Abstract
Mucuna pruriens belongs to the Fabaceae family and is ordinarily known as velvet bean, in English cowitch and Hindi Kawaanch. The restorative quality of this bean makes it an excellent component in pharmaceutical and therapeutic applications. Apart from high protein and starch content, these beans contain (l-Dopa) 3, 4-dihydroxy-l-phenylalanine, which exhibits several medicinal properties. However, it is poisonous when ingested by ruminants. The obstruction to the advancement of Mucuna as nutrition or food is the nearness of antinutrients, which are high as opposed to other uncommon vegetables. Also, this legume is considered as a future restorative herb because of its anticholesterolemic, anti-Parkinson, antioxidant, antidiabetic, sexual enhancing, anti-inflammatory, antimicrobial, and antivenom activities. It also exhibits anticancer activities, but very few studies have been done. The seeds of Mucuna pruriens also contain a vast range of phytochemical constituents such as alkaloids, glycosides, saponins, reducing sugars, and tannins, which provide an avenue to explore it for wider applications. This review sheds light on the possible mechanism of action of Mucuna pruriens on some diseases (hypoglycemia, Parkinson’s disease, microbial diseases and tumor). and also fills the gap in the studies of Mucuna pruriens. and Further more in vitro and in vivo studies should be done to explore the potential of these seeds against many diseases, its application as a food source, its antinutrient, and harmful properties as well as its nutraceutical perspective.
Keywords: Mucuna pruriens, l-Dopa, Nutraceutical, Phytochemicals, Antioxidant, Parkinson’s ailment
Introduction
Traditional medicinal plants and herbs play an important role in meeting worldwide health-care needs, and their needs will increase in the future (Sharma et al. 2016). Plants have been extensively utilized for therapeutic purposes, particularly in developing nations. India stands out among the most important nations in the world in terms of floristic diversity (Chawla et al. 2017). About 54% of the country’s land is under farming for food, ornamental, and medicinal plant crops, and about 19% area has been covered with forest vegetation (Pathania et al. 2018). Medicinal herbs have various meanings, but in the simplest form, it refers to “crude drugs of vegetable origin utilized for the treatment of various diseases, often of a chronic nature, or to maintain a condition of improved wellbeing” (Boadu and Asase 2017). Mucuna pruriens (Kawaanch) is a yearly climbing vegetable that is excessively utilized as a nutraceutical, pharmaceutic, and food commodity (Szabo and Tebbett 2002). Its natural habitat is in tropical areas, in the region of Africa, South America, and Asia. Mucuna can be considered a good blend of nutrients and medicine. These plants are additionally utilized as a very successful green fertilizer to signify the fundamental issues of soils (Duke 1981). Moreover, over the past decades, it has also been grown all through the southeastern USA for animals and as green compost until the 1950s when the approach of less expensive inorganic manures and soybean feast sources prompted its destruction (Eilitta and Carsky 2003). Apart from its use for animal consumption and as green fertilizer, these beans are processed into flour or eaten as a vegetable by a large population of tropical nations. Also, these beans consist of high starch content, protein, and fiber (Taylor 2005), which significantly enhances its functional applications for the formulation of different food products.
Also, Mucuna is widely used as an anticarcinogenic, antiviral, antimicrobial, anti-inflammatory, antihypertensive, anti-Parkinson, antioxidant agent and also acts against nervous disorders (Adebowale and Lawal 2003; Sadh et al. 2018). Several reports have explored its utilization in Indian Ayurveda, which prescribes it as an effective medicine for the treatment of worms, loose bowels, the runs, snakebite, sexual debility, tuberculosis, ineptitude, rheumatic issue, aches, gonorrhea, sterility, gout, tumor, wooziness, dysmenorrhea, and diabetes. About 30% of the whole plant variety is utilized for therapeutic goals (Ezeagu et al. 2003). Many studies suggest that its seed may have therapeutic value because of several properties against many diseases in different tissues of the body. A variety of preparations made from Mucuna seeds are used for the management of several free radical-mediated diseases such as aging, rheumatoid arthritis, diabetes, male infertility, and nervous disorders (Rakesh and Praveen 2020). The exact mechanism by which these diverse effects occur is still unknown. In our present review, we discuss the possible mechanisms of action in some cases such as hypoglycemia, Parkinson’s disease, tumor, and microbial disease. Due to many useful properties of Mucuna pruriens, as highlighted in this review, the useful compounds from this seed may be used for designing suitable medications or effective drugs for these disorders. As the monetary significance of the medicinal plants is considerable, there is a need to learn about the importance of Mucuna pruriens. Therefore, the present review emphasizes on the food and functional applications of Mucuna beans and its therapeutic importance in the treatment of various disorders.
Distribution
This plant is widely distributed throughout the world, but more in tropical regions, especially Asia, Africa, West Indies, tropical America, the Pacific Islands, and the USA (Fung et al. 2011). In the Indian sub-continent, it is enormously distributed in dry evergreen low forests and throughout the plain regions and found in different forms of bushes and hedges, respectively. Mainly in India, this legume has been considered as a medicinal plant grown in some parts of Madhya Pradesh, Uttar Pradesh, Andaman, and the Nicobar Islands. It naturally grows in the entire tropical plains of India from the lower Himalayan range (Muralia and Pathak 2003). Residents of these states use these legumes as traditional medicine and for the formulation of traditional food products.
Taxonomy and species characteristics of Mucuna pruriens
M. pruriens is a constituent of the domain Eukaryota, which includes kingdom Plantae; class Magnoliopsida; order Fabales; family Fabaceae; subfamily Faboideae (Rajeshwar et al. 2005); tribe Phaseoleae; genus Mucuna; Species pruriens (Taylor 2005). The most commonly cited species include M. deeringiana Merrill, M. utilis Wallich (Bengal velvet bean), M. pruriens, M. Nivea, M. Hassjoo (Yokohama velvet bean), M. aterrima Holland (Mauritius and Bourbon velvet bean), M. capitata, and M. diabolica (Capo-chichi et al. 2003). More recent taxonomists have considered all cultivars of the velvet bean as Mucuna pruriens variety utilis (St Laurent et al. 2002). The plant involves legumes that have an annual and continuous origin, and their breeding traditions first started in China and eastern India, where these plants are often used as green legumes (Gurumoorthi et al. 2003). Mucuna is an important plant genus of family Leguminosae that contains 150 species distributed throughout the tropical and subtropical regions of the world (Pugalenthi et al. 2005). "Cowitch" is a regular English name of Mucuna variety whose sheath has prolonged orange thread-like hair that cause irritation on contacting skin scalp. These hairy non-stinging cultivars, known by its English name as velvet bean. The itchiness is potentially produced by mucunain, which is mainly due to an enzyme, i.e., neurotransmitter serotonin (Akhondzadeh et al. 2009). The plants of Mucuna are perennial climbing legumes with long slender branches, trifoliate leaves, and produce either clustered or long inflorescence. The flowers are white or purple, which hang in large groups or the pyramid. They bear green, brown pod which are covered with soft or rigid hair causing intense irritation (Leelambika et al. 2010). There is a diversity of different varieties in the species with an appearance of pubescence on the sheath, and the bean shaded white, dim, or dark in color after planting until the sheath matures (Rajeshwar et al. 2005). Various species of Mucuna are reported with many versatile medicinal and agronomic applications to humankind (Patil et al. 2015). M. pruriens, one from the Indian subcontinent, nine species, and three varieties of Mucuna were reported by Wilmot-Dear (1984). Further, a new variety of M. pruriens (L.) DC.var. thekkadiensis from Kerala state of India and one new species, M. sanjappae, have been documented (Aitawade and Yadav 2012). Consequently, at present, the genus is represented by ten species, in which one species M. pruriens has four different varieties in India (Natarajan et al. 2012).
Nutritional aspects of Mucuna beans
This legume has a high content of lipids, minerals, carbohydrates, fiber as well as amino acids. In order to improve the nutritional status of this bean, several types of Mucuna that have been developed as nourishment in numerous parts of the world according to the nutritional thickness of the beans (Ezeagu et al. 2003) (Fig. 1). The Mucuna composition ranges between 42.79 and 64.88% crude carbohydrate, 4.1 and 14.39% crude lipid, 5.3 and 11.5% crude fiber, and 2.9 and 5.5% ash content (Ravindran and Ravindran 1988). Mucuna beans are rich in minerals, particularly potassium (806–2790 mg/100 g), magnesium (85–477 mg/100 g), calcium (104–900 mg/100 g), iron (1.3–15 mg/100 g) sodium (4–70 mg/100 g), phosphorus (98–498 mg/100 g), copper (0.33–4.34 mg/100 g), zinc (1–15 mg/100 g) and manganese (0.56–9.26 mg/100 g) (Siddhuraju et al. 1996).
Fig. 1.

