin | Vinblastina | Increased serum levels of P-glycoprotein; |
| Increased toxicity (neutropenia, myalgias, constipation) | ||
| Chloroquine | Doxorubicina | cardiomyopathy or conduction system abnormalities |
| Chloroquine | Taxanes | Increase in Plasma concentrations |
| Chloroquine | Trastuzumab | cardiomyopathy or conduction system abnormalities |
| Anakinra | Fluorouracile | Increased immunosuppressive action |
| Anakinra | Durvalumab | Therapeutic effect reduction |
| Emapalumab, Sarilumab | no interactions with antineoplastic drugs | |
| Favipiravir | Enzalutamide | Interference with cytochromes: reduce efficacy of antivirals |
| Lopinavir | ||
| Ritonavir | ||
| Ribavirin | ||
| Umifenovir |
Enzalutamide, androgen receptor signaling inhibitors (ARSI), commonly used in prostate cancer could potentially interfere with antiviral drugs due to interference on cytochromes (Nhean et al., 2018).
Table 4 shows interferences with tocilizumab, ruxolitinib and colchicine. Tocilizumab increases immunosuppressive action and inhibits Fluouracil-resistance (Ying et al., 2015); it interferes with pharmacodynamic activity and the therapeutic efficacy of durvalumab, nivolumab, atezolizumab (Ying et al., 2015; Jin et al., 2020; Bersanelli, 2020). Moreover it is important to emphasize that tocilizumab decreases the concentration of several medications as a cytochrome P450 (CYP), isoenzyme CYP3A4 inducer (De Felice et al., 2020, Akladios et al., 2020, Banna et al., 2020; Al-Shamsi et al., 2020; Zhang et al., 2020; Hanna et al., 2020; Ying et al., 2015; Author 17, 2020). Therefore if drugs in lung cancer are used, such as ceritinib, crizotinib, brigatinib, gefitinib, docetaxel, a reduction in the anticancer drug may become noticeable, as well as paying attention in the case of concomitant use of tyrosine kinase inhibitors.
Table 4.
Pharmacological interactions of the Tocilizumab, Ruxolitinib and colchicine with the most common antineoplastic drugs.
| Antineoplastic agents | Drug interaction with antiCOVID-19 agents | Mechanism of interaction |
|---|---|---|
| Abiraterone | Tocilizumab | Tocilizumab reduces abiraterone as a CYP3A4 inducer. |
| Coclchicine | Increase abiraterone as CYP3A4 inhibitor | |
| Alectinib | Ruxolitinib | Increased bradycardic effect. |
| Axitinib | Tocilizumab | Tocilizumab reduces axitinib as a CYP3A4 inducer. |
| Bosutinib | Tocilizumab | Tocilizumab reduces bosutinib as a CYP3A4 inducer. |
| Brigatinib | Ruxolitinib | Increased bradycardic effect. |
| Tocilizumab | Tocilizumab reduces brigatinib serum as a CYP3A4 inducer. | |
| Cabozantinib | Tocilizumab | Tocilizumab reduces cabozantinib as a CYP3A4 inducer. |
| Ceritinib | Colchicine | Increased colchicine serum concentration. |
| Ruxolitinib | Increased ruxolitinib serum concetration. | |
| Tocilizumab | Tocilizumab reduces ceritinib as a CYP3A4 inducer. | |
| Cisplatin | Colchicine | adjust doses because uricemia increases |
| Cobimetinib | Tocilizumab | Tocilizumab reduces cobimetinib, as a CYP3A4 inducer. |
| CPI | Ruxolitinib | Therapeutic effect reduction or synergism (nivolumab) |
| Baricitinib | Interference with pharmacodynamic activity and therapeutic efficacy of these molecules | |
| Tocilizumab | ||
| Crizotinib | Colchicine | Increased colchicine and |
| Ruxolitinib | increased bradycardic effect as CYP3A4 inhibitor | |
| Tocilizumab | Tocilizumab reduces crizotinib as a CYP3A4 inducer | |
| Dasatinib | Tocilizumab | Tocilizumab reduces dasatinib as a CYP3A4 inducer. |
