Abstract
Magnetic hyperthermia is one of the most promising techniques for treating gynecological cancer, where magnetite (Fe3O4) is the most common nanomaterial used as a magnetic hyperthermia agent. Here, we demonstrate that optimal Fe3O4 nanorods (NRs) can act as a magnetic hyperthermia agent with higher specific absorption rate (SAR), which is mostly attributed to their enhanced surface anisotropy. As a result, Fe3O4 NRs could effectively hinder the growth of gynecological cancer cells in nude mice models, again demonstrating its good magnetic heating properties. These results provide a powerful basis for the development of an ideal magnetic hyperthermia agent with enhanced SAR, thereby effectively treating gynecological cancer in clinical practice.
Subject terms: Biophysics, Cancer
Introduction
Currently, gynecological cancers are seriously affecting women’s health and safety due to environmental pollution and poor eating habits1–5. Hence, early diagnosis and effective treatment of gynecological cancer is the optimal means to reduce mortality6–8. Magnetic hyperthermia, as one of the new hyperthermia methods, shows good prospects for gynecological diseases and cancer treatment due to the characteristics of targeting, simple administration, reduced dosage, and minimal side effects9–13. During magnetic hyperthermia treatment, the magnetic nanoparticles are usually injected or targeted into the tumor lesion area, and the nanoparticles are heated by applying an alternating magnetic field (AMF). As a result, the tumor cells are destroyed while normal cells remain alive14–17. Most recently, the application of biomedical magnetic nanoparticles in gynecological tumor hyperthermia has received extensive attention, attributed to a series of features including good biosafety, surface modification, special in-vitro properties, and unique magnetism18–20.
Magnetic iron oxide nanoparticles, one of the significant biomaterials among novel biofunctional nanoparticles are widely considered the most suitable magnetic hyperthermia agents owing to their high saturation magnetization (Ms)21–24. However, high synthesis process cost and weak thermal conversion efficiency restrict their extensive usage. Recently, the application of tunable nanomaterials is also becoming more widespread, and there are many mature preparation methods including sol-gel synthesis, hydrothermal reaction, sonochemical reaction and laser pyrolysis25–29. Generally, the traditional method is thermal decomposition of iron salts, which needs more energy and higher cost30. Hydrothermal synthesis, one of the preferred solutions to prepare biomedical Fe3O4 nanomaterials owing to the low cost and feasibility, while it can effectively regulate the size of nanomaterials by controlling hydrothermal time or reactant concentration31–33. In addition, nanorod materials have excellent magnetic properties due to their adjustable aspect ratio and strong anisotropy, thereby improving the heating efficiency of materials8,34.
Based on the foundation of enhanced magnetic hyperthermia by adjusting the aspect ratio of Fe3O4 nanorods (NRs), while hydrophilic graphene oxide (GO) was chosen as the counterpart for avoiding aggregation between Fe3O4 NRs. Hence, the different sizes of Fe3O4 NRs were synthesized by a simple hydrothermal method with different reaction times and post-annealing method at 340 °C. The Ms value of hydrophilic Fe3O4 NRs-GO with a length of 350 nm reaches the largest (60 emu/g). While it shows excellent magnetic hyperthermia performance with large SAR of 1045 W/g for a concentration of 0.2 mg/mL at 360 kHz, 308 Oe. Moreover, as a magnetic hyperthermia agent, Fe3O4 NRs can effectively slow the growth rate of gynecological cancer in nude mice. These results provide a feasible research basis for the application of high-performance magnetic hyperthermia agent in clinical practice.
