Background
There is much debate on the use of angiotensin receptor blockers (ARBs) in severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2)–infected patients. Although it has been suggested that ARBs might lead to a higher susceptibility and severity of SARS-CoV-2 infection, experimental data suggest that ARBs may reduce acute lung injury via blocking angiotensin-II–mediated pulmonary permeability, inflammation, and fibrosis. However, despite these hypotheses, specific studies on ARBs in SARS-CoV-2 patients are lacking.
Methods
The PRAETORIAN-COVID trial is a multicenter, double-blind, placebo-controlled 1:1 randomized clinical trial in adult hospitalized SARS-CoV-2–infected patients (n = 651). The primary aim is to investigate the effect of the ARB valsartan compared to placebo on the composite end point of admission to an intensive care unit, mechanical ventilation, or death within 14 days of randomization. The active-treatment arm will receive valsartan in a dosage titrated to blood pressure up to a maximum of 160 mg bid, and the placebo arm will receive matching placebo. Treatment duration will be 14 days, or until the occurrence of the primary end point or until hospital discharge, if either of these occurs within 14 days. The trial is registered at clinicaltrials.gov (NCT04335786, 2020).
Summary
The PRAETORIAN-COVID trial is a double-blind, placebo-controlled 1:1 randomized trial to assess the effect of valsartan compared to placebo on the occurrence of ICU admission, mechanical ventilation, and death in hospitalized SARS-CoV-2–infected patients. The results of this study might impact the treatment of SARS-CoV-2 patients globally.
The world is currently facing the challenges of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2). SARS-CoV-2 results in acute lung injury and acute respiratory distress syndrome (ARDS), frequently necessitating mechanical ventilation and intensive care unit (ICU) admission and ultimately causing high morbidity and mortality.1
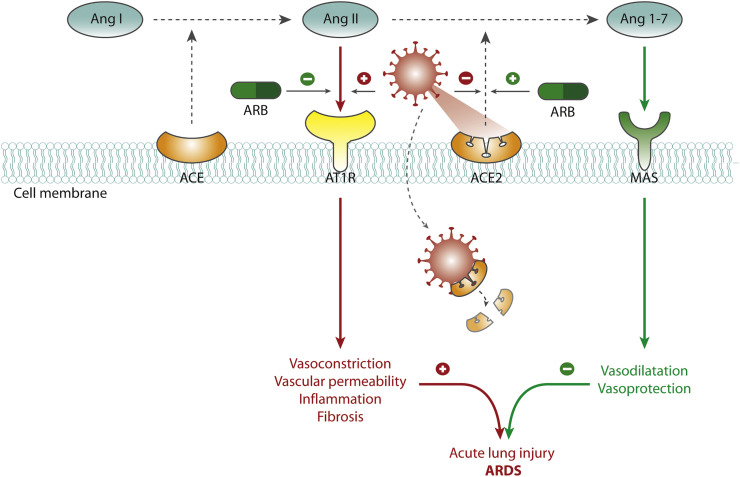
Development of ARDS in SARS-CoV-2 is attributed to changes in the renin-angiotensin system (RAS).2 The RAS is delicately balanced by the counteracting angiotensin-converting enzyme (ACE) and ACE2, which among others regulate concentrations of the vasoconstrictor angiotensin II (Ang-II) and the vasodilator angiotensin 1-7 (Ang-1-7). Increased ACE activity leads to higher Ang-II concentrations, whereas ACE2 breaks down Ang-II to Ang-1-7 (Figure 1 ).
Figure 1.
Study rationale and hypothesis.
Legend: The RAS is delicately balanced by counteracting enzymes ACE and ACE2, which regulate concentrations of the vasoconstrictor Ang-II and the vasodilator Ang-1-7. Increased ACE activity leads to higher Ang-II concentrations, whereas ACE2 breaks down Ang-II to Ang-1-7. The SARS-CoV-2 virus uses ACE2 as the cell entry site for internalization. It then decreases ACE2 and consequently increases Ang-II concentrations with deleterious effects such as increased vascular permeability, inflammation, and fibrosis. These pathways are thought to contribute to acute lung injury and ARDS. ARBs may attenuate acute lung injury in SARS-CoV-2 infectious disease by the following mechanisms. First and foremost, blockade of the AT1R may reduce the detrimental effects of Ang-II. Second, administration of ARBs may increase ACE2 expression, which may reduce the detrimental effects of Ang-II.