Muccuna pruriens (velvet bean) seeds
Different proteins and amino acids are also found in M. pruriens, such as threonine, proline, tyrosine, phenylalanine, tryptophan, glutamic corrosive, aspartic corrosive, serine, lysine, histidine and arginine (Sridhar and Bhat 2007). The crude protein concentration of natural Mucuna bean ranges between 21 and 38% (Flores et al. 2000). The protein content differs mainly due to factors such as variety, growth environment, and maturity. Protein fixation may spoil as the bean creates, just because of nitrogen accessibility throughout bean filling and the last bean measure. Beans that have not matured have 37% crude protein, while mature beans have 24% (Wanjekeche et al. 2003). The amino acid content of these beans is comparable to other legume vegetable beans, from 18 to 44% dry weight (Kay 1979). A couple of studies show that Mucuna protein made up of albumin and globulins, which ordinarily have a positive illustration of necessity amino acids (Bressani 2002). Because of their high lysine content, these beans are extraordinary examples of extra protein monogastric food as compared to oat grains and root crops, which are low in protein and lysine (Adebowale and Lawal 2003). The protein digestibility of this bean resembles that of another grain legume. Therefore, these beans have a reasonable protein digestibility extending from 69 to 82% in rats (Siddhuraju et al. 1996). The protein digestibility studies conducted in human subjects and showed that digestibility is a problem in grain legumes due to enzyme-inhibiting polyphenols and the concentration of dietary fiber has increased during the cooking of beans. Such polyphenols clarify the difference in the shade of Mucuna beans (Gurumoorthi et al. 2003). Colors are white to dull, reflecting differences in amino acid absorbability. In this manner, white beans have the most effective absorbability (62.1%) as compared to dull and red beans (49.6 and 55.7%) (Divya et al. 2017). This is because greater concentrations of the digestibility-inhibiting polyphenolic compounds in the seed coat are present in black or red than in white beans. Various nutritional chemicals present in seeds are listed in Table 1 (Ravindran and Ravindran 1988).
Table 1.
Nuritional properties associated with Mucuna pruriens (Ravindran and Ravindran 1988)
| Nutritional properties | Composition |
|---|---|
| Crude protein | 24–31.44% |
| Crude carbohydrate | 42.79–64.88% |
| Crude lipid | 4.1–14.39% |
| Crude fiber | 5.3–11.5% |
| Ash | 2.9–5.5% |
| Potassium | 0.806–2.790% |
| Sodium | 4–70 mg/100 g |
| Calcium | 104–900 mg/100 g |
| Phosphorus | 98–498 mg/100 g |
| Magnesium | 85–477 mg/100 g |
| Iron | 1.3–15 mg/100 g |
| Copper | 0.33–4.34 mg/100 g |
| Zinc | 1–15 mg/100 g |
Phytochemical constituents
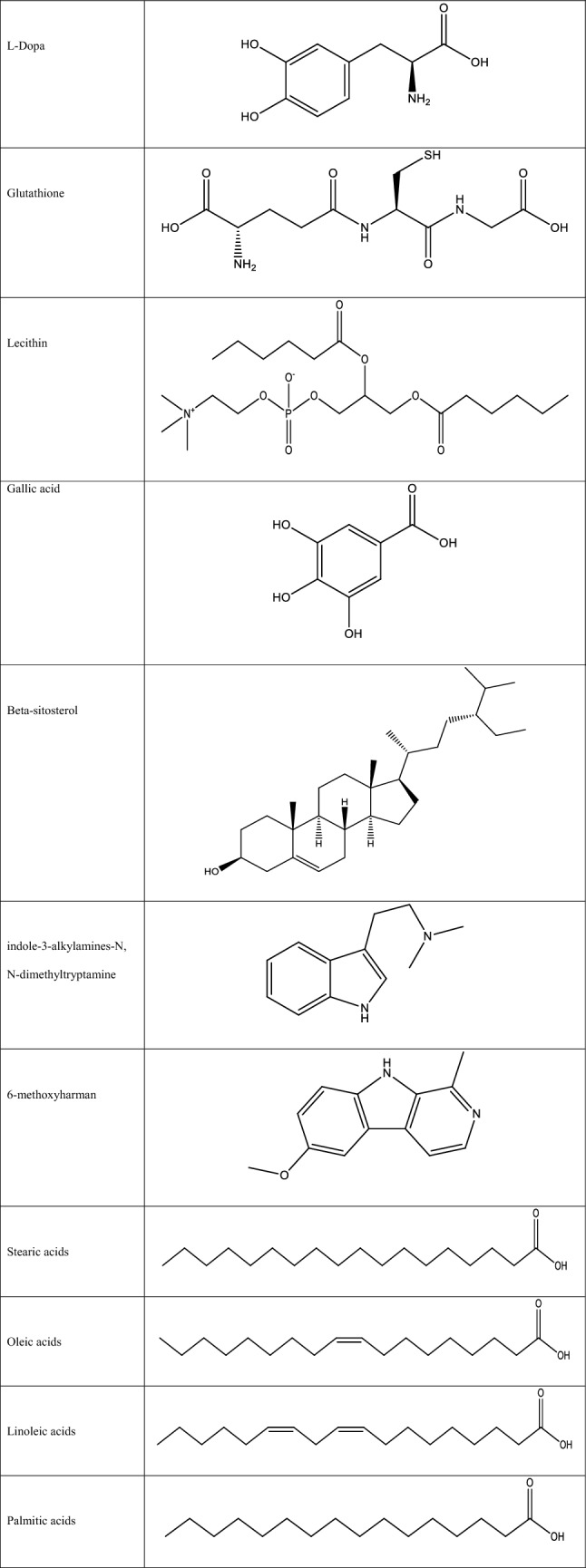
Mucuna seeds are known to produce the unusual nonprotein amino acid 3-(3,4 dihydroxyl phenyl)-l-alanine (l-Dopa), a potent neurotransmitter precursor that is, at least in part, believed to be responsible for the toxicity of Mucuna seeds (Cilia et al. 2017). Besides, it also contains some other compounds such as glutathione, lecithin, gallic acid, and beta-sitosterol. The pods, seeds, leaves, and roots include indole-3-alkylamines-N, N-dimethyltryptamine (Misra and Wagner 2004). Leaves have 6-methoxyharman and serotonin is only present in pods. The seeds also contain oils, including stearic, oleic, linoleic, and palmitic acids. Due to the abundance and toxic nature of l-Dopa, it is of a more significant concern than other antinutrient components of Mucuna (Szabo 2003). Whole pods contain up to 4% l-Dopa, and this concentration decreases with maturity. It is evident from the literature that the highest concentration of l-Dopa is present in the seed, which is the central part of the plant used in monogastric diets. The structures of the chemical constituents were described by Siddhuraju et al. (2000) and Sharma et al. (2005), and these are shown in Table 2.
Table 2.
Structure of phytochemical constituents of Mucuna pruriens (Siddhuraju et al. 2000; Sharma et al. 2005)
Mechanism of action of l-Dopa in humans
l-Dopa is an intermediary product in the enzymatic synthesis of dopamine from l-tyrosine. Dopamine regulates functions in the brain (neurotransmitter), heart (an inotropic increase of cardiac output), vascular system (vessel dilator), and kidney (diuretic) (Grover et al. 2001). l-Dopa is widely distributed in muscle, kidney, and liver, and present across the blood–brain barrier in the central nervous system due to de novo synthesis. Many of these physiological effects are correlated with the de novo synthesis and level of intake of l-Dopa (Mishra and Wagner 2006).
Szabo and Tebbett (2002) provided the following description of the pharmacokinetics in humans: approximately 33% of an orally administered dose of l-Dopa is absorbed from the human gastrointestinal tract, primarily from the jejunum. Peak plasma concentrations occur within 1–3 h of ingestion with levels that may vary as much as tenfold among individuals. l-Dopa undergoes decarboxylation into dopamine extra- and intracerebrally. A majority of the successfully absorbed l-Dopa is converted to dopamine in the periphery, mainly in the intestinal mucosa via decarboxylation by the enzyme l-aromatic amino acid decarboxylase (LAAD). In addition to dopamine, peripheral l-Dopa is metabolized to melanin, norepinephrine, 3-methoxytyramine, methyldopa, 3,4-dihydroxyphenylacetic acid, and 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid or HVA) (Pugalenthi and Vadivel 2007). These metabolites are rapidly excreted in the urine, such that about 80% of an administered dose of l-Dopa is excreted within 24 h, less than 1% of which consists of the original compound.
Of the l-Dopa metabolized and secreted in the urine, roughly 50% consists of dihydroxyphenylacetic acid (DOPAC) and HVA, and 10% of dopamine. In comparison, less than 30% occurs as 3-O-methyldopa Dopamine produced by decarboxylation of l-Dopa by LAAD in the periphery does not cross the blood–brain barrier, but is further metabolized by monoamine oxidase (MAO) in endothelial cells into DOPAC (Aware et al. 2017). Less than 1% of the administered dose crosses the blood–brain barrier into the central nervous system and the basal ganglia. In the brain’s basal ganglia, it is converted to dopamine by LAAD or dopa decarboxylase. Dopamine subsequently undergoes enzymatic inactivation catalyzed by MAO and catechol-O methyltransferase (COMT). The COMT in the glial cells methylates dopamine to 3-methoxytyramine, while MAO oxidizes dopamine to DOPAC (Sathiyanarayanan and Arulmozhi 2007). The COMT also methylates about 10% of oral l-Dopa to 3-O-methyldopa in the red blood cells and the liver. The COMT and MAO together convert dopamine to 3-methoxy-4-hydroxyphenyl acetic acid and HVA.