Experiment
Synthesis of Fe3O4 NRs
Different sizes of Fe3O4 NRs were fabricated by a one-step hydrothermal method and reduction reaction35. First, 6.0 mM FeCl3 · 6H2O was added to 60 mL of deionized water and stirring continued for 0.5 h to form a homogeneous solution, then transferred to a Teflon-lined stainless-steel autoclave and reacted at 100 °C for 4, 6, and 10 h. The β-FeOOH precursors were collected by centrifugation and washed with deionized water and alcohol three times, then dried at 60 °C. Second, 20 mg of different sized β-FeOOH NRs was uniformly dispersed in 6 mL trioctylamine, then 200 μL oleic acid was added to the mixture and kept stirring for 2 h, respectively. Then the yellow gelatinous mixture was collected by centrifugation at 7500 rpm for 10 min. Finally, under the flow of mixed gas of 95% Ar and 5% H2 gas, the as-prepared precursors were transferred to a tube furnace for the reduction reaction at 340 °C and kept for 2 h, then naturally cooled to room temperature. The different sizes of Fe3O4 NRs were collected though centrifuging, washed with hexane, and dried at 60 °C. The 460, 350, and 250 nm Fe3O4 NRs correspond to different hydrothermal times of 4, 6, and 10 h, respectively.
Synthesis of Fe3O4 NRs-GO
First, the as-prepared Fe3O4 NRs was dissolved in chloroform at a concentration of 10 mg/mL, and 5 mg/mL aqueous solution of graphene oxide (GO) was prepared. Then octadecylamine (20 mg) and chloroform (1 mL) were fully dissolved by ultrasound for 1-2 min, then Fe3O4 NRs solution (100 μL), deionized water (4 mL) and GO (1 mL) were added to further ultrasound for 30 min. Finally, the obtained solution was placed on a shaker for 6 h at 55 °C to remove chloroform, then the Fe3O4 NRs-GO dispersion system was prepared.
Materials characterization
The phase structure, morphology, and chemical composition on the surface of the Fe3O4 NRs were analyzed by X-ray diffraction (XRD) patterns (X’pert Pro Philips), scanning electron microscope (SEM, Hitachi S-4800), transmission electron microscope (TEM, Tecnai G2 F30), and X-ray photoelectron spectroscopy (XPS, Kratos AXIS Ultra), respectively. Vibrating sample magnetometer (VSM Model EV9, MicroSense, LLC) and SQUID systems (MPMSXL-7) were carried out to measure hysteresis curves and zero field cooled (ZFC) and field cooled (FC) magnetization plots. Further, the hydrodynamic diameters and zeta potential of samples were measured on a Malvern Zetasizer Nano-ZS. The magnetic heating properties of Fe3O4 NRs were analyzed by a radiofrequency heating machine (EASYHEAT-5060, Ambrell), the specific absorption rate (SAR) value was calculated based on the following equation: , where is the initial slope of the temperature versus time curve, C is the specific heat capacity of water, and mFe is the weight fraction of Fe in solution.
In vivo experimental sequences
Sixteen female 6–8 weeks old nude mice, weighing about 20 g, purchased from Beijing Weitong lihua Experimental Animal Technology Co., Ltd, were kept in a sterile environment. Four mice were placed in each autoclaved cage and the animals were fed with a special diet, given water, and kept at an appropriate temperature. 0.1 mL of cancer cell suspension (about 1*107 cells) was injected subcutaneously into the right lower extremities of mice to induce tumors and observe the growth of mice. Then mice were randomly divided into 4 groups with a tumor nodule volume as long as about 80 mm3, and 4 mice in each group. Nude mice in the experimental group were injected subcutaneously with 0.1 mL (Fe concentration of about 1.2 mg/mL) of Fe3O4 NRs-GO-AFM and Fe3O4 NRs-GO, respectively. While in the control group, nude mice were injected with 0.1 mL of PBS buffer. Before treatment, the initial tumor volume of each nude mouse was measured and recorded (Volume = length * width * Width/2)36,37. Then three groups of nude mice were placed under a magnetic field of 308 Oe for 10 min. Finally, nude mice were sacrificed after 20 days, and tumor volume changes of each nude mouse were measured every 2 days before euthanasia.