The SARS-CoV-2 virus spike protein binds to ACE2 as the cell entry site and forms a complex for internalization.3., 4., 5., 6. This internalization results in a decrease of ACE2 concentrations and consequently elevated Ang-II concentrations with deleterious effects such as increased vascular permeability, inflammation, and fibrosis. These pathways are thought to contribute to acute lung injury and ARDS in COVID-192 , 7 , 8 (Figure 1). In light of this proposed mechanism, there is extensive debate on the use of RAS-inhibitors (ie, angiotensin receptor blockers [ARBs] and ACE inhibitors [ACE-i]) in SARS-CoV-2 infection.
Currently, the role of these drugs in SARS-CoV-2 infection is unclear. Observations that older patients with cardiovascular disease, in whom ARBs and ACE-i are frequently prescribed, are at a higher risk for more severe SARS-CoV-2 infection made some investigators to speculate that RAS inhibitors might lead to a higher susceptibility and severity of SARS-CoV-2 infection.9 , 10 Moreover, select experimental studies suggested that RAS inhibitors may increase ACE2 expression.11., 12., 13. These concerns led some media sources and health systems to discourage the use of RAS inhibitors in SARS-CoV-2 patients.
Contrastingly, several other studies counter such statements, and present data rather suggest a beneficial effect of RAS inhibitors in SARS-CoV-2 patients, which has been put forward in various excellent reviews.14., 15., 16., 17., 18., 19., 20. Data suggest that ARBs may attenuate acute lung injury in SARS-CoV-2 infectious disease by the following mechanisms.14., 15., 16., 17., 18., 19., 20. First and foremost, blockade of the angiotensin-II type 1 receptor (AT1R) may reduce the detrimental effects of Ang-II.11., 12., 13., 14., 15., 16., 17., 18. , 21 Second, administration of ARBs may increase ACE2 expression, which may reduce the detrimental effects of Ang-II11., 12., 13., 14., 15., 16., 17., 18. , 21 (Figure 1). Lastly, it has been hypothesized that AT1R blockade at the cell surface may reduce the internalization of the virus and thereby limit the decrease of ACE2 caused by the infection.5 , 21
To date, no studies have evaluated the effects of RAS inhibition in COVID-19 patients. Therefore, health care authorities such as the European Society of Cardiology, Heart Failure Society of America, American College of Cardiology, and American Heart Association stated that there is a lack of evidence to withhold RAS inhibitors in SARS-CoV-2 patients, and call for studies specifically addressing the safety and efficacy of ACE-i and ARBs in COVID-19.14 , 22 , 23 These drugs are cheap and widely available. In case it is demonstrated in robust clinical trials that ARBs are effective and safe for treatment of COVID-19, they may provide a feasible treatment option for SARS-CoV-2 infectious disease, accessible for many patients all over the world.
To address this gap in knowledge, we designed a double-blinded, placebo-controlled randomized clinical trial to investigate the effect of ARBs in patients with SARS-CoV-2 infection on the occurrence of ICU admission, mechanical ventilation, and death.
Methods
We followed the SPIRIT guidelines in the design of our study protocol.24
Aim of the study
The primary aim of this study is to investigate the effect of valsartan compared to placebo in hospitalized SARS-CoV-2–infected patients on the occurrence of ICU admission, use of mechanical ventilation, or death within 14 days of randomization.
Setting and overview of the study design
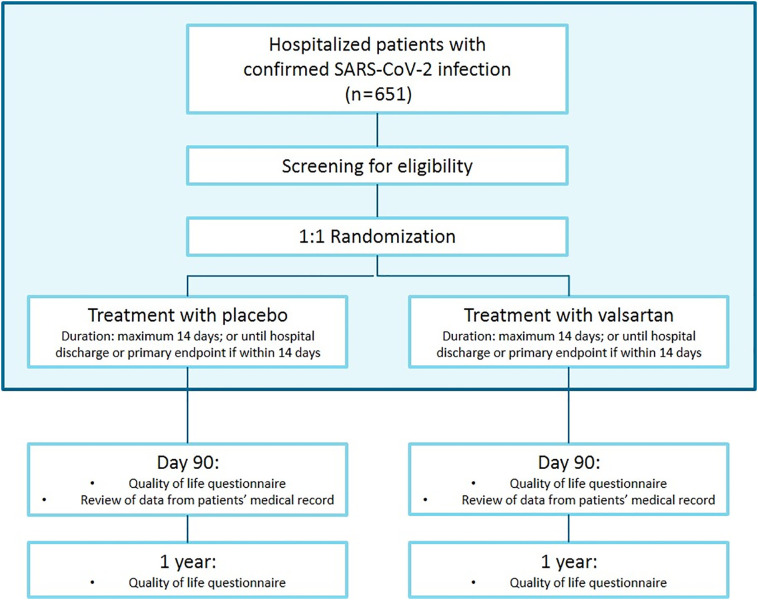
The present study will be performed in both academic and general hospitals in the Netherlands. This study is designed as a double-blind, placebo-controlled 1:1 randomized clinical trial, with randomization to either valsartan or matching placebo. Treatment duration will be 14 days, or until the occurrence of the primary end point or until hospital discharge, if either of these occurs within 14 days. Follow-up for mortality will be done up to 1 year after randomization. The study flowchart is depicted in Figure 2 .