Therapeutic importance of Mucuna pruriens
Mucuna pruriens is utilized throughout the world for many purposes. For example, it is used as an ornamental crop that forms a thick covering with visually appealing purple blossoms. Mucuna pruriens is also one of the most popular multipurpose legumes among small farmers in the tropics because it is an excellent source of green manure, to some extent because of its ability to fix atmospheric nitrogen (N) thereby restoring soil fertility. It likewise includes huge amounts of organic matter (< 30 tons) to the soil (Flores et al. 2002). Mucuna's rich shallow roots, thick leaves, and vines have the potential to lessen soil disintegration and moderate soil dampness. The therapeutic importance of M. pruriens is summarized in Table 3.
Table 3.
Medicinal properties of Mucuna pruriens
| Activity | Extract | Therapeutic effects | References |
|---|---|---|---|
| Aphrodisiac effect | Methanolic | Increase in the mounting frequency, ejaculation latency and decrease in the mounting latency, post-ejaculatory interval and interintromission interval | Suresh et al. (2009) |
| Antioxidant effect |
Ethanolic Methanolic |
Antilipid peroxidation property Inhibition of ascorbate-induced peroxidation. Decrease in the levels of lipid peroxidation and increase in the levels of glutathione, superoxide dismutase, and catalase |
Tripathi and Upadhyay (2002) Kumar et al. (2010) |
| Antitumor effect | Methanolic |
Decreased tumor volume, packed cell volume and viable cell count Prolonged the mean survival time. A decrease in WBC count and an increase in RBC count |
Rajeshwar et al. (2005) |
| Antidiabetic effect | Aqueous | Reduced the blood glucose levels | Bhasker et al. (2008) |
| Antibacterial effect | Methanolic | Broad-spectrum antimicrobial activity against Gram-positive bacteria Staphylococcus aureus and Gram-negative Proteus vulgaris | Verma et al. (2014) |
| Antiprotozoal effect | Methanolic | Eradicate Licthyophtirius multifilis infection in goldfish | Ekanem et al. (2004) |
| Anti Snake venom effect | Ethanolic | Protects rat heart and liver blood vessels against cobra venom-induced damages. Neutralize the lethalities of Naja sputatrix venoms | Fung et al. (2010) |
| Analgesic and anti-inflammatory effect | Ethanolic | Increased the pain threshold and decreased body temperature. Inhibits carrageenin-induced edema and showed anti-inflammatory activity | Tan et al. (2009) |
Nutraceutical versatility
Mucuna pruriens has been utilized for broad-spectrum nutraceutical applications for a considerably long period. It shows valuable features in pharmaceutics due to the presence of l-Dopa, which have been broadly utilized in the treatment of Parkinson's disorder (Ngatchic et al. 2013). Mucuna tree has reportedly been utilized against bugs, as a bug repellent, and its l-Dopa concentration may make it a powerful anthelmintic (Jorge et al. 2007).
Antioxidant property
A portion of the healing properties is yet to be checked systematically. However, research has approved some verifiable cases. Tripathi and Upadhyay (2002) carried out in vitro and in vivo studies to exploit the cell reinforcement properties of an alcoholic extract of M. pruriens beans. The impact was also examined on iron-instigated lipid peroxidation, oxidation of glutathione, and its cooperation with hydroxyl and superoxide radicals. There was no adjustment or no change in the rate of airborne oxidation of glutathione; however, the concentrate restrained iron sulfate-actuated lipid peroxidation. It hindered the appropriate chemical responses initiated by superoxide radicals. The methanolic extract of the beans has an antilipid peroxidation activity, which is interceded throughout the expulsion of hydroxyl and superoxide radicals (Kumar et al. 2010).
Antivenin property
Mucuna pruriens is one of the plants that is active against snake venom (Kumar et al. 2016). Mucuna leaf extracts follow up on thrombin and fibrinogen to upgrade blood thickening, which makes such concentrates an effective treatment against snake venoms (Houghton and Skari 1994). Hence, its seeds are used in traditional medicine to prevent the toxic effects of snake bites, which are mainly triggered by potent toxins such as neurotoxins, cardiotoxins, cytotoxins, phospholipase A2 (PLA2), and proteases. The mechanisms of the protective effects exerted by M. pruriens seed aqueous extract (MPE) were investigated in detail in a study involving the effects of Echis carinatus venom (EV) (Guerranti et al. 2008). A few in vivo studies of these beans and their neutralizing properties have been shown by Guerranti et al. (2002) and that the protection against the poison is evident at 24 h (short-term) and 1 month (long-term) after injection of M. pruriens seed extract. MPE protects mice against the toxic effects of EV through an immune mechanism. The impact of this M. pruriens separated on prothrombin initiation after EV organization in vitro was examined, and an expansion in the procoagulant movement was found. MPE contains an immunogenic component, a multiform glycoprotein, which stimulates the production of antibodies that bind to certain venom proteins (Guerranti et al. 2004). Thus, aqueous extracts of M. pruriens seeds possess compounds, which inhibit the activity of cobra and blue krait venoms (Meenatchisundaram and Michael 2010).
Fertility-improving and growth-advancing property
Mucuna pruriens is utilized as a key ingredient in marketable tablets for curing infertility and is found to have a positive effect on treatment. It is known to augment the sperm count and motility, along with the enhanced conversion of spermatocytes to sperm (Pulikkalpura et al. 2015). It improves male fertility by its action on the hypothalamus–pituitary–gonadal axis. Misra and Wagner (2007), considered how to best concentrate l-Dopa from M. pruriens for its utilization in decreasing male impotence and as a nerve tonic. They found that l-Dopa can best be removed with a 1:1 (ethanol–water) blend utilizing ascorbic acid as a protector, while thin layer chromatography (TLC) fingerprinting might be utilized to verify the plant material in the homegrown industry. The utilization of M. pruriens as an aphrodisiac was approved through clinical investigations in India. M. pruriens increases sexual intensity, partially because it increases sperm count and testosterone levels (Siddhuraju et al. 1996). As a result of similarity with l-Dopa, these beans can reportedly be utilized as a sexual enhancer and prophylactic operator in patients experiencing oligospermia to lift sperm check in men and enhance ovulation in ladies. The Mucuna bean additionally enhances sperm motility (Sridhar and Bhat 2007). M. pruriens determined l-Dopa and dopamine: both are additionally successful inhibitors of prolactin, a hormone discharged by the pituitary organ that is responsible for 70–80% of erection disappointments in man (Vaidya et al. 1978). In one examination, oral admission of M. pruriens beans in 56 human men enhanced erection, the term of sex, and post-coital fulfillment following a month of treatment (Shukla et al. 2009). M. pruriens likewise has anabolic and development hormone invigorating properties. The presence of L-Dopa and therefore, dopamine in the human system stimulates the release of growth hormone by the pituitary gland (Mesko 2002), and the anabolic impact of the bean is because of its capacity to expand testosterone secretion.
Hypoglycemic property
Supplements of M. pruriens seed extract have a synergistic effect in reducing cholesterol and blood sugar levels. It has been considered as an excellent source for treating thehypoglycemic condition. Several reports demonstrate the benefit of M. pruriens seed for diabetes, a reduction in blood glucose level has been seen significantly by the use of its extract (Majekodunmi et al. 2011). Also, a few in vivo investigations of bean hypoglycemia-instigating impact approve the conventional utilization of the Mucuna plant for diabetes treatment. Bhasker et al. 2008 proved the hypoglycemic effects of M. pruriens in rats having normal glucose level, and streptozotocin-induced conditions. The decrease in the blood glucose (mg/dl) levels in rats was seen after the treatment with the extracts. Studies point out that the existence of wider active compounds in M. pruriens may be a reason for the hypoglycemic effect.
Feeding of M. pruriens seeds with fewer carbs for 1 week to rats diminished fasting blood glucose levels by 39% (Grover et al. 2002). Plasma glucose concentration in mice were decreased by 9% when M. pruriens was administerd and these seeds when taken releases glucagon by pancreatic alpha-cells through glucose transporter 2 (GLUT2) (Fig. 2), and by this route liver releases glucose, and it has been oxidized to pyruvate by glycolysis. Therefore, insulin-containing vesicles migrate to the plasma membrane, which releases insulin into the blood. Besides, these methanolic and ethanolic extract of the leaf (5 g/kg) or bean diminished aggregate cholesterol fixation in rats (Grover et al. 2001).
Fig. 2.