Results and discussion
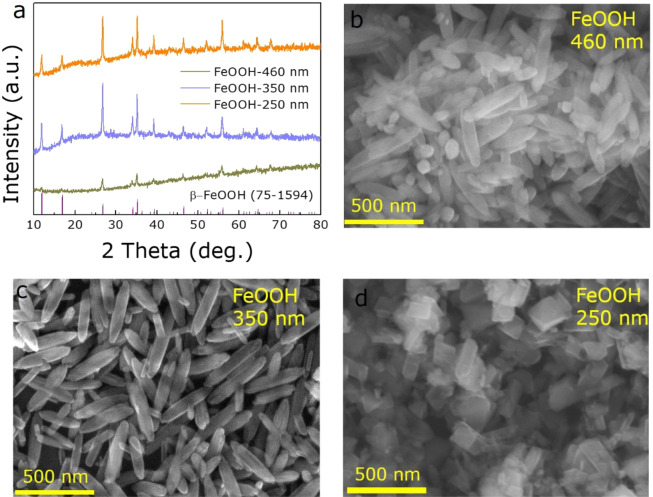
Different size of FeOOH NRs were prepared by a simple hydrothermal method with various reaction times. Figure 1a shows the X-ray diffraction (XRD) patterns of the prepared precursors of FeOOH NRs, all the diffraction peaks are well indexed to the β-FeOOH (JCPPS no. 75-1594). Figure 1b–d illustrate the scanning electron microscope (SEM) images of FeOOH NRs with lengths of 460 nm, 350 nm, and 250 nm, respectively, which correspond to the experimental conditions of 4 h, 6 h, and 10 h hydrothermal times, respectively.
Figure 1.
The structure and morphology of FeOOH precursors. (a) XRD patterns of FeOOH. (b–d). SEM images of 460, 350 and 250 nm FeOOH NRs, respectively.
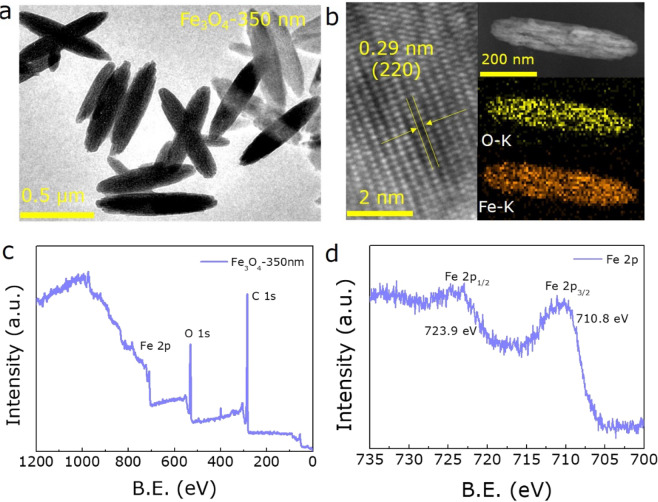
After annealing at 340 °C for 2 h in a mixed gas of 95% Ar and 5% H2, FeOOH precursors were reduced to Fe3O4 NRs as confirmed by XRD patterns (Fig. 2a). All the diffraction patterns of three samples correspond to the Fe3O4 (JCPPS no. 74-0748), where no impurity phase or second phase (e.g. FeO, Fe, or FeOOH) are produced, indicating that the FeOOH are completely reduced to Fe3O4 after calcination. Besides, the average size and morphology of Fe3O4 NRs are basically unchanged compared to the FeOOH NRs precursors, as described by SEM and TEM images shown in Figs. 2b–d and 3a, where a slight surface roughness change is possibly caused by the incomplete reaction of oleic acid as the capping agent12,31.
Figure 2.
The structure and morphology of Fe3O4 samples. (a) XRD patterns of Fe3O4. (b–d) SEM images of 460 nm, 350 nm and 250 nm Fe3O4 NRs, respectively.
Figure 3.
The detailed characterizations of 350 nm Fe3O4 NRs sample. (a) TEM image. (b) HRTEM image and element mappings of 350 nm Fe3O4 NRs. XPS spectra of (c) wide and (d) Fe 2p spectrum for 350 nm Fe3O4 NRs.