| Docetaxel | Tocilizumab | Tocilizumab reduces docetaxel as a CYP3A4 inducer. |
| Coclchicine | Iincrease docetaxel as a CYP 3A4 inhibitor. | |
| Enzalutamide | Tocilizumab | Tocilizumab reduces enzalutamide as a CYP3A4 inducer. |
| Erlotinib | Tocilizumab | Tocilizumab reduces erlotinib as a CYP3A4 inducer. |
| Everolimus | Tocilizumab | Tocilizumab reduces serum concentration as a CYP3A4 inducer. |
| Fluorouracil | Tocilizumab (eculizumab) | Increased immunosuppressive action; |
| Fluorouracil-resistance inhibition | ||
| Exemestane | Tocilizumab | Tocilizumab reduces exemestane, as a CYP3A4 inducer. |
| Gefinitib | Tocilizumab | Tocilizumab reduces gefitinib as a CYP3A4 inducer. |
| Ibrutinib | Tocilizumab | Tocilizumab reduces ibrutinib as a CYP3A4 inducer. |
| Idelalisib | Tocilizumab | Tocilizumab reduces idelasib as a CYP3A4 inducer. |
| Ixazomib | Tocilizumab | Tocilizumab reduces ixazomib as a CYP3A4 inducer. |
| Lapatinib | Colchicine | Lapatinib increases colchicine as a glycoproteine-P, ABCB1 inhibitor. |
| Tocilizumab | Tocilizumab reduces lapatinib as a CYP3A4 inducer. | |
| Neratinib | Colchicine | Neratinib increases colchicine as glycoproteine-P ABCB1 inhibitor. |
| Tocilizumab | Tocilizumab reduces neratinib as a CYP3A4 inducer. | |
| Nilotinib | Colchicine | Nilotinib increases colchicine as a CYP 3A4 inhibitor. |
| Ruxolitinib | Nilotinib increases ruxolitinib as a CYP 3A4 inhibitor. | |
| Tocilizumab | Tocilizumab reduces nilotinib as a CYP3A4 inducer. | |
| Olaparib | Tocilizumab | Tocilizumab reduces olaparib as a CYP3A4 inducer. |
| Paclitaxel | Tocilizumab | Tocilizumab reduces paclitaxel serum as a CYP3A4 inducer |
| Palbociclib | Tocilizumab | Tocilizumab reduces palbociclib as a CYP3A4 inducer. |
| Panobinostat | Tocilizumab | Tocilizumab reduces panobinostat as a CYP3A4 inducer. |
| Pazopanib | Tocilizumab | Tocilizumab reduces pazopanib as a CYP3A4 inducer. |
| Pomalidomide | Tocilizumab | Increased immunosuppressive effect. |
| Regorafenib | Tocilizumab | Tocilizumab reduces regorafenib as a CYP3A4 inducer. |
| Ribociclib | Colchicine | Ribociclib increases colchicine as a CYP 3A4 inhibitor. |
| Ruxolitinib | Ribociclib increases colchicine as a CYP 3A4 inhibitor. | |
| Tocilizumab | Tocilizumab reduces ribociclib, as a CYP3A4 inducer. | |
| Ruxolitinib | Tocilizumab | Tocilizumab reduces ruxolitinib as a CYP3A4 inducer. |
| Sunitinib | Tocilizumab | Tocilizumab reduces sunitinib as a CYP3A4 inducer. |
| Tamoxifene | Colchicine | Tamoxifene increases colchicine as a glycoproteine-P, ABCB1 inhibitor. |
| Tocilizumab | Tocilizumab reduces tamoxifene as a CYP3A4 inducer. | |
| Vandetanib | Tocilizumab | Tocilizumab reduces vandetanib as a CYP3A4 inducer. |
| Venetoclax | Tocilizumab | Tocilizumab reduces venetoclax as a CYP3A4 inducer. |
| Alcaloids of the vinca | Colchicine | Synergy of action and enhancement of toxicity. |
CPI: Check Point inhibitors.
Anakinra and ruxolitinib could interfere with checkpoint inhibitors (CPI) in terms of antagonisms or synergy but this is not yet entirely clear (Ying et al., 2015; Bersanelli, 2020; Author 13, 2020). Several drugs, such as CYP3A4 inhibitors, increase concentration of colchicine and attention should be paid when the latter is used with cisplatin or vinca alkaloids (La Regina et al., 2019). Furthermore, one should not forget a concomitant use of antidiabetic drugs with antineoplastics that require cortisone (de Bono et al. 2011) or cytostatic drugs that reduce the value of platelets with the concomitant use of heparin (Thachil, 2020).
