Abstract
The unique physicochemical characteristics of nanoparticles have recently gained increasing attention in a diverse set of applications, particularly in the biomedical field. However, concerns about the potential toxicological effects of nanoparticles remain, as they have a higher tendency to generate excessive amounts of reactive oxygen species (ROS). Due to the strong oxidation potential, the excess ROS induced by nanoparticles can result in the damage of biomolecules and organelle structures and lead to protein oxidative carbonylation, lipid peroxidation, DNA/RNA breakage, and membrane structure destruction, which further cause necrosis, apoptosis, or even mutagenesis. This review aims to give a summary of the mechanisms and responsible for ROS generation by nanoparticles at the cellular level and provide insights into the mechanics of ROS-mediated biotoxicity. We summarize the literature on nanoparticle toxicity and suggest strategies to optimize nanoparticles for biomedical applications.
Keywords: Reactive oxygen species, Nanoparticles, Oxidative stress, Biotoxicity
Introduction
Nanoparticles (NPs) are a class of novel synthetic particles with dimensions < 100 nm. Depending on their shape and size, the distinct physical and chemical characteristics give NPs different functions. NPs are widely used in many consumer products, including textiles, cosmetics, water purification, and food packaging [1, 2]. They are also used in the engineering of photocatalysts, energy, and optoelectronics [3–6].
In particular, NPs have become a favored material in biomedical materials and are widely used in biosensors, siRNAs delivery, targeted gene knockdown, drug delivery, and in bio-filling medical materials [7–11]. Further uses of NPs are still being discovered. For example, Duan et al. [12] showed that Fe3O4-polyethylene glycol-polyamide-amine-matrix metalloproteinase2@ chlorin e6 (Fe3O4-PEG-G5-MMP2@Ce6) nanoprobes significantly inhibited gastric tumor growth. In another case, pDNA-polyethylenimine CeO nanoparticles (pDNA-PEI-CeO NPs) could induce more fibrosarcoma cell apoptosis [13]. Furthermore, hollow silica-Fe-polyethylene glycol-human epidermal growth factor receptor 2 nanoparticles (HS-Fe-PEG-HER2 NPs) could selectively bind tumor cells and were used as imaging agents to distinguish normal tissue from cancerous cells [14]. Finally, silver nanoparticles (Ag NPs) serve as nano-antibiotics, which efficiently combat resistant bacterial biofilm-associated infections [15].
Despite the potential for positive applications of NPs in various fields, an increasing number of studies have indicated their adverse effects on organisms [16, 17] and cells following NP exposure [18, 19]. The toxic potential of NPs is dependent on their size and shape, which determined their propensity to induce the generation of reactive oxygen species (ROS) [20, 21]. The excess generation ROS may induce an array of physiopathologic outcomes, including genotoxicity, apoptosis, necrosis, inflammation, fibrosis, metaplasia, hypertrophy, and carcinogenesis [18, 22, 23]. The toxicity of NPs has also been shown to enhance the expression of pro-inflammatory cytokines and activate inflammatory cells, such as macrophages, which further increase the generation of ROS [23, 24]. The increased generation of ROS following exposure to NPs has been also shown to induce the modulation of cellular functions, with fatal results in some cases [17, 23, 25]. In this review, we discuss the main mechanisms underlying the ROS bursts induced by NPs, analyze the primary reasons for the cytotoxicity of NPs, and summarize the potential pathogenic effects of NPs. Our present review provides overwhelming evidence that the over-production of ROS is the major cause of the biotoxicity of NPs. Therefore, novel research should aim to reduce the cytotoxicity of NPs by designing NPs which induce low ROS production.
The Application of NPs in the Biomedical Field
NPs have been used in a variety of medical applications, and several novel NPs exhibit properties which are promising for their use in novel biomedical materials. As summarized in Table 1, Nano-C60 can be used as an anticancer agent, which inhibits cancer cell proliferation, both in vivo and in vitro [26]. ZnO NPs have been used as fillers in orthopedic and dental implants [38]. TiO2 can be used as antibacterial agents, in air and water purification, and for dental prostheses [52–54]. Davaeifar et al. reported that a phycocyanin-ZnO nanorod could protect the cell by decreasing endogenous ROS generation [68]. Pacurari et al. pointed out that SWCNTs could be applied as a clinical diagnostic agent and as bioengineering materials [88]. Beyond that, numerous NPs can be used as antimicrobial agents, which kill bacteria by inducing ROS bursts (Table 1).