Figure 2.
Study flowchart of the PRAETORIAN-COVID trial.
Legend: The blue box comprises the primary end point analysis.
Participants
The source population will comprise all adult SARS-CoV-2–infected patients admitted to one of the participating centers. Further inclusion and exclusion criteria are shown in Table I .
Table I.
Inclusion and exclusion criteria of the PRAETORIAN-COVID trial
| Inclusion criteria | |
| 1 | Age ≥8 y |
| 2 | Admitted to the hospital of any participating center |
| 3 | Confirmed SARS-CoV-2 infection with either positive laboratory test result for SARS-CoV-2* or positive computed tomography (CT) thorax diagnostic test result for SARS-CoV-2 infection according to the prevailing criteria. *In case there is a lack of laboratory tests for SARS-CoV-2 in the participating center of the potentially eligible patient, a positive laboratory test result for SARS-CoV-2 will no longer be required. In that case, the potentially eligible patient needs to meet the prevailing criteria for the diagnosis of SARS-CoV-2 infection of that participating center, such as typical abnormalities on pulmonary CT in the setting of high clinical suspicion of SARS-CoV-2 infection. |
| Exclusion criteria | |
| 1 | Admitted to ICU prior to randomization |
| 2 | Currently taking an ARB, angiotensin receptor neprilysin inhibitor (ARNI), aliskiren, fluconazole, rifampicin, ciclosporin, or ritanovir |
| 3 | Use of other investigational drugs at the time of enrollment |
| 4 | Prior reaction or intolerance to an ARB or ARNI, or severe intolerance to an ACE-i, defined as angioedema requiring medical intervention |
| 5 | Systolic blood pressure <105 mm Hg or diastolic blood pressure <65 mm Hg |
| 6 | Potassium greater than 5.5 mEq/L within 4 wk of study enrollment |
| 7 | Estimated glomerular filtration rate of <30 mL/min/1.73 m2 within 4 wk of study initiation |
| 8 | A known history of renal artery stenosis |
| 9 | Aspartate aminotransferase and/or alanine aminotransferase >3 times the upper limit of normal within 4 wk of study enrollment. In case of mild to moderate liver dysfunction, valsartan dosage will be limited to a maximum of 80 mg. |
| 10 | Severe liver dysfunction, biliary cirrhosis, or cholestasis |
| 11 | Severe volume depletion or severe acute kidney injury that, in the opinion of the investigator, would preclude administration of valsartan |
| 12 | Inability to obtain informed consent |
| 13 | Pregnancy or breastfeeding |
| 14 | In females of childbearing age, unwillingness to use birth control or to be sexually abstinent for the duration of the study |
Randomization and data management
Randomization will take place either within 24 hours of confirmed in-hospital SARS-CoV-2 infection diagnosis or within 24 hours of hospital admission in case of prehospital confirmed SARS-CoV-2 infection. Participants will be randomized using the online Castor Electronic Data Capture system.25 Randomization will be double-blind using a variable block randomization algorithm. Castor Electronic Data Capture will also be used for data management. Castor data will be exported for analysis.
Interventions
Participants will be randomized (1:1) to either valsartan or matching placebo. Titration of the study drug will be done according to the titration scheme (Supplement 1). In order to safely titrate the study drug, blood pressure will be measured prior to each administration of the study drug. Treatment duration will be 14 days; or until the occurrence of the primary end point or until hospital discharge, if either of these occurs within 14 days.