For the 350 nm Fe3O4 NRs, the high-resolution TEM (HRTEM) image and EDX mapping analysis were also acquired as shown in Fig. 3b. The clear lattice fringes with d-spacing of 0.29 nm, correspond to (220) crystal plane of Fe3O4, demonstrating that the 350 nm Fe3O4 NRs has excellent crystallinity. Additionally, the EDX mapping analysis illustrates that the Fe and O atoms are uniformly distributed in the Fe3O4 NRs matrix. Furthermore, X-ray photoelectron spectroscopy (XPS) was performed to determine the surface chemical composition of the 350 nm Fe3O4 NRs sample. As seen in the survey scan (Fig. 3c), Fe, O, and C elements coexist, corresponding to the chemical composition of Fe2O3. Figure 3d further shows the Fe 2p spectrum, where the binging energy peaks at 723.9 and 710.8 eV match well with the Fe 2p1/2 and Fe 2p3/2 levels, respectively, confirming the existence of pure Fe3O4. The typical satellite peaks at 729.5 and 719.0 eV have not been identified, which further illustrates the full reduction of β-FeOOH precursor and high purity of Fe3O4 NRs38,39.
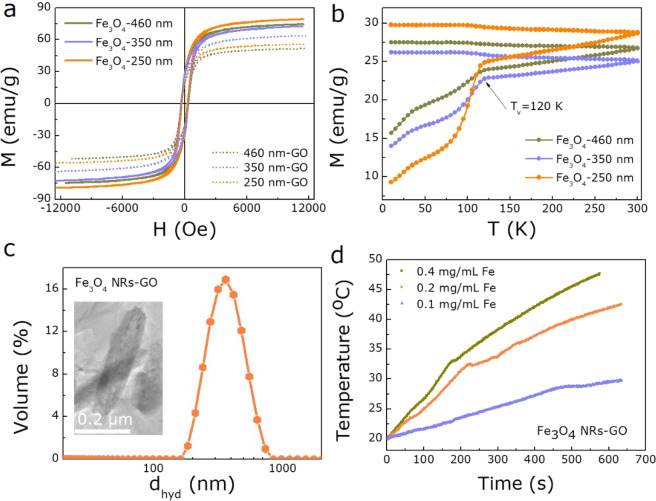
Subsequently, a vibrating sample magnetometer (VSM) was used to estimate the magnetic properties of Fe3O4 NRs. Figure 4a shows the room temperature M(H) hysteresis loop of samples, the saturation magnetization (Ms) of 460, 350, and 250 nm Fe3O4 NRs are measured to be 72, 71 and 77 emu/g, respectively. Almost equal coercivity (Hc) and remanence (Mr) were obtained for three samples. However, the Ms value is not changed significantly with the change of size for Fe3O4 NRs, and lower than that of bulk Fe3O4 (90 emu/g) at room temperature, which is possibly caused by the tiny uncompensated surface spin or the disordered surface microstructures40. The ZFC and FC magnetization plot of three Fe3O4 NRs samples were measured to further confirm the phase structure and crystallinity of Fe3O4. The Verwey transition temperature (Tv) is detected at 120 K as shown in Fig. 4b, which is one of the criteria for judging Fe3O441,42, in accordance with the above XRD, XPS results.
Figure 4.
The magnetothermal performances of Fe3O4 NRs. The magnetization hysteresis loops (a) and temperature-dependent ZFC-FC curves (b) of all Fe3O4 NRs. (c) Hydrodynamic size of 350 nm Fe3O4 NRs-GO. The inset: The morphology of Fe3O4 NRs-GO. (d) Heating curves for 350 nm Fe3O4 NRs at the concentration of 0.1, 0.2, and 0.4 mg/mL.