Schematic representation of the possible mechanism against hypoglycemia (insulin secretion in pancreatic β-cells). Glucose enters the pancreatic β-cells with the assistance of facilitative glucose transporter (GLUT 2) and the glycolysis pathway occurs. As a result, glycolysis leads to the generation of high energy molecules like ATP, increasing within the ATP:ADP ratio. The increased ATP:ADP ratio inhibits the ATP-sensitive potassium channel (KATP), causing depolarization of the cell membrane, which allows the influx of calcium into the cell. This, therefore, results in the release of insulin by exocytosis from the secretory granules. GLUT2 glucose transporter 2, ATP adenosine triphosphate, ADP adenosine diphosphate, KATP ATP-sensitive potassium channel, cAMP cyclic adenosine monophosphate
Anthelmintic property
Sources from different nations assert that M. pruriens has high anthelmintic properties, yet there is insufficient proof for this claim. Jalalpure et al. (2007) revealed a huge increment in loss of motion of worms because of the use of M. pruriens oil extract. In a study, Mucuna was substituted for soybean in the feed to sheep (Chikagwa-Malunga et al. 2009) to bring down coccidian oocyst scores (p < 0.05) and a 52% numerical reduction in fecal egg counts (FEC). This emphasizes the requirement to encourage further scientific investigation of the anthelmintic properties of M. pruriens. Such examinations are especially important given the expanding issue of parasite protection from antiparasitic drugs and the expanded worry about medication deposits in animals and nature. These issues make the scan for natural anthelmintics a need. The most infamous helminth in tropical and sub-tropical ruminant generation is Haemonchus contortus, which infects sheep, goats, deer, and other ruminants and has been a critical reason for the economic loss to ruminant producers around the world (Lange et al. 2006). It is hence essential to look at the anthelmintic impact of M. pruriens on this parasite nematode. The significant egg-laying capacity of Haemonchus contortus is maintained by grown-ups feeding on blood. The immature larvae of late stage additionally feast upon blood and blood loss can result in anemia, anorexia, loss of condition, and in the long run, the demise of the host (Miller and Horohov 2006). The indicated anthelmintic properties of M. pruriens require logical approval. On the off chance that such claims are demonstrated legitimate, research would also be expected to recognize the dynamic segments to build the remedial utilization of M. pruriens. The effects of chronic ingestion of abnormal amounts of Mucunal-Dopa also warrant additional research (Duke 1981).
Antiparkinson property
Parkinson is a neurodegenerative ailment, and it is second most common after Alzheimer’s disease (Abushouk et al. 2017). It is caused by the harm of nerve cells that results in the reduction of dopamine production in the brain, which subsequently decreases the capacity of the body to manage its further development and feelings (Neta et al. 2018). A condition portrayed by idleness, diminished responsiveness to stimuli, an inclination to keep up an immobile posture, and diminished reactivity to pain, has been regularly arising in individuals with Parkinson, called catalepsy (Dar et al. 2012). The lack of efficient pharmaceutical treatments had led the scientific community to exploit Mucuna as a Parkinson's disease therapeutic agent in several animal studies and a limited number of human clinical trials (Johnson et al. 2018). Throughout the years, individuals have considered finding an alternative prescription that can bring down reactions and lower costs, for example, the usage of plant extracts. M. pruriens has been reported as a potential plant to lower catalepsy due to the content of l-dopa in the seed (Champatisingh et al. 2011). The extract of M. pruriens used for antiparkinson’s diseases (MPE) is thought to include, among several additives, about 12.55% l-dopa, in comparison to equal doses of l-dopa (Kasture et al. 2013). The dopamine content material in brain tissue is decreased when the conversion of tyrosine to l-dopa is blocked (Fig. 3). l-Dopa, the precursor of dopamine, can cross the blood–brain barrier and go through a conversion to dopamine, restoring neurotransmission (Liu et al. 2016).
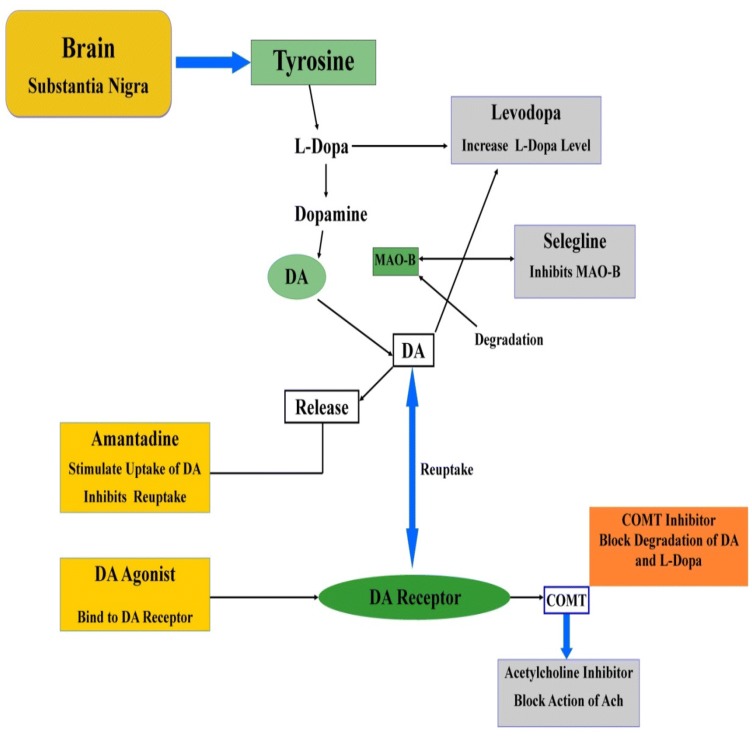
Fig. 3.
Metabolic pathway of l-dopa agent against Parkinson’s disease. Degeneration of the substantia nigra occurs in patients with Parkinson's disease. l-Dopa is converted to dopamine with the help of enzyme DPOPA decarboxylase. This happens both in thecentral nervous system and within the peripheral circulation after levodopa has crossed the blood–brain barrier. Once converted to dopamine, it activates postsynaptic dopaminergic receptors and compensates for the decrease in endogenous dopamine. (MAO-B inhibitors decrease DA levels,which can ensure its rapid breakdown by the catalytic enzyme monoamine oxidase-B (MAO-B), whose level is increased within the Parkinson's disease. Selegiline is well tolerated used MAO-B inhibitor, which, when administered with l-DOPA, may sustain the response of l-DOPA. Dopamine agonists can increase DA levels within the brain and are most active during the early stages of Parkinson's disease. They will even be combined with l-DOPA in the late stages of Parkinson's disease to extend the lifetime of l-DOPA). l-DOPA L-3, 4-dihydroxyphenylalanine, DA dopamine, DA Agonist dopamine agonist, MAO-B monoamine oxidase B, COMT catechol-O-methyltransferase, Ach acetylcholine.
Powdered seeds have been used against parkinsonism properties, maybe as a direct result of the proximity to l-dopa (4–6%). It is exceptional that dopamine is a neurotransmitter. These beans build l-dopa, which exhibited activity against Parkinson’s (Rijntjes 2019). Around 30 g of Mucuna seed powder found to have good capacity in treating Parkinson's disease patients which has been compared to the traditional established standard drugs, particularly the regular wellspring of l-dopa may have central focuses over standard drugs in a whole to deal with Parkinson’s disease (Nagashayana et al. 2000). Cotyledon powder of these beans extended the development and densities of different brain regions, yet did not impact the low activity form of the gene that encodes the activity of monoamine oxidase (in vitro) due to having nicotinamide adenine dinucleotide (NADH) and coenzyme Q-10 in the cotyledon powder, which seem to possess a therapeutic benefit in Parkinson's disease (Nagashima et al. 2016). The cotyledon powder of beans restored and help in treating the endogenous levodopa, serotonin, dopamine, etc. (Manyam et al. 2004). The other finding of a neurorestorative advantage through M. pruriens cotyledon powder on the degenerating dopaminergic neurons within the substantia nigra can be because of accelerated complex-I activity and the presence of NADH and coenzyme Q-10 (Katzenschlager et al. 2004).
Aphrodisiac development property
Extracts of M. pruriens are known to enhance the aphrodisiac effects in humans, and also several commercially available preparations for various sexual disorders use it as an ingredient (Chauhan et al. 2014). Studies done on Wistar male albino rats by Muthu and Krishnamoorthy 2011 shows the androgenic effect of M. pruriens. The use of M. pruriens extract ahowed significant increase in the weight of the sexual organs like the testis and also increased the level of testosterone in turn to alkaline phosphatase activity and protein content.
The ethanolic extract of these beans has been tested on rats in a general sense significantly increased the mounting frequency, ejaculation latency and intromission frequency and decreased the mounting latency, post-ejaculatory interval and inter-intromission interval. The quality analysis on an elementary level extended erections, energetic spin, and involuntary actions. The ethanolic extract of these seeds augment the sexual activity in male rats at an appropriate dose of 200 mg/kg when compared to the control (Suresh et al. 2009). It showed a change in sperm flexibility and check. There is a colossal increment in superoxide dismutase (SOD), glutathione (GSH), glandular activity, and levels of catalase in males (Shukla et al. 2010). All these specifications suggested that these seeds significantly reduce psychological stress and also improve the sperm count and motility.