Outcome measures and other parameters
The primary outcome measure is the first occurrence of ICU admission, mechanical ventilation, or death within 14 days of randomization. This primary composite end point is based on the landmark study by Guan et al.1 Death is defined as all-cause mortality. All primary end points will be adjudicated by an independent event committee.
The key secondary outcome measures are death within 14 days, 30 days, 90 days, and 1 year, defined as all-cause mortality, mechanical ventilation within 14 days, ICU admission within 14 days, time to the primary composite end point and each of its components, and occurrence of acute kidney injury within 14 days defined as a 50% decline in estimated glomerular filtration rate relative to baseline, or decrease of >30 mL/min/1.73 m2 and to a value below 60 mL/min/1.73 m2.
Other study parameters
At baseline, we will acquire data on, for example, age, sex, comorbidity, vital parameters, and concomitant medication. For exploratory purposes, we will collect biomarker data (eg, C-reactive protein, creatinine, troponin, NT-proBNP, according to standard practice) and data on oxygen use, oxygen saturation, blood pressure, and quality of life. Furthermore, we will assess data on the occurrence of ARDS (according to the Berlin criteria), hypoxic respiratory failure, myocarditis, depressed systolic or diastolic function according to prevailing criteria, and data on length of stay in-hospital (at the ICU and non-ICU wards). Such data may be collected according to standard practice. Follow-up questionnaires after 3 and 12 months will focus on quality of life using Short Form–36 questionnaires. In case new insights provide a sound basis to prefer another questionnaire for SARS-CoV-2–infected patients, this will be considered.
Follow-up assessment
During hospital admission, clinical follow-up will be performed on a daily basis. In case of hospital discharge before the occurrence of the primary end point and within 14 days, clinical follow-up for the primary end point will be performed at 14 days after randomization by telephone contact. For follow-up on the secondary end point of mortality at 30 days, 90 days, and 1 year, we will use the national database of Statistics Netherlands. The other secondary outcomes will be collected from patients’ medical records at 90 days. Data on quality of life will be collected at 90 days and 1 year with standardized questionnaires, either through electronic surveys or by telephone contact.
Study organization
Radboud Technology Centre of Clinical Studies (Nijmegen, the Netherlands) will be responsible for data monitoring. Monitoring will be done according to a predefined monitoring plan in accordance with the prevailing guidelines. The review and adjudication of all primary end points will be conducted by an independent event adjudication committee blinded for study group. This work is supported by the Netherlands Heart Institute, the Dutch Heart Foundation, the Dutch CardioVascular Alliance, and Novartis Pharma BV.
Statistical considerations
Baseline descriptive statistics will be presented by treatment arm. Continuous variables will be assessed for normal distribution and reported as mean (SD) or median (interquartile range), whichever is appropriate. Continuous data will be compared using a Student t test or Mann-Whitney U test, whichever is appropriate. Categorical variables will be reported as numbers (%) and compared using χ2 or Fisher exact test, whichever is appropriate.
Primary end point analysis
The analysis of the binary primary outcome will be summarized by estimating the difference in proportions between groups and will be presented with a relative risk reduction, absolute risk reduction, and number needed to treat (with contingency table). Differences in proportion of primary outcome between groups will be tested at a prespecified α at each interim-stage analysis (P value of .001) and final-stage analysis (P value of .05) according to the Haybittle-Peto method.
Secondary end point analysis
The analysis of the secondary binary outcomes will be performed accordingly with a P value of .05. Time-to-event secondary outcomes will be analyzed using a Cox proportional hazards model, with, if necessary, baseline covariate adjustment in case of unexpected differences in baseline descriptive statistics. The estimated treatment effect will be presented in the form of hazard ratios with 95% CIs. Kaplan-Meier plots will be used to present the pattern of events per treatment group over the follow-up period. The assumption of proportional hazards will be checked using interaction of independent variable(s) with log(time) and graphical diagnostics based on the Schoenfeld residuals.
Exploratory analyses
The following subgroups were prespecified for analysis of the respective outcome measures: age group (above/below median age; above/below 65 years), gender (male/female), admission hospital (study site), known history of prehospital hypertension (yes/no), known history of prehospital diabetes (yes/no), oxygen saturation at baseline (above/below median), treatment with ACE-i (yes/no), and duration of symptoms prior to hospital admission (above/below median).