4. Discussion
Based on the increased risk of disease aggression of COVID-19 in patients with cancer it is important to describe which anti COVID-19 drugs could be administered and which not in cancer patients. The current review attempts to provide a first systematic glance at this issue. There are certain emergent points of attention that have already been described.
Specifically, particular attention to the potential cardiotoxicity of chloroquine with anthracyclines is necessary. Hydroxychloroquine is an antimalarial that has become a mainstay in the management of systemic lupus erythematosus and rheumatoid arthritis. Similar to chloroquine in structure, hydroxychloroquine is used more frequently because it confers lower toxicity. Cardiotoxicity manifests as restrictive or dilated cardiomyopathy or with conduction system abnormalities such as atrioventricular and bundle-branch block (Gautret et al., 2020). Recently, some doubts related to cardiovascular health have emerged in COVID-19 patients. In fact cardiac rhythm disturbances and cardiac arrest in some French hospitals are currently are under consideration by the French National Agency for the Safety of Medicines (Author 16, 2020).
If one considers that the metabolism of most drugs is catalyzed by the enzyme group of CYP, composed of a large number of isoenzymes, the most important of which in the metabolism of drugs are CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. Enzalutamide, recognized as a strong CYP3A4 and a moderate CYP2C9 and CYP2C19 inducer, significantly reduces concentrations of several anti-HIV agents such as darunavir, ritonavir, etravirine, and raltegravir. The latter drugs are the subject of a series of trials in progress and could be soon active in the fight against COVID-19 (Nhean et al., 2018).
It is important also to note that some chemotherapeutic agents or hormonal therapies such as abiraterone require concomitant steroids that are immunosuppressive agents and could weaken anticitokines drugs such as tocilizumab (de Bono et al. 2011). However in some cases, the use of steroids could be reduced without interfering with the efficacy and toxicity profile. Tocilizumab decreases concentrations of many anticancer drugs due to interference with CYP (Author 17, 2020).
Deserving to underline that today many patients carry out CPI, currently used in daily practice for the treatment of solid tumors. CPI, activating T-lymphocytes, increase the risk of autoimmune disease such as pneumoniae (Gerson et al., 2018). Few case-reports have described significant reduction in IL-6 and CPI immunorelated toxicity after tocilizumab administration (Naqash et al., 2018). To date the interaction between CPI and anti COVID-19 agents is not well-described. Consideration could be given to either extending the timing of the doses or delaying pretreated patients.
The present review should be interpreted in light of its limitations. Firstly, there are no studies with COVID-19 therapies in cancer populations being treated with antineoplastic drugs and for this reason the informations collected are indirect and from studies in which these antiviral experimental agents have been used in cancer patients for other indications. Thus more research on this topic is needed before concrete recommendations can be made.
Secondly, the type and dosage of therapeutic agents varied between studies.
Thirdly the data are based on retrospective findings, while the results of prospective cohorts are being awaited and expected to shed more light.
Moreover considering the rapid evolution of information related to the pandemic we conducted a search on 2 arxiv and biorxiv databases indentifying 50 articles of interest; these are preliminary reports that have not been peer-reviewed. Among the 50 articles only 2 were considered as relevant (Yu et al., 2020; Williams et al., 2020) and subsequently 1 has been published in peer-reviewed journal (Yu et al., 2020), testifying to the current urgent need of relevant, well-designed studies.
5. Conclusions
The literature so far available provides an inconclusive picture on potential preferred treatments for COVID-19 and their interactions with antineoplastic agents.
In order to continue treating cancer patients likely to be infected with COVID-19, we believe that it is appropriate to modify available therapeutic choices and arrive at a new therapeutic protocol that provides:
-
1)
where there are no interactions to continue or delay antineoplastic therapy by 1-2 weeks;
-
2)
where there are interactions and the patient has already done many cycles to stop the administration of the anticancer drug;
-
3)
for newly diagnosed cancer patients to prefer antineoplastic drugs without interactions;
-
4)
where further attention is given to CPI, the choice of which may vary from case to case.
This new therapeutic proposal awaits the constructive suggestions of the oncological community in order to make improvements, such as to better protect our patients, already fragile because of cancer. While the information flow has been largely uninhibited during the pandemic, findings from current and future clinical trials are still needed to better understand the interactions between different pharmaceutical agents in the context of COVID-19 infections.
Declaration of conflicting interests
All authors declare no conflict of interest.