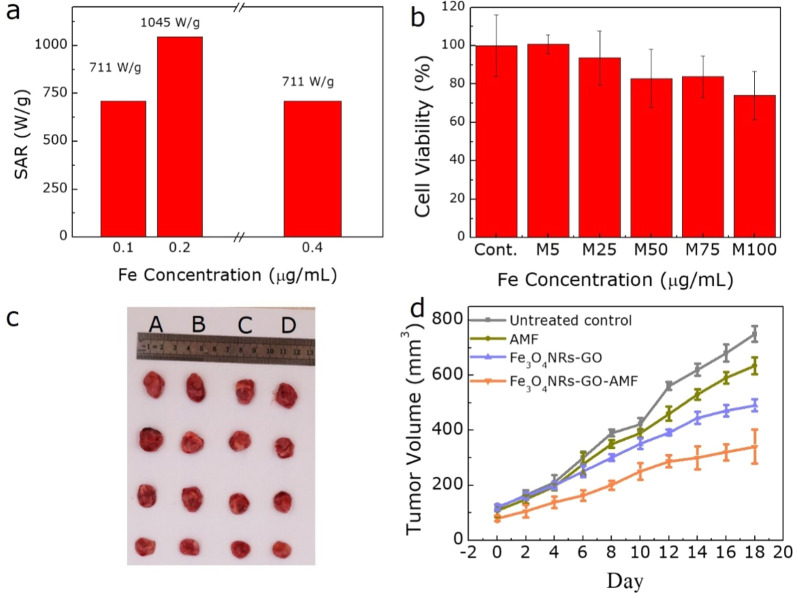
To further understand the hyperthermia performance of samples, the transformation of the aqueous phase was first performed to evenly disperse the Fe3O4 NRs in water43. Then the magnetic properties of as-prepared samples were again analyzed as shown in Fig. 4a, the Ms of 350 nm Fe3O4 NRs-GO reaches a maximum of 60 emu/g, suggesting it has the best hyperthermia performance44. Besides, Fig. 4c shows that the Fe3O4 NRs-GO dispersion is without obvious aggregation and stability, which is demonstrated by hydrodynamic diameter of 370 nm and zeta-potential of 50.1 mV, and the morphology of Fe3O4 NRs-GO as shown in the inset of Fig. 4c. Therefore, to study the effect of different concentrations of 350 nm Fe3O4 NRs on heating efficiency, the SAR value was measured and analyzed at 308 Oe and 360 KHz. Figure 4d shows the heating performance as a function of temperature for 350 nm Fe3O4 NRs with different concentrations of 0.1, 0.2, and 0.4 mg/mL Fe, where a shorter time of 10 min is observed to easily reach 42 °C for 0.2 mg/mL Fe3O4 NRs, indicating that it is suitable for high temperature magnetothermal application. Further, the heating rate significantly accelerates as the concentration of Fe increases to 0.4 mg/mL. Correspondingly, the sample with 0.2 mg/mL Fe achieves the largest SAR value of 1045 W/g, superior to other concentration samples as shown in Fig. 5a, demonstrating the excellent magnetic hyperthermia performance of Fe3O4 NRs, further providing a powerful basis for the development and utilization of a magnetic hyperthermia agent. The reduction in the heating efficiency with increasing concentration of 0.4 mg/mL Fe maybe due to the higher aggregation of the Fe3O4 NRs, which tends to negatively affect their heating capacity35,45. In addition, Fe3O4 NRs have a low cytotoxic effect for gynecological cancer cells with the concentration of Fe from 5 to 100 μg/mL (Fig. 5b), revealing the good biocompatibility of biomedical Fe3O4 NRs. Therefore, further study on cell magnetic hyperthermia was performed. All protocols for animal research conformed to the Guide for the Care and Use of Laboratory Animals, and approved by the Lanzhou University Ethics Committee. All the in vivo studies were conducted according to guidelines that were approved by the Institutional Animal Care and Use Committee of the Tsinghua University. After 10 min of magnetic hyperthermia with Fe3O4 NRs-GO, the growth rate of tumors is significantly slowed by tracking the growth of tumors in nude mice for 18 days (Fig. 5c,d). These results indicate that Fe3O4 NRs can be used as a high-performance magnetic hyperthermia agent to effectively limit the growth of gynecological cancer.
Figure 5.
(a) The SAR values for 350 nm Fe3O4 NRs at the concentration of 0.1, 0.2, and 0.4 mg/mL. (b) Biocompatibility of 350 nm Fe3O4 NRs. (c) Photograph of nude mice xenografted with gynecological cancer cells before treatment and 18 days after treatment with the untreated control, 350 nm Fe3O4 NRs hyperthermia, respectively and (d) the corresponding plot of tumor volume versus days after treatment.