Antimicrobial property
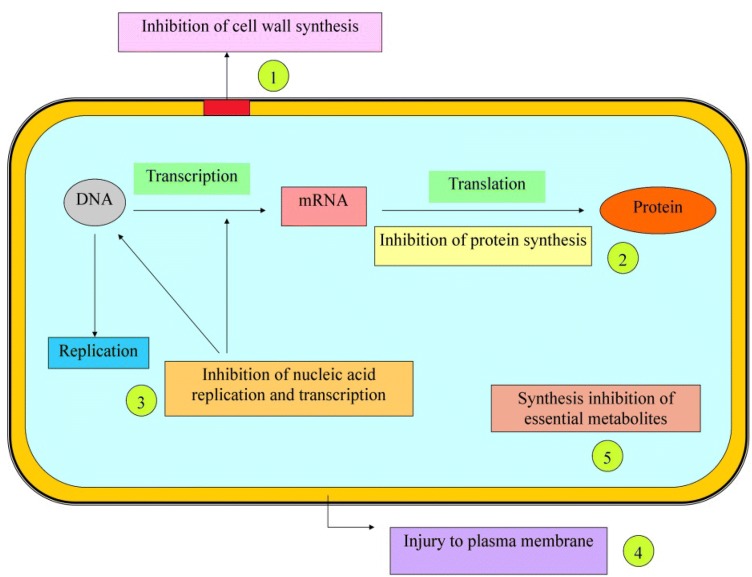
The root and seed of M. pruriens have been incorporated for antibacterial and antifungal activity. The root and seed extract, hexane and petroleum ether, has been used against many bacteria such as Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Bacillus subtilis (Verma et al. 2014). The extract showed a broad spectrum of activity against Bacillus subtilis having a maximum zone of inhibition 18 mm as compared to other bacterial pathogens. Antimicrobial treatment is done with the help of selective toxicity, i.e., selective inhibition of the growth of the microorganism without damage to the host. Selective toxicity is therefore attained by exploiting the differences between the metabolism and structure of the microorganism and the corresponding features of human cells. These seeds work in the binding of the components involved in the process of DNA or RNA synthesis (Fig. 4), which causes interference of the normal cellular processes and ultimately compromise bacterial multiplication and survival. However, the methanolic extract showed high antibacterial activity against Xanthomonas campestris and had very high antagonistic infectious activity against fungal plant pathogens Fusarium oxysporum, Penicillium expansum, and Rhizoctonia solani (Kavitha and Thangamani 2014). The results exhibit that the concentrates have a distinctive degree of essential inhibitory effect against the living things (Murugan and Mohan 2011).
Fig. 4.
Possible mechanism of Mucuna seed against microbial strains. (It can act on five main targets: (1) inhibition of cell wall synthesis (2) inhibition of protein synthesis (3) inhibition of nucleic acid replication and transcription (4) injury to the plasma membrane (5) synthesis inhibition of essential metabolites).
Antitumor property
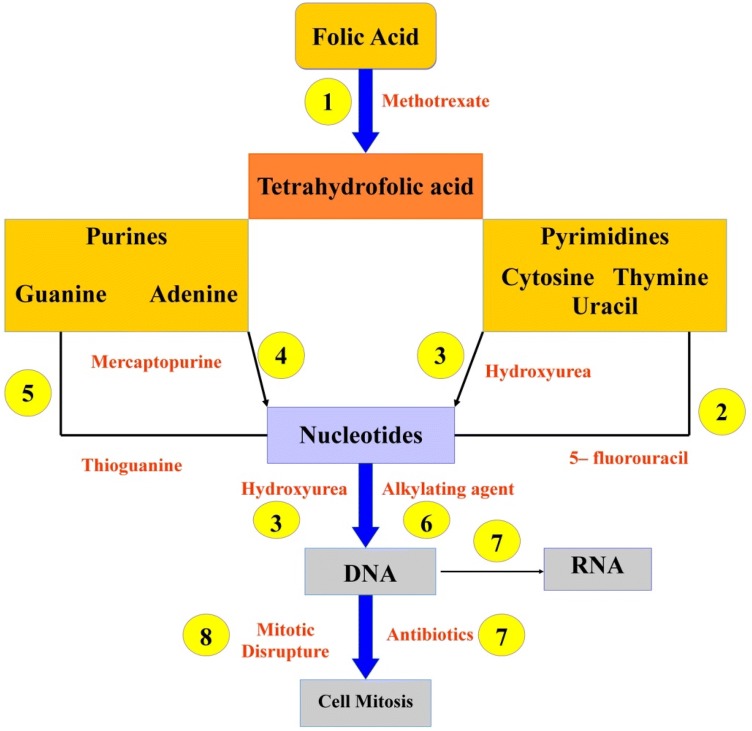
The methanolic concentrate of M. pruriens seeds at 125 mg/kg body weight controlled the tumor in tumor-induced Swiss albino mice. Folic acid is an essential dietary factor (Fig. 5), which is present and has been converted by enzymatic reduction to a series of tetrahydrofolate cofactors that provide carbon groups for the synthesis of precursors of DNA and RNA (Rajeshwar et al. 2005). When the tumor volume was decreased, the viable cell count and amount of cell volume were observed in extract-treated animals when compared with treated animals. Treatment with extract at estimations of 125 and 250 mg/kg extended the mean survival time to 29.5 ± 0.55 and 34 ± 0.2 days, respectively. The focus moreover reduced the body weight of the mice bearing a tumor. There was a huge reduction in leukocytes and total erythrocyte count in treated creatures when contrasted and EAC treated. The study was similarly correlated with the liver biochemical parameters, for example, lipid peroxidation (LPO), and cell support chemicals like SOD and catalase. The levels after treating reduced the lipid peroxidation and extended the levels of superoxide dismutase and catalase (Natarajan et al. 2012). These studies suggested that these seeds have basic anticancer and malignancy counteractive action.
Fig. 5.
Possible mechanism of action of Mucuna seeds against tumor. Folic acid can undergo a reduction to the biologically active coenzyme, tetrahydrofolic acid. Also, antimetabolites are directly incorporated into DNA, which inhibits DNA synthesis. Accordingly, these drugs are generally grouped into derivatives of folic acid that inhibit enzymes that utilize folic acid or synthesize the precursors of purine and pyrimidine, which are necessary for the growth of the cell. These drugs disrupt the formation of those mitotic spindles and, as a result, interrupt cell division.
Mucuna pruriens as a food and feed source
M. pruriens is an outstanding dietary protein (Mohan and Janarandhan 1995). Dietary M. pruriens has assumed a critical part in avoiding a lack of healthy diet in Central American nations, for example, Honduras (Flores et al. 2002) and African nations Benin, Nigeria (Diallo et al. 2000). During the eighteenth and nineteenth centuries, the utilization of M. pruriens as a nourishment crop has likewise been described in Ghana and Mozambique. M. pruriens was developed broadly as a green legume in the regions of the lower eastern Himalayas, and both green sheath and developed beans were bubbled and eaten (Sridhar and Bhat 2007). M. pruriens was finally replaced as a vegetable in Asia by more delicious vegetables. In northeastern India, it is utilized as a special vegetable for nourishment.
In the mid-1980s, the ladies of the World Neighbors' advancement program in El Rosario, Honduras, utilized M. pruriens as substitutes for wheat flour, espresso, and cocoa (Lim 2012) and created 22 formulas that were modest, simple to plan, profoundly dietary, and with locally accessible ingredients. The program reported, different advantages, the positive effect of M. pruriens construct nutrichocolate on the milk production of nursing mothers whose breastfed babies progressed in two months from having second-degree malnutrition to no malnutrition at all (Carew and Gernat 2006). In Mexico, M. pruriens has customarily been cooked and ground to make Nescafé, the principle espresso in a lot of Central America. However, worries about the pharmacological properties of compounds in M. pruriens have been relieved by more extensive utilization of M. pruriens as a nourishment source.
It is not clear if ingested l-Dopa accumulates in the tissues of monogastric animals consumed by people. However, they do not appear to gather in ruminant tissues (Baloyi et al. 2008). They nourished 40 Rambouillet whether sheep on M. pruriens or soybean diets and demonstrated that muscle l-Dopa concentrations of all the sheep were deficient and inside the typical range (< 5 ng l-Dopa/g), showing that ingestion of M. pruriensl-Dopa did not collect in the creatures' muscle tissue contraction. The creators presumed that meat items from sheep fed M. pruriens beans containing around 2% l-Dopa 15 h previous to slaughter was all right for human utilization. Equivalent examinations are required for monogastric animals (Jorge et al. 2007).