Acknowledgements
None.
References
- Akladios C., Azais H., Ballester M., Bendifallah S., Bolze P.A., Bourdel N., Bricou A., Canlorbe G., Carcopino X., Chauvet P., Collinet P., Coutant C., Dabi Y., Dion L., Gauthier T., Graesslin O., Huchon C., Koskas M., Kridelka F., Lavoue V., Lecointre L., Mezzadri M., Mimoun C., Ouldamer L., Raimond E. Touboul Recommendations for the surgical management of gynecological cancers during the COVID-19 pandemic - FRANCOGYN group for the CNGOF. J Gynecol Obstet Hum Reprod. 2020;(Apr 1) doi: 10.1016/j.jogoh.2020.101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M., Wolff R., Nuhad I.K., Chua MLK, Hotte SJ, Meyers B.M., Elfiki T., Curigliano G., Eng C., Grothey A., Xie C. A Practical Approach to the Management of Cancer Patients During the Novel Coronavirus Disease 2019 (COVID-19) Pandemic: An International Collaborative Group. Oncologist. 2020;(Apr 3) doi: 10.1634/theoncologist.2020-0213. [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int Immunopharmacol. 2020;83(Apr 2):106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid 19 global consequences for oncology. Lancet Oncology. 2020;(April) doi: 10.1016/S1470-2045(20)30175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.fda.gov/drugs/fda-approves-emapalumab-hemophagocytic-lymphohistiocytosis last access. 8 april 2020.
- https://www.drugs.com/history/kevzara.html. last access.8 april 2020.
- https://www.drugs.com/searchterm=eculizumab last access 8 april 2020.
- https://clinicaltrials.gov/ct2/show/NCT04330638. Last access 8 april 2020.
- www.adnkronos.com/salute/farmaceutica/2020/04/04/coronavirus-Antiparassitario.
- https://www.mediterranee-infection.com/wpcontent/uploads/2020/03/COVID-IHU-2-1.pdf.
- https://www.newsweek.com/hydroxychloroquine-coronavirus-france-heart-cardiac-1496810. Last access 12 april 2020.
- https://www.drugs.com/drug-interactions/tocilizumab.html. Last access. 2 May 2020.
- https://www.pharmastar.it/news/altri-studi/covid-19-a-bergamo-lo-studio-sullanticorpo-monoclonale-siltuximab-31702.
- https://www.aifa.gov.it/documents/20142/1131319/Tocilizumab_Documenti.zip.
- www.http://www.protezionecivile.gov.it/. Last Accessed May 2, 2020.
- https://www.aifa.gov.it/documents/20142/1131319/RCT-TCZ-COVID19_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/TOCIVID-19_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/Sarilumab_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/Sobi.IMMUNO-101_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/TOCIVID-19_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/GS-US-540-5774_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/GS-US-540-5773_documenti.zip. Last access. 8 april 2020.
- https://www.aifa.gov.it/documents/20142/1131319/BARCHID_documenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/CoICoviddocumenti.zip.
- https://www.aifa.gov.it/documents/20142/1131319/INHIXACOVID_documenti.zip.
- https://www.who.int/health-topics/coronavirus#tab=tab_1. Last Accessed May 2, 2020.
- https://arxiv.org/search/?query=coronavirus&searchtype=all. last access 25 april 2020.
- https://www.biorxiv.org/. Last access 25 april 2020.
- https://scholar.google.com. Last access 25 april 2020.
- https://www.aifa.gov.it/sperimentazioni-cliniche-covid-19 Last access. 23 april 2020.
- https://www.nature.com/articles/d41586-020-01295-8. Last access 1 May 2020.
- https://www.drugs.com/history/actemra.html last Access 8 april 2020.