Sample size calculation and interim analyses
Based on Dutch national data, we expect 41% of the patients to develop the primary event in the control group and 30% in intervention group.26 , 27 To test this difference at a P value of .05 and power of 80%, we need a total of 592 evaluable patients. We will perform 2 interim-stage analyses and 1 final-stage analysis for efficacy according to the Peto-Haybittle method. We will use predefined type I error boundary points with P values of .001 at each interim analysis and P value of .05 at the final analysis. First interim analysis will be performed when data are available on 200 patients, second when data are available on 400 patients, and final when data are available on all 592 patients. Anticipating a dropout rate of 10%, we aim to enroll 651 patients. Analyses will be performed using R for statistical computing and graphics (R Foundation, Vienna, Austria).
Data safety monitoring board
A data safety monitoring board (DSMB) will be established to perform (unblinded) analyses according to the DSMB charter (Supplement 2). The DSMB will be composed of a chair clinical expert, a second clinical expert, and a statistician with experience in randomized controlled trials, independent of the sponsor. Criteria on which the DSMB may decide to terminate the trial prematurely are defined in the DSMB charter. The advice(s) of the DSMB will only be sent to the sponsor of the study. Should the sponsor decide not to fully implement the advice of the DSMB, the sponsor will send the advice to the reviewing METC, including a note to substantiate why (part of) the advice of the DSMB will not be followed.
Ethical considerations
The study will be conducted according to the principles of the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects act. The research ethics committee of the Radboud University Medical Center has reviewed this study on the basis of the Dutch Code of conduct for health research, the Dutch Code of conduct for responsible use, the Dutch Personal Data Protection Act, and the Medical Treatment Agreement Act. The ethics committee has passed a positive judgment on the study. All participants will provide oral informed consent before participating in the trial according to prevailing standards during the COVID-19 pandemic, and a written informed consent as soon as possible. The trial is registered at clinicaltrials.gov (NCT04335786, 2020).
Discussion
The PRAETORIAN-COVID trial has been initiated as a project to provide highly awaited evidence regarding the controversy on the effects of ARBs in patients with SARS-CoV-2 infectious disease. Facing the current COVID-19 pandemic, we aim to assess the effect of the cheap and widely available treatment option valsartan on morbidity and mortality. In the setting of a double-blind, placebo-controlled, 1:1 randomized trial, we will compare valsartan with placebo for the occurrence of ICU admission, mechanical ventilation, and death in hospitalized SARS-CoV-2–infected patients.
The role of the RAS in SARS-CoV-2 infection
Similar to SARS-CoV-1 (responsible for the SARS 2002-2004 pandemic), SARS-CoV-2 interacts with the RAS through ACE2, an enzyme that physiologically counters RAS activation but also functions as a receptor for both SARS viruses.3., 4., 5., 6. , 16 ACE2 is expressed in lung epithelium. It is postulated that the virus binds to ACE2 and consequently enters the cell via endocytosis.5 Subsequently, ACE2 is degraded.5 Before lysis of the complex, the virus moves in the cytosol where it spreads from cell to cell and infiltrates the epithelium of the lung.28 Thus, internalization of the COV-ACE2 complex decreases the expression of ACE2 and its inhibitory effect on the activated RAS.7 Consequently, SARS-CoV-2 shifts the delicate RAS balance into increased levels of Ang-II because degradation to Ang-1-7 is limited.7
Elevated levels of unblocked Ang-II, possibly in addition to disruptions of the kinin-kallikrein system,29 increase pulmonary vascular permeability, inflammation, and fibrosis, which may eventually lead to ARDS.2 , 7 , 8 In concordance with various other research groups, we hypothesize that ARBs may attenuate acute lung injury by mitigation of the detrimental Ang-II–mediated cascade2 , 7 , 11., 12., 13., 14., 15., 16., 17., 18. , 21 , 30., 31., 32. (Figure 1).
Rationale for the intervention
In this study, we chose to use an ARB over an ACE-I for modulation of RAS for the prevention of acute lung injury. ACE-I may decrease Ang-II concentrations by inhibition by conversion of angiotensin I to Ang-II, but there is evidence that ACE-I does not have the supposed beneficial effect of increasing ACE2.16 , 33 , 34 ARBs have been demonstrated to increase ACE2 expression in various settings, which may counterbalance the breakdown of ACE2 by the SARS-CoV-2 virus.11., 12., 13., 14., 15., 16., 17., 18. , 21 A relative increase in ACE2 expression by ARBs would lead to increased breakdown of Ang-II to protective Ang-1-7.11., 12., 13., 14., 15., 16., 17., 18. , 21
Besides ARBs, other treatments targeting ACE2 have been suggested as potential agents against SARS-CoV-2, such as chloroquine or exogenous recombinant ACE2. Previous research showed efficacy of chloroquine when administered prior to or shortly after being infected, however, with reduced effect in progressing infections.35 Promising results have been demonstrated with exogenous recombinant ACE2 in a phase II trial in ARDS patients.32 However, this is currently not a widely available treatment option.