Antinutritional and toxic properties
Antinutritional property in M. pruriens beans is mainly due to the presence of L-Dopa and amylase inhibitors. M. pruriens is likewise rich in indolic alkaloids, saponins, and sterols (Manyam et al. 2004). The thread-like hairs of the seed sheath contain the phytochemical mucunain, which causes skin irritation and tingling properties. This extreme tingling effect is just because of the signaling in nerve cells, which had been found in M. pruriens units (Szabo 2003). Because of their regular indole ring structure and appealing properties, M. pruriens has additionally been of concern due to the presence of indolic alkaloids. Semisynthetic indoles, for example, neurotransmitter serotonin, which is an alkaloid, have been identified in different parts of the plant (Daxenbichler et al. 1972). In M. pruriens roots, stems, leaves, and bean, low convergences of estimated alkaloids are roughly reported to be 0.001%. Serotonin was found somewhere in crispy leaves and stemmed around ~ 0.001% but not in the bean. Be that as it may, broke down roots, cases, stems, leaves, and beans for different indole alkylamines and revealed organic compound indoles which have to be available at about weight 0.0001%; these levels have been lower than that beforehand estimated. Numerous herbs contain serotonin, including ordinarily eaten natural products, for example, apples (17 mg/g) and bananas (15 mg/g). By examination, these elevations are substantially higher than those revealed in M. pruriens bean (Szabo and Tebbett 2002). The majority of tryptamine which is a monoamine alkaloid subordinates which are additionally described by reduced circumstances and quick fringe digestion; in this manner, the nearness of low-level indole alkylamines is probably not going to influence the utilization of M. pruriens as a nourishment and food source (Ngatchic et al. 2013).
Detoxification of Mucuna pruriens
Preparing strategies have been produced to encourage supplemental use of many sustenance grains and vegetables for both human and animal utilization. Certain techniques can fulfill the need of detoxifying the M. pruriens bean by diminishing the l-Dopa fixation to a safe level while keeping up its substantial advantages (Fig. 6) (Polanowska et al. 2019). A portion of the preparation strategies utilized with M. pruriens incorporates absorbing water, acids, and different cooking techniques, including dry warming (simmering), bubbling, and additionally sprouting or aging (Bressani 2002).
Fig. 6.
Steps involved in detoxification of Mucuna pruriens
Thermal preparing strategies incorporate cooking beans at atmospheric pressure, without or with previous soaking in water, which regularly decreases cooking time. These techniques can lessen l-Dopa concentration. Cooking builds dietary fiber fixation from roughly 19–26%, and the fiber traps protein and, therefore, likely makes it unavailable. Cooking also initiates losses of vitamins (25–30%) and minerals (10–15%). Even though several thermal processing methods are effective in reducing l-Dopa levels and rendering the bean safe for monogastric utilization, applying sufficient heat requires energy, time, and expense (Emenalom and Udedibie 2005). Many energy sources for rapidly heating M. pruriens would be costly or unavailable in developing countries where M. pruriens consumption is likely to be most beneficial. Therefore, it is important to examine different methods.
Conclusion and future perspectives
In conclusion, Mucuna pruriens exhibits remarkable food and pharmaceutics application, which make it an excellent functional product. Till now, various researchers are exploring valuable components of this legume to a large extent. Because this legume is an excellent source of vital macromolecules, protein isolates with a higher biological value of this legume could be a better replacement for soy protein isolates. Apart from macromolecules, Mucuna beans are an excellent source of micronutrient. Therefore, further research on micronutrients of this legume could provide a valuable source of micronutrients for every age group of the population. The presence of bioactive compounds such as polyphenols and tannins indicates the variation in the component of this herb. These variations occur due to differences in topographical area, atmospheric condition, yield, preparing system, and some different components. Therefore, there is a need to explore the phytochemical qualities of Mucuna beans that can be used to help animal and human nutrition and health benefits as well as the environment through the use of these compounds as natural weed and pest control management. Hence, more clinical examinations should be done to explore its exact potential. Apart from these, Mucuna beans have been effective against aging, rheumatoid, arthritis, diabetes, male infertility, and nervous disorders. Hence there is a need for further research to know about the precise mechanism about how Mucuna beans have been effective against these diseases and shortly can be utilized for designing and developing effective therapeutics for these diseases. Collectively, the investigations referred to in this review propose that this plant has numerous restorative values. So, we can state that it has been demonstrated as an important medication because of its multidirectional effects.
Acknowledgements
The authors gratefully acknowledge Shoolini University, Solan, Himachal Pradesh, India.
Author contributions
All the authors have contributed equally to the manuscript.
Compliance with ethical standards
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Ethical statement
The article does not include any ethical study.
Contributor Information
Ruhi Pathania, Email: ruhipathania6696@gmail.com.
Prince Chawla, Email: princefoodtech@gmail.com.
Huma Khan, Email: humakhan309@gmail.com.
Ravinder Kaushik, Email: ravinder_foodtech2007@rediffmail.com.
Mohammed Azhar Khan, Email: mk.azhar1@gmail.com.
References
- Abushouk AI, Negida A, Ahmed H, Abdel-Daim MM. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: future applications in Parkinson’s disease. Biomed Pharmacother. 2017;85:635–645. doi: 10.1016/j.biopha.2016.11.074. [DOI] [PubMed] [Google Scholar]
- Adebowale KO, Lawal OS. Foaming, gelation and electrophoretic characteristics of mucuna bean (Mucuna pruriens) protein concentrates. Food Chem. 2003;83:237–246. [Google Scholar]
- Aitawade MM, Yadav SR. Mucuna sanjappae, a new species from the north-Western Ghats, India. Kew Bull. 2012;67:539–543. [Google Scholar]
- Akhondzadeh S, Jafari S, Raisi F, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Aware C, Patil R, Gaikwad S, Yadav S, Bapat V, Jadhav J. Evaluation of l-dopa, proximate composition with in vitro anti-inflammatory and antioxidant activity of Mucuna macrocarpa beans: a future drug for Parkinson treatment. Asian Pac J Trop Med. 2017;7(12):1097–1106. [Google Scholar]
- Bayoli JJ, Ngongoni NT, Hamudikuwanda H. Chemical composition and ruminal degradability of cowpea and silver leaf desmodium legumes harvested at different stage of maturity. Trop Subtrop Agroecosyst. 2008;8:1–11. [Google Scholar]
- Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia. 2008;79:539–543. doi: 10.1016/j.fitote.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Boadu AA, Asase A (2017) Documentation of herbal medicines used for the treatment and management of human diseases by some communities in southern Ghana. Evid Based Complement Alternat Med 2017:3043061 [DOI] [PMC free article] [PubMed]
- Bressani R. Factors influencing nutritive value in food legumes, Mucuna compared to other grain legumes Food and feed mucuna: current use and the way forward. Proceedings of an international work shop on food and feed from mucuna: current uses and the way forward. Tegucigalpa Honduras. 2002;2000:164–188. [Google Scholar]
- Capo-chichi LJ, Eilitta M, Carsky RJ, Gilbert RA, Maasdorp B. Effect of genotype and environment on l-dopa concentration in Mucunas (Mucuna sp.) Seeds. Trop Subtrop Agroecosyst. 2003;1:319–328. [Google Scholar]
- Carew LB, Gernat AG. Use of velvet beans, Mucuna spp., as a feed ingredient for poultry: a review. Worlds Poult Sci J. 2006;62:131–144. [Google Scholar]
- Champatisingh D, Sahu PK, Pal A, Nanda GS. Anticataleptic and antiepileptic activity of ethanolic extract of leaves of Mucuna pruriens: a study on role of dopaminergic system in epilepsy in albino rats. Indian J Pharmacol. 2011;43:197. doi: 10.4103/0253-7613.77368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan NS, Sharma V, Dixit VK, Thakur M (2014) A review on plants used for improvement of sexual performance and virility. BioMed research international 2014:868062 [DOI] [PMC free article] [PubMed]
- Chawla P, Bhandari L, Sadh PK, Kaushik R. Impact of solid-state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black-eyed pea (Vigna unguiculata) Seed Flour. Cereal Chem. 2017;94(3):437–442. [Google Scholar]
- Chikagwa-Malunga SK, Adesogan AT, Szabo NJ, et al. Nutritional characterization of Mucuna pruriens: 3. Effect of replacing soybean meal with Mucuna on intake, digestibility, N balance and microbial protein synthesis in sheep. Anim Feed Sci Technol. 2009;148:107–123. [Google Scholar]
- Cilia R, Laguna J, Cassani E, Cereda E, Pozzi NG, Isaias IU, Contin M, Barichella M, Pezzoli G. Mucuna pruriens in Parkinson disease: a double-blind, randomized, controlled, crossover study. Neurology. 2017;89(5):432–438. doi: 10.1212/WNL.0000000000004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar PA, Shabir A, Parray G, Jafri M. Concept of catalepsy (jamood/shakhoos) in Greeko-Arab medicine: a review. Int J Pharma Sci. 2012;2:26–31. [Google Scholar]
- Daxenbichler ME, VanEtten CH, Earle FR, Tallent WH. 1-Dopa recovery from Mucuna seed. J Agric food chem. 1972;20:1046–1048. doi: 10.1021/jf60183a002. [DOI] [PubMed] [Google Scholar]
- Diallo OK, Kante S, Myhrman R et al (2000) Increasing farmer adoption of Mucuna pruriens as human food and animal feed in the Republic of Guinea. In: Proceedings of international workshop on food and feed from MUCUNA. Tegucigalpa, 60–72
- Divya BJ, Suman B, Venkataswamy M, ThyagaRaju K (2017) The traditional uses and pharmacological activities of Mucuna pruriens (L.) DC: a comprehensive review Indo Am j pharm 7(01)
- Duke JA. Legume species. In handbook of legumes of world economic importance. Boston: Springer; 1981. [Google Scholar]
- Eilitta M, Carsky RJ. Efforts to improve the potential of Mucuna as a food and feed crop: background to the workshop. Trop Subtrop Agroecosyst. 2003;1:47–55. [Google Scholar]
- Ekanem AP, Obiekezie A, Kloas W, Knopf K. Effects of crude extracts of Mucuna pruriens (Fabaceae) and Carica papaya (Caricaceae) against the protozoan fish parasite Ichthyophthirius multifiliis. J Parasitol Res. 2004;92:361–366. doi: 10.1007/s00436-003-1038-8. [DOI] [PubMed] [Google Scholar]
- Emenalom OO, Udedibie AB. Evaluation of different heat processing methods on the nutritive value of Mucuna pruriens (Velvet Bean) seed meals for broilers. Int J Poult Sci. 2005;4:543–548. [Google Scholar]
- Ezeagu IE, Maziya-Dixon B, Tarawali G. Seed characteristics and nutrient and antinutrient composition of 12 Mucuna accessions from Nigeria. Trop Subtrop Agroecosyst. 2003;1:129–139. [Google Scholar]
- Flores B, Eilitta M, Myhrman R, Carew LB, Carsky R (2002) Food and feed from mucuna: current uses and the way forward; proceedings. In International Workshop on Food and Feed from Mucuna: Current Uses and the Way Forward 26-29 Abr 2000 Tegucigalpa (Honduras) 2002 (No. 633.3 I61). CIDICCO, Tegucigalpa (Honduras) IITA, Cotonou (República de Benin) World Hunger Research Center, Elgin, IL (EUA). Judson College.