- Banna G., Curioni-Fontecedro A., Friedlaender A., Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5(Apr (2)) doi: 10.1136/esmoopen-2020-000765. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;(Mar 26) doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018;41(Oct (10)):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., Hanna G., Jagsi R., Kaidar Person O., Kirby A., Mjaaland I., Meattini I., Luis A.M., Marta G.N., Offersen B., Poortmans P., Rivera S. International Guidelines on Radiation Therapy for Breast Cancer During the COVID-19 Pandemic. Clin Oncol (R Coll Radiol). 2020;32(May (5)):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs S.E., Belka C., Niyazi M., Corradini S., Pigorsch S., Wilkens J., Grosu A.L., Guckenberger M., Ganswindt U., Bernhardt D. First statement on preparation for the COVID-19 pandemic in large German Speaking University-based radiation oncology departments. Radiat Oncol. 2020;15(Apr 7(1)):74. doi: 10.1186/s13014-020-01527-1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L., Chi K.N., Jones R.J., Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M., Efstathiou E, Zivi A, Bianchi D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(May 26(21)):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F., Polimeni A., Valentini V. The impact of Coronavirus (COVID-19) on head and neck cancer patients’ care. Radiother Oncol. 2020;147(Mar 24):84–85. doi: 10.1016/j.radonc.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debureaux P.E., Arrondeau J., Bouscary D., Goldwasser F. Nivolumab combined with ruxolitinib: antagonism or synergy? Ann Oncol. 2020;29(5):1334–1335. doi: 10.1093/annonc/mdy077. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Ko C.W., Nie L., Zirkelbach J.F., Zhao L., Bullock J., Mehrotra N., Del Valle P., Saber H., Sheth C., Gehrke B., Justice R., Farrell A., Pazdur R. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res. 2015;21(Mar 1(5)):950–954. doi: 10.1158/1078-0432.CCR-14-1678. [DOI] [PubMed] [Google Scholar]
- Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020;(Mar 11) doi: 10.1016/j.jinf.2020.03.002. pii: S0163-4453(20)30113-30114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Saverio S., Pata F., Gallo G., Carrano F., Scorza A., Sileri P., Smart N., Spinelli A., Pellino G. Coronavirus pandemic and Colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020;(Mar 31) doi: 10.1111/codi.15056. [DOI] [PubMed] [Google Scholar]
- Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gerson J.N., Ramamurthy C., Borghaei H. Managing adverse effects of immunotherapy. Clin Adv Hematol Oncol. 2018;16(May (5)):364–374. [PubMed] [Google Scholar]
- Givi B., Schiff B.A., Chinn S.B., Clayburgh D., Iyer N.G., Jalisi S., Moore M.G., Nathan C.A., Orloff L.A., O’Neill J.P., Parker N., Zender C., Morris LGT, Davies L. Safety Recommendations for Evaluation and Surgery of the Head and Neck During the COVID-19 Pandemic. JAMA Otolaryngol Head Neck Surg. 2020;(Mar 31) doi: 10.1001/jamaoto.2020.0780. [DOI] [PubMed] [Google Scholar]
- Hanna TP, Evans GA, Booth CM. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;(Apr 2) doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162(Mar 26) doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAK Inhibitors: Prospects in Connective Tissue Diseases, You H., Xu Zhao J, Li J., Wang Q, Tian X, Li M, Zeng X. Clin Rev Allergy Immunol. 2020;(Mar 28) doi: 10.1007/s12016-020-08786-6. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- Jin Xh, Zheng Ki, Pan Kh, Xie Yp, Zheng Mh. COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7(Apr (4)):e351–e352. doi: 10.1016/S2352-3026(20)30074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A. Treating COVID-19—Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA. 2020 doi: 10.1001/jama.2020.4742. Published online March 24. [DOI] [PubMed] [Google Scholar]
- La Regina G., Coluccia A., Naccarato V., Silvestri R. Towards modern anticancer agents that interact with tubulin. Eur J Pharm Sci. 2019;131(Apr 1):58–68. doi: 10.1016/j.ejps.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Landmann A., et al. Cancer patients in SARS-CoV-2 infection: a nation wide analysis in China. Lancet. 2020 Published Online February 14, 2020 https://doi.org/10.1016/ S1470-2045(20)30096-6. [Google Scholar]
- Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H., Li S., He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(Mar(3)):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Jaegyun, Jeon Seunghyun, Shin Hyun-Young, Jung Kim Moon, Min Seong Yu, Jun Lee Wang, Choe Kang-Won, Min Kang Yu, Lee Baeckseung, Park Sang-Joon. Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35(Feb 17(6)):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohile N.A., Blakeley J.O., Gatson NTN, Hottinger AF, Lassman A.B., Ney D.E., Olar A., Schiff D., Shih H.A., Strowd R., van den Bent M.J., Ziu M. Urgent Considerations for the Neuro-oncologic Treatment of Patients with Gliomas During the COVID-19 Pandemic. Neuro Oncol. 2020;(Apr 11) doi: 10.1093/neuonc/noaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqash A.R., Yang L.V., Sanderlin E.J., Atwell D.C., Walker P.R. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol. 2018;57(May (5)):705–708. doi: 10.1080/0284186X.2017.1406668. [DOI] [PubMed] [Google Scholar]
- Nhean S., Bravo J., Sheehan NL, Walmsley S., David Tilley, Tseng Alice L. Successful use of the potent enzyme inducer enzalutamide in a treatment-experienced HIV-positive male with prostate cancer. AIDS. 2018;32:2640–2642. doi: 10.1097/QAD.0000000000002019. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., et al. Covid 19 and Italy: whatnext? Lancet. 2020 doi: 10.1016/S0140-6736(20)30627-9. Published Online March 12, 2020 https://doi.org/10.1016/S0140-6736(20)30627-9 Pag. 4 di 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14(Mar 30):1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salako O., Okunade K., Allsop M., Habeebu M., Toye M., Oluyede G., Fagbenro G., Salako B. Upheaval in cancer care during the COVID-19 outbreak. cancermedicalscience. 2020;14(Apr 1) doi: 10.3332/ecancer.2020.ed97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(Jan 10(1)):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbin J., Phelan A., Griffin I., Tucher C., Oechsle O., Smith D. Covi d19: combining antiviral and anti inflammatory disease. Lancet Infectious Disease. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L.A., Clarke M., Rovers M., et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. Jama. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;(Apr 2) doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Martins R., Hendrie P.C., McDonnell T., Crews J.R., Wong T.L., McCreery B., Jagels B., Crane A., Byrd D.R., Pergam S.A., Davidson N.E., Liu C., Stewart F.M. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J Natl Compr Canc Netw. 2020;20(Mar):1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;(Mar 16) doi: 10.1093/cid/ciaa272. pii: ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Li-sheng, Wang Yi-ru, Ye Da-wei, Liu Qing-quan. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Calvez KL, Mi L., Chen J., Dadhania S., et al. Pakdzad-Shahabi L. Estimating the Risks from COVID-19 Infection in Adult Chemotherapy Patients. medRxiv. 2020 doi: 10.1101/2020.03.18.20038067. medrxiv.org. [DOI] [Google Scholar]
- Yang G., Zhang H., Yang Y. Challenges and Countermeasures of Integrative Cancer Therapy in the Epidemic of COVID-19. Integr Cancer Ther. 2020;19(Jan-Dec) doi: 10.1177/1534735420912811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J., Tsujii M., Kondo J., Hayashi Y., Kato M., Akasaka T., Inoue T., Shiraishi E., Inoue T., Hiyama S., Tsujii Y., Maekawa A., Kawai S., Fujinaga T., Araki M., Shinzaki S., Watabe K., Nishida T., Iijima H., Takehara T. The effectiveness of an anti-human IL-6 receptor monoclonal antibody combined with chemotherapy to target colon cancer stem-like cells. Int J Oncol. 2015;46(Apr (4)):1551–1559. doi: 10.3892/ijo.2015.2851. [DOI] [PubMed] [Google Scholar]
- Yu J., Wen O., Chua MLK, Conghua X. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. Medrxhiv. 2020 doi: 10.1101/2020.02.22. 20025320d. ( JAMA Oncol 2020, 25 March 10.1001/jamaoncol.2020.0980) [DOI] [Google Scholar]
- Zaorsky N.G., Yu JB, McBride SM, Dess R.T., Jackson W.C., Mahal B.A., Chen R., Choudhury A., Henry A., Syndikus I., Mitin T., Tree A., Kishan A.U., Spratt D.E. Prostate Cancer Radiotherapy Recommendations in Response to COVID-19. Advances in Radiation Oncology. 2020 doi: 10.1016/j.adro.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H.Q., Peng L., Chen Y., Peng P., Zhang P., Chu Q., Shen Q., Wang Y., Xu S.Y., Zhao J.P., Zhou M. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2000 doi: 10.1016/j.annonc.2020.03.296. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, Wang J, Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(Apr 14(7)):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.X., Ma S.J., Xiong Y.H., Fan X.G. The Efficacy and Safety of Direct-Acting Antiviral Regimens for HCV/HIV Co-infection: A Systematic review and Network meta-analysis. J Gastroenterol Hepatol. 2020;(Apr 4) doi: 10.1111/jgh.15051. [DOI] [PubMed] [Google Scholar]