Major advantages of ARBs are the low costs and wide availability. ARBs may provide a feasible treatment option for SARS-CoV-2 infectious disease, accessible for many patients globally. To our knowledge, there is no evidence to suggest one ARB over another. Based on local availability, we chose to use valsartan. Besides our study, other trials in this particular field of interest are registered (NCT04312009, NCT04311177, NCT04338009, NCT04330300, NCT04366050). The combination of these trials may provide insight into whether there is an overall class effect of ARBs or ACE-i or that there may be a difference between particular ARBs and ACE-i.
Potential limitations of the intervention
The use of ARBs in SARS-CoV-2 infection may be hampered by their blood pressure–lowering effect, hyperkalemia, and liver or kidney dysfunction. Based on the concept that Ang-II–mediated effects cause the clinical presentation of SARS-CoV-2 infection, it is likely that patients will present with a syndrome of hyperaldosteronism with hypertension and hypokalemia. Therefore, we do not expect hypotension and hyperkalemia to be a major obstacle in this trial. Liver dysfunction may be present in SARS-CoV-2–infected patients and could limit enrolment and potential future use of valsartan.36
Based on previous literature, we expect a drop of 16 mm Hg in systolic and of 12 mm Hg in diastolic blood pressure at a maximum dosage of valsartan. A blood pressure at enrolment of 105/65 mm Hg should ensure a mean arterial pressure allowing adequate end-organ perfusion.37 Moreover, dosage of study treatment will be titrated based on systolic blood pressure according to predefined criteria, whereas based on the proposed mechanism of action, vasopressive support is not contraindicated.
Study setting, design, and outcome measures
In response to calls from several healthcare authorities, we will investigate the effect of valsartan compared to a placebo in the setting of a double-blind randomized clinical trial to provide the highest level of evidence. Our primary end point is in concordance with the landmark paper by Guan et al and focuses on respiratory failure, which is currently the most profound therapeutic challenge in the treatment of SARS-CoV-2 infection.1
Our study is designed for patients in-hospital but prior to ICU admission. In this setting, we can adequately target patients with substantial morbidity and mortality and in whom there may still be time to attenuate the development of severe complications of SARS-CoV-2 infection. Of note, treatment with an ARB in an outpatient setting may be an attractive approach to mitigate the adverse cascade of SARS-CoV-2 infection at the most early stage and should be addressed in future studies.
The rationale for inclusion of patients currently taking an ACE-I is based on the evidence that the combination of an ARB with ACE-I can be performed safely.38 Appreciating the in-hospital study setting and the careful titration scheme, potential adverse effects such as hypotension will be carefully monitored. Moreover, we feel that inclusion of this likely substantial proportion of SARS-CoV-2 patients increases the external validity of the trial results.1
The duration of follow-up for the primary analysis is 14 days, based on the available experience that most of the SARS-CoV-2–infected patients have either developed progressive severe disease or started to recover within that time frame. Similar approaches are adopted in other registered trials in COVID-19 (NCT04312009, NCT04311177, NCT04366050). A short time frame also allows for swift analyses and reports on the primary end point, which increases the speed of data availability in light of the global COVID-19 crisis.
The longer duration of follow-up for some of the secondary outcome measures may increase the number of events and may provide more sensitivity to explore an effect of valsartan over placebo. These secondary end points focus on safety as well as efficacy to provide data for adequate risk/benefit analyses. Furthermore, we will conduct questionnaires in response to concerns raised on (social) media regarding the quality of life following SARS-CoV-2 infection. We will conduct additional analyses on clinically important subgroups to explore whether the treatment effect may differ according to specific patient characteristics.
Implications
The PRAETORIAN-COVID trial will provide scientifically robust data on the use of ARBs in SARS-CoV-2 infection and may provide important insights that can be used to formulate evidence-based guidelines on this important topic.