- Flores L, Esnaola MA, Myhrman R (2002) Growth of pigs fed diets with Mucuna bean flour (Mucuna pruriens) compared to soybean meal. In: Mucuna Workshop held in April 26. pp 26–29
- Fung SY, Tan NH, Sim SM, et al. Mucuna pruriens Linn. Seed extract pretreatment protects against cardiorespiratory and neuromuscular depressant effects of Naja sputatrix (Javan spitting cobra) venom in rats. Indian J Exp Biol. 2011;49:254–259. [PubMed] [Google Scholar]
- Fung SY, Tan NH, Sim SM. Protective effects of Mucuna pruriens seed extract pretreatment against cardiovascular and respiratory depressant effects of Calloselasma rhodostoma (Malayan pit viper) venom in rats. Trop Biomed. 2010;27:366–372. [PubMed] [Google Scholar]
- Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76:233–238. doi: 10.1016/s0378-8741(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Guerranti R, Aguiyi JC, Neri S, et al. Proteins from Mucuna pruriens and enzymes from Echis carinatus Venom characterization and cross-reactions. J Biol Chem. 2002;277:17072–17078. doi: 10.1074/jbc.M201387200. [DOI] [PubMed] [Google Scholar]
- Guerranti R, Aguiyi JC, Ogueli IG, et al. Protection of Mucuna pruriens seeds against Echis carinatus venom is exerted through a multiform glycoprotein whose oligosaccharide chains are functional in this role. Biochem Biophys Res Commun. 2004;323:484–490. doi: 10.1016/j.bbrc.2004.08.122. [DOI] [PubMed] [Google Scholar]
- Guerranti R, Ogueli IG, Bertocci E, et al. Proteomic analysis of the pathophysiological process involved in the antisnake venom effect of Mucuna pruriens extract. Proteomics. 2008;8:402–412. doi: 10.1002/pmic.200700265. [DOI] [PubMed] [Google Scholar]
- Gurumoorthi P, Senthil Kumar S, Vadivel V, Janardhanan K. Studies on agrobotanical charecters of different accessions of velvet bean collected from Western Ghats, south India. Trop Subtrop Agroecosyst. 2003;2:105–115. [Google Scholar]
- Houghton PJ, Skari KP. The effect on blood clotting of some west African plants used against snakebite. J Ethnopharmacol. 1994;44:99–108. doi: 10.1016/0378-8741(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Jalalpure SS, Alagawadi KR, Mahajanashetti CS, Shah BN, Singh V, Patil JK. In vitro anthelmintic property of various seed oils against Pheritima posthuma. Indian J Pharm Sci. 2007;69:158–160. [Google Scholar]
- Johnson SL, Park HY, DaSilva NA, Vattem DA, Ma H, Seeram NP. Levodopa-reduced Mucuna pruriens seed extract shows neuroprotective effects against Parkinson’s disease in murine microglia and human neuroblastoma cells, Caenorhabditis elegans, and Drosophila melanogaster. Nutrients. 2018;10(9):1139. doi: 10.3390/nu10091139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge MA, Eilitta M, Maasdorp PFJ, et al. Mucuna species: recent advances in application of biotechnology. Fruit, vegetable and cereal science and biotechnology. Glob Sci Books. 2007;1:80–94. [Google Scholar]
- Kasture S, Mohan M, Kasture V. Mucuna pruriens seeds in treatment of Parkinson’s disease: pharmacological review. Orient Pharm Exp Med. 2013;13:165–174. [Google Scholar]
- Katzenschlager R, Evans A, Manson A, et al. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672–1677. doi: 10.1136/jnnp.2003.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha C, Thangamani C. Amazing bean Mucuna pruriens: a comprehensive review. J Med Plants Res. 2014;8:138–143. [Google Scholar]
- Kay DE. Hyacinth bean-food legumes. Ind Crops Prod. 1979;3:184–196. [Google Scholar]
- Kumar A, Gupta C, Nair DT, Salunke DM. MP-4 contributes to snake venom neutralization by Mucuna pruriens seeds through an indirect antibody-mediated mechanism. J Biol Chem. 2016;291:11373–11384. doi: 10.1074/jbc.M115.699173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DS, Muthu AK, Smith AA, Manavalan R. Free radical scavenging activity of various extracts of whole plant of Mucuna pruriens (Linn): an in vitro evaluation. J Pharm Res. 2010;3:718–721. [Google Scholar]
- Lange KC, Olcott DD, Miller JE, et al. Effect of sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental Haemonchus contortus infections in lambs. Vet Parasitol. 2006;141:273–278. doi: 10.1016/j.vetpar.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leelambika M, Mahesh S, Jaheer M, Sathyanarayana N. Comparative evaluation of genetic diversity among Indian Mucuna species using morphometric, biochemical and molecular approaches. World J Agric Sci. 2010;6:568–578. [Google Scholar]
- Lim TK (2012) Mucuna pruriens. In Edible medicinal and non-medicinal plants. Springer, Dordrecht, pp 779–797
- Liu W, Ma H, DaSilva NA, et al. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem Int. 2016;100:164–177. doi: 10.1016/j.neuint.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majekodunmi SO, Oyagbemi AA, Umukoro S, Odeku OA. Evaluation of the anti-diabetic properties of Mucuna pruriens seed extract. Asian Pac J Trop Med. 2011;4(8):632–636. doi: 10.1016/S1995-7645(11)60161-2. [DOI] [PubMed] [Google Scholar]
- Manyam BV, Dhanasekaran M, Hare TA. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2004;18:706–712. doi: 10.1002/ptr.1514. [DOI] [PubMed] [Google Scholar]
- Meenatchisundaram S, Michael A. Antitoxin activity of Mucuna pruriens aqueous extracts against Cobra and Krait venom by in vivo and in vitro methods. Int J PharmTech Res. 2010;2:870–874. [Google Scholar]
- Mesko CA (2002) US Patent No. 6,340,474. US Patent and Trademark Office, Washington, DC U.S.