Conclusions
Highly crystalline and tunable size Fe3O4 NRs have been successfully synthesized by reducing β-FeOOH precursor at 340 °C, while their magnetic and inductive heating properties have been researched. At room temperature, 460, 350, and 250 nm Fe3O4 NRs could display relatively high Ms values, where the 350 nm Fe3O4 NRs exhibits a large SAR value of 1045 W/g at low magnetic field (308 Oe), attributing to the high Ms and effective anisotropy of nanorod materials. As a result, it reveals good biocompatibility, which is a precondition for magnetic hyperthermia investigation on cancers in the future. Furthermore, as a magnetic hyperthermia agent, the as-prepared Fe3O4 NRs could effectively alleviate the growth of gynecological cancer in the nude mice model, providing an effective approach for the treatment of clinical gynecological cancer in the future.
Acknowledgements
This work is supported by the National Natural Science Foundation of China Nos. 81960278 and 81601351, the Science and Technology plan of Chengguan District of Lanzhou City (2017KJGG0014), lzujbky-2019-cd02.
Author contributions
Yongxiu Yang, Daqiang Gao and Xiaolei Liang planned and design the study. Mengwei Huang and Jinmei Qian collected data and analyzed results. Yongxiu Yang, Daqiang Gao and Xiaolei Liang helped in the experiments. Jinmei Qian and Daqiang Gao wrote the manuscript. All authors discussed the results.
Data availability
The datasets generated during and/or analyzed during the current research are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/3/2025
A Correction to this paper has been published: 10.1038/s41598-025-18195-4
References
- 1.Sailor, M. J. & Park, J. H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater.24, 3779–3802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, X. L. et al. Innovative magnetic nanoparticle platform for magnetic resonance imaging and magnetic fluid hyperthermia applications. Curr. Opin. Chem. Eng.4, 38–46 (2014). [Google Scholar]
- 3.Jun, Y.-w et al. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc. Chem. Res.41, 179–189 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Wu, W. et al. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mat.16, 023501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandia, D. et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small15, 1902626 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Barreto, J. A. et al. Nanomaterials: applications in cancer imaging and therapy. Adv. Mater.23, H18–H40 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Pankhurst, Q. et al. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys42, 224001 (2009). [Google Scholar]
- 8.Mohapatra, J. et al. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale7, 9174–9184 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst, Q. A. et al. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys.36, R167 (2003). [Google Scholar]
- 10.Barreto, J. A. et al. Colloidal stability and 64 Cu labeling of iron oxide nanoparticles bearing different macrocyclic ligands. New J. Chem.35, 2705–2712 (2011). [Google Scholar]
- 11.Lim, E.-K. et al. Nanomaterials for theranostics: recent advances and future challenges. Chem. Rev.115, 327–394 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Geng, S. et al. Anisotropic magnetite nanorods for enhanced magnetic hyperthermia. Chem. Asian J.11, 2996–3000 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Das, R. et al. Magnetically tunable iron oxide nanotubes for multifunctional biomedical applications. J. Alloy. Comp789, 323–329 (2019). [Google Scholar]
- 14.Rosensweig, R. E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater.252, 370–374 (2002). [Google Scholar]
- 15.Glöckl, G. et al. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Mat18, S2935 (2006). [Google Scholar]
- 16.Martinez-Boubeta, C. et al. Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci. Rep3, 1652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huong, P. T. L. et al. Magnetic iron oxide-carbon nanocomposites: Impacts of carbon coating on the As (V) adsorption and inductive heating responses. J. Alloy. Comp739, 139–148 (2018). [Google Scholar]
- 18.Petros, R. A. et al. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov.9, 615 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Mehdaoui, B. et al. Optimal size of nanoparticles for magnetic hyperthermia: a combined theoretical and experimental study. Adv. Funct. Mater.21, 4573–4581 (2011). [Google Scholar]