In case of a beneficial effect of valsartan, this could provide a cheap and easily available treatment option for SARS-CoV-2 patients. Our study design is statistically powered to demonstrate an approximately 28% relative risk reduction in the occurrence of the primary outcome measure. Besides the effect on morbidity and mortality, this would substantially limit the use of scarce medical equipment and personnel. However, regardless of the outcome of the trial, the gathered data can be used to conduct risk/benefit analyses for patients that are chronically using ARBs.
Potential limitations
The in-hospital study setting may limit generalizability of our results to SARS-CoV-2 patients in an outpatient setting and for those who are directly admitted to the ICU. For patients who present at the emergency ward, are admitted to the general ward, and then deteriorate rapidly, it may also be difficult to be included in the study and receive study treatment in a presumably adequate dose. Second, it is unsure what would be an adequate amount of ARB that is likely to result in a beneficial effect. It seems plausible that patients would need ARB treatment for a few days to have a substantial effect. Our study will provide additional insight in this respect. The assumptions of our sample size calculation are based on limited data available in the Netherlands.26 Data from China, the United States, and Italy have been taken into consideration but probably do not apply to our Dutch study cohort.1 , 39., 40., 41.
Conclusion
The PRAETORIAN-COVID trial is a double-blind, placebo-controlled, 1:1 randomized trial to assess the effect of valsartan compared to placebo on the occurrence of ICU admission, mechanical ventilation, and death in hospitalized SARS-CoV-2–infected patients. This study will provide valuable insights into the use of ARB in SARS-CoV-2–infected patients and may contribute to improved treatment recommendations for a large group of patients in this time of the global COVID-19 pandemic.
The following are the supplementary data related to this article.
Study drug titration scheme.
DSMB charter.
Acknowledgements
We thankfully acknowledge Sander Damen, Stijn van Vugt, Peter-Paul Zwetsloot, Haldun Bulut, and Xander Staal for their critical appraisal of the manuscript and study protocol. We also thankfully acknowledge Vincent Aengevaeren and Esmee Bakker for agreeing to contribute to the emergency deblinding procedure.
Footnotes
Funding: This work is supported by the Netherlands Heart Institute, the Dutch Heart Foundation, the Dutch CardioVascular Alliance, and Novartis Pharma BV.
Declaration of interest: All authors have provided conflict of interest forms.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wit E., van Doremalen N., Falzarano D. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshotels M.R., Xia H., Sriramula S. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L., Karakiulakis G., Roth M. 2020. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommerstein R, Gra¨ni C. Rapid response: re: preventing a covid-19 pandemic: ACE inhibitors as a potential risk factor for fatal Covid-19. BMJ 2020. https:// www.bmj.com/content/368/bmj.m810/rr-2
- 11.Furuhashi M., Moniwa N., Mita T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 12.Klimas J., Olvedy M., Ochodnicka-Mackovicova K. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med. 2015;19(8):1965–1974. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishiyama Y., Gallagher P.E., Averill D.B. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 14.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 15.Patel A.B., Verma A. 2020. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. [DOI] [PubMed] [Google Scholar]
- 16.Vaduganathan M., Vardeny O., Pharm D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuster G.M. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? European Heart Journal. 2020;0:1–3. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020:1–4. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battistoni A., Volpe M. 2020. Might renin-angiotensin system blockers play a role in the COVID-19 pandemic? Eur Heart J Cardiovasc Pharmacother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.closingthedoortocorona.org/endorsements/
- 22.https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19
- 23.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 24.Chan A.W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of internal medicine. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciwit B.V. 2016. Castor electronic data capture. Amsterdam. [Google Scholar]
- 26.https://www.rivm.nl/
- 27.Zhang P., Zhu L., Cai J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circulation Research. 2020 doi: 10.1161/CIRCRESAHA.120.317242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan Y., Shang J., Graham R. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van de Veerdonk F.L., Netea M.G., van Deuren M. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. 2020. https://www.preprints.org/
- 30.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125(Pt A):21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M.L., Yang J.M., Sun Y.P. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):219–222. doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Khan A., Benthin C., Zeno B. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell D.J., Zeitz C.J., Esler M.D. Evidence against a major role for angiotensin converting enzyme-related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens. 2004;22(10):1971–1976. doi: 10.1097/00004872-200410000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Rice G.I., Thomas D.A., Grant P.J. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham H.M., White C.M., White W.B. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38(1):33–54. doi: 10.1007/s40264-014-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeffer M.A., Swedberg K., Granger C.B. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study drug titration scheme.
DSMB charter.