- Miller JE, Horohov DW. Immunological aspects of nematode parasite control in sheep 1. J Anim Sci. 2006;84:124–132. doi: 10.2527/2006.8413_supple124x. [DOI] [PubMed] [Google Scholar]
- Misra L, Wagner H. Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry. 2004;65:2565–2567. doi: 10.1016/j.phytochem.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Misra L, Wagner H. Lipid derivatives from Mucuna pruriens seeds. Indian J Chem. 2006;45:801–804. [Google Scholar]
- Misra L, Wagner H. Extraction of bioactive principles from Mucuna pruriens seeds. Indian J Biochem Biophys. 2007;44:56–60. [PubMed] [Google Scholar]
- Mohan VR, Janardhanan K. Chemical analysis and nutritional assessment of lesser known pulses of the genus, Mucuna. Food Chem. 1995;52:275–280. [Google Scholar]
- Muralia S, Pathak AK (2003) Database of medicinal plant used in ayurveda. Medicinal and aromatic plants cultivation and uses. pp 185–187
- Murugan M, Mohan VR. Antibacterial activity of Mucuna pruriens (L.) Dc. var. pruriens—an ethnomedicinal plant. Sci Res Rep. 2011;1:69–72. [Google Scholar]
- Muthu K, Krishnamoorthy P. Evaluation of androgenic activity of Mucuna pruriens in male rats. Afr J Biotechnol. 2011;10(66):15017–15019. [Google Scholar]
- Nagashayana N, Sankarankutty P, Nampoothiri MR, Mohan PK, Mohanakumar KP. Association of l-DOPA with recovery following Ayurveda medication in Parkinson’s disease. J Neurol Sci. 2000;176:124–127. doi: 10.1016/s0022-510x(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Nagashima Y, Kondo T, Sakata M, Koh J, Ito H. Effects of soybean ingestion on pharmacokinetics of levodopa and motor symptoms of Parkinson's disease—in relation to the effects of Mucuna pruriens. J Neurol Sci. 2016;361:229–234. doi: 10.1016/j.jns.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Narayanan N, Ravichandran N. Review on “mucuna”-the wonder plant. Int J Pharm Sci Rev Res. 2012;17:86–93. [Google Scholar]
- Neta F, Da Costa I, Lima F, Fernandes L, Cavalcanti J, Freire M, Lucena ED, Do Rêgo AM, De Azevedo E, Guzen F (2018) Effects of Mucuna pruriens (L.) supplementation on experimental models of Parkinson's disease: a systematic review. Pharmacogn Rev 12(23):78
- Ngatchic JT, Sokeng SD, Njintang NY, et al. Evaluation of some selected blood parameters and histopathology of liver and kidney of rats fed protein-substituted mucuna flour and derived protein rich product. Food Chem Toxicol. 2013;57:46–53. doi: 10.1016/j.fct.2013.02.045. [DOI] [PubMed] [Google Scholar]
- Pathania R, Kaushik R, Khan MA. Essential oil nanoemulsions and their antimicrobial and food applications. Curr Res Nutr Food Sci J. 2018;6(3):626–643. [Google Scholar]
- Patil RR, Gholave AR, Jadhav JP, Yadav SR, Bapat VA. Mucuna sanjappae Aitawade et Yadav: a new species of Mucuna with promising yield of anti-Parkinson’s drug L-DOPA. Genet Resour Crop Evol. 2015;62:155–162. [Google Scholar]
- Polanowska K, Łukasik RM, Kuligowski M, Nowak J. Development of a sustainable, simple, and robust method for efficient l-DOPA extraction. Molecules. 2019;24(12):2325. doi: 10.3390/molecules24122325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugalenthi M, Vadivel V. A non-protein toxic amino acid in Mucuna pruriens seeds. Food. 2007;1:322–343. [Google Scholar]
- Pugalenthi M, Vadivel V, Siddhuraju P. Alternative food/feed perspectives of an underutilized legume Mucuna pruriens var utilis—a review. Plant Foods Hum Nutr. 2005;60:201. doi: 10.1007/s11130-005-8620-4. [DOI] [PubMed] [Google Scholar]
- Pulikkalpura H, Kurup R, Mathew PJ, Baby S. Levodopa in Mucuna pruriens and its degradation. Sci Rep. 2015;5:11078. doi: 10.1038/srep11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshwar Y, Gupta M, Mazumder UK. Antitumor activity and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich ascites carcinoma in Swiss albino mice. Iran J Pharm Ther. 2005;4:46–50. [Google Scholar]
- Rakesh B, Praveen N (2020) Chapter-10 biotechnological approaches for the production of l-DOPA: a novel and potent anti-Parkinson’s Drug from Mucuna pruriens (L.) DC. Chief Editor: 179
- Ravindran V, Ravindran G. Nutritional and anti-nutritional characteristics of mucuna (Mucuna utilis) bean seeds. J Sci Food Agric. 1988;46:71–79. [Google Scholar]
- Rijntjes M (2019) Knowing your beans in Parkinson’s disease: a critical assessment of current knowledge about different beans and their compounds in the treatment of parkinson’s disease and in animal models. Parkinson’s Disease 2019:1349509 [DOI] [PMC free article] [PubMed]
- Sadh PK, Chawla P, Duhan JS. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018;22:113–120. [Google Scholar]
- Sathiyanarayanan L, Arulmozhi S. Mucuna pruriens Linn.-A comprehensive review. Pharmacogn Rev. 2007;1:157. [Google Scholar]
- Sharma PC, Yelne MB, Dennis TJ. Database on medicinal plants used in Ayurveda, vol 3. New Delhi: CCRAS; 2005. pp. 130–131. [Google Scholar]
- Sharma S, Kaushik R, Sharma P, et al. Antimicrobial activity of herbs against Yersinia enterocolitica and mixed microflora. Ann Univ Dunarea de Jos of Galati Fascicle VI Food Technol. 2016;40(2):118–134. [Google Scholar]
- Shukla KK, Mahdi AA, Ahmad MK, et al. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. J Evid Based Complementary Altern Med. 2010;7:137–144. doi: 10.1093/ecam/nem171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla KK, Mahdi AA, Ahmad MK, Shankhwar SN, Singh R, Jaiswar SP. Mucuna pruriens improves male fertility by its action on the hypothalamus–pituitary–gonadal axis. Fertil Steril. 2009;92:1934–1940. doi: 10.1016/j.fertnstert.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K, Makkar HP. Studies on the nutritional composition and antinutritional factors of three different germplasm seed materials of an under-utilized tropical legume, Mucuna pruriens Var. Utilis J Agric Food Chem. 2000;48:6048–6060. doi: 10.1021/jf0006630. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Vijayakumari K, Janardhanan K. Chemical composition and protein quality of the little-known legume, velvet bean (Mucuna pruriens (L.) DC.) J Agric Food Chem. 1996;44:2636–2641. [Google Scholar]
- Sridhar KR, Bhat R. Agrobotanical, nutritional and bioactive potential of unconventional legume–Mucuna. Livest Res Rural Dev. 2007;19:126–130. [Google Scholar]
- St Laurent L, Livesey J, Arnason JT, Bruneau A (2002) Variation in l-Dopa concentration in accessions of Mucuna pruriens (L.) DC and in Mucuna brachycarpa Rech. In: Food and feed from Mucuna: current uses and the way forward, Proceedings of an International Workshop, CIDICCO, CIEPCA, World Hunger Research Center, Tegucigalpa, Honduras 2002, pp 352–373
- Suresh S, Prithiviraj E, Prakash S. Dose-and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122:497–501. doi: 10.1016/j.jep.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Szabo NJ. Indolealkylamines in Mucuna species. Trop Subtrop Agroecosyst. 2003;1:295–307. [Google Scholar]
- Szabo NJ, Tebbett IR (2002) The chemistry and toxicity of Mucuna species. In: Flores BM, Eilitta M, Myhrman R, Carew LB, Carsky RJ (eds) Food and feed from Mucuna: current uses and the way forward. Proceedings of the Centro Internacional de Informacion sobre Cultivos de Cobertura (CIDICCO) Workshop, Tegucigalpa, Honduras, pp 120–141
- Tan NH, Fung SY, Sim SM, et al. The protective effect of Mucuna pruriens seeds against snake venom poisoning. J Ethnopharmacol. 2009;123:356–358. doi: 10.1016/j.jep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Taylor L. The healing power of rainforest herbs: a guide to understanding and using herbal medicinals. New York: Square One Publishers; 2005. [Google Scholar]
- Tripathi YB, Upadhyay AK. Effect of the alcohol extract of the seeds of Mucuna pruriens on free radicals and oxidative stress in albino rats. Phytother Res Int J Devot Pharmacol Toxicol Eval Nat Prod Deriv. 2002;16:534–538. doi: 10.1002/ptr.962. [DOI] [PubMed] [Google Scholar]
- Vaidya RA, Sheth AR, Aloorkar SD, et al. The inhibitory effect of the cowhage plant-Mucuna pruriens-and l-dopa on chlorpromazine-induced hyperprolactinaemia in man. Neurol India. 1978;26:177–178. [PubMed] [Google Scholar]
- Verma SC, Vashishth E, Singh R, Pant P, Padhi MM. A review on phytochemistry and pharmacological activity of parts of Mucuna Pruriens used as an ayurvedic medicine. World J Pharm Res. 2014;3:138–158. [Google Scholar]
- Wanjekeche E, Wakasa V, Mureithi JG. Effect of alkali, acid and germination on nutritional composition and antinutritional factors of Mucuna (Mucuna pruriens) Trop Subtrop Agroecosyst. 2003;1:183–192. [Google Scholar]
- Wilmot-Dear CM. A revision of Mucuna (Leguminosae-Phaseoleae) in China and Japan. Kew Bull. 1984;39:23–65. [Google Scholar]