- 20.Nemati, Z. et al. Iron oxide nanospheres and nanocubes for magnetic hyperthermia therapy: a comparative study. J. Elec. Mater46, 3764–3769 (2017). [Google Scholar]
- 21.Yu, J. et al. Multifunctional Fe5C2 nanoparticles: a targeted theranostic platform for magnetic resonance imaging and photoacoustic tomography‐guided photothermal therapy. Adv. Mater.26, 4114–4120 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Gao, J. et al. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Accounts Chem. Res.42, 1097–1107 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Lee, N. et al. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev.115, 10637–10689 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Nemati, Z. et al. Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J. Phys. Chem. C122, 2367–2381 (2018). [Google Scholar]
- 25.Bao, L. et al. Colloidal synthesis of magnetic nanorods with tunable aspect ratios. J. Mater. Chem.22, 7117–7120 (2012). [Google Scholar]
- 26.Sun, H. et al. Solvothermal synthesis of tunable electroactive magnetite nanorods by controlling the side reaction. J. Phys. Chem. C116, 5476–5481 (2012). [Google Scholar]
- 27.Deng, Y. et al. Preparation of magnetic polymeric particles via inverse microemulsion polymerization process. J. Magn. Magn. Mater.257, 69–78 (2003). [Google Scholar]
- 28.Mukh-Qasem, R. A. et al. Sonochemical synthesis of stable hydrosol of Fe3O4 nanoparticles. J.Colloid Interf. Sci284, 489–494 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Nemati, Z. et al. Enhanced magnetic hyperthermia in iron oxide nano-octopods: size and anisotropy effects. J. Phys. Chem. C120, 8370–8379 (2016). [Google Scholar]
- 30.Lee, J.-H. et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. nanotechnol6, 418 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Si, J.-C. et al. Solvothermal synthesis of tunable iron oxide nanorods and their transfer from organic phase to water phase. CrystEngComm16, 512–516 (2014). [Google Scholar]
- 32.Khurshid, H. et al. Mechanism and controlled growth of shape and size variant core/shell FeO/Fe3O4 nanoparticles. Nanoscale5, 7942–7952 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Nemati, Z. et al. Core/shell iron/iron oxide nanoparticles: are they promising for magnetic hyperthermia? RSC Adv6, 38697–38702 (2016). [Google Scholar]
- 34.Kopwitthaya, A. et al. Biocompatible PEGylated gold nanorods as colored contrast agents for targeted in vivo cancer applications. Nanotechnology21, 315101 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Das, R. et al. Tunable high aspect ratio iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C120, 10086–10093 (2016). [Google Scholar]
- 36.Euhus, D. M. et al. Tumor measurement in the nude mouse. J. Surgi. Oncol31, 229–234 (1986). [DOI] [PubMed] [Google Scholar]
- 37.Tomayko, M. M. et al. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemoth. Pharm24, 148–154 (1989). [DOI] [PubMed] [Google Scholar]
- 38.Liu, S. et al. Polarity and surface structural evolution of iron oxide films. RSC Adv2, 9938–9943 (2012). [Google Scholar]
- 39.Radu, T. et al. X-ray photoelectron spectroscopic characterization of iron oxide nanoparticles. Appl. Surf. Sci.405, 337–343 (2017). [Google Scholar]
- 40.Sun, S. et al. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc.124, 8204–8205 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Garaio, E. et al. A multifrequency eletromagnetic applicator with an integrated AC magnetometer for magnetic hyperthermia experiments. Meas. Sci. Technol.25, 115702 (2014). [Google Scholar]
- 42.Poddar, P. et al. First-order metal-insulator transition and spin-polarized tunneling in Fe3O4 nanocrystals. Phys. Rev. B65, 172405 (2002). [Google Scholar]
- 43.Liu, X. et al. Ultrasonication-Triggered Ubiquitous Assembly of Magnetic Janus Amphiphilic Nanoparticles in Cancer Theranostic Applications. Nano Lett. 2019. [DOI] [PubMed]
- 44.Conde-Leboran, I. et al. A single picture explains diversity of hyperthermia response of magnetic nanoparticles. J. Phys. Chem. C119, 15698–15706 (2015). [Google Scholar]
- 45.Andreu, I. et al. Nano-objects for addressing the control of nanoparticle arrangement and performance in magnetic hyperthermia. ACS Nano9, 1408–1419 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current research are available from the corresponding author on reasonable request.