Table 1.
NPs played their biologic role by inducing ROS burst in cells
| No. | Type of NPs | Potential applications | ROS | Dose | Molecule mechanism of biotoxicity | References |
|---|---|---|---|---|---|---|
| 1 | Nano-C60 | Antibacterial agents, Anticancer agents. | ↑ | 1 μg/mL | Necrosis, apoptosis, autophagy, DNA fragmentation, cell membrane damage. | [26–28] |
| 2 | Carbon-based nanodots | Antibacterial agents. | ↑ | > 1 mg/mL | Oxidize the phospholipids, destroy the membranes. | [29] |
| 3 | Ag | Antibacterial agents. | ↑ | 150 μg/mL | Intracellular oxidation, membrane potential variation, membrane permeability disruption, DNA damage, genomic instability, cell cycle arrest, cellular contents release, inactivate proteins, autophagy, disturb electron transfer process. | [30–36] |
| 4 | Gold-silver nanocage | Antibacterial agents. | ↑ | 2.5 μg/mL | Destruction of cell membrane, apoptosis. | [37] |
| 5 | ZnO | Wastewater purification, antibacterial agents, antitumor agents, fillers in orthopedic, and dental implants. | ↑ | 20 μg/mL | Disintegration the cell membrane, inhibition enzyme activity, inhibition DNA synthesis, DNA damage, interruption of energy transduction, mitochondrial damage, apoptosis, intracellular outflow, mitotic arrest, carcinogenic. | [38–45] |
| 6 | Gold | Anticancer agents, antibacterial agents. | ↑ | 20 μM | Collapse membrane potential, inhibit ATPase activities, inhibit the subunit of ribosome. | [46, 47] |
| 7 | MgO | Antibacterial agents, anticancer agents. | ↑ | 100 mg/mL | Lipid peroxidation, apoptosis. | [48, 49] |
| 8 | Fe3O4 | Antibacterial agents. | ↑ | 32 μg/mL | DNA cleavage. | [50] |
| 9 | CdSe | Antibacterial agents. | ↑ | Inhibition proliferation. | [51] | |
| 10 | TiO2 | Antimicrobial agents, air and water purification, dental prosthesis. | ↑ | 10 μg/mL | Loss respiratory activity, interfere oxidative phosphorylation, DNA lesions, mitochondrial dysfunction, carcinogenicity. | [52–57] |
| 11 | Al2O3 | Antibacterial agents, cross-linker. | ↑ | 0.16 mg/L | DNA damage, mutagenesis. | [58, 59] |
| 12 | VO2 | Antimicrobial agents. | ↑ | 2.5 μg/mL | Mitochondrial dysfunction apoptosis. | [60, 61] |
| 13 | V2O5 | Antimicrobial agents. | ↑ | 20 mg/L | Interruption mitochondrial function. | [62, 63] |
| 14 | PCAE | Antimicrobial agents. | ↑ | 30 μg/mL | Membrane damages. | [64] |
| 15 | Co-ZnO | Antimicrobial agents. | ↑ | 20 μg/mL | Low toxicity. | [65] |
| 16 | Hybrid Gold/Polymer | Antimicrobial agents. | Unknown | Unknown | No cytotoxicity. | [66] |
| 17 | Ag-Fe NPs | Antimicrobial agents. | ↑ | 100 mg/L | LDH release, disruption membrane integrity. | [67] |
| 18 | Phycocyanin-ZnO nanorod | Protect cell. | ↓ | 50 μg/mL | Decrease in ROS production. | [68] |
| 19 | Ag/lyz-Mt | Antimicrobial agents, water disinfection. | ↑ | 160 μg/mL | Damage cell membrane. | [69] |
| 20 | PEGylated ZnO | Antimicrobial agents, biological labeling. | ↑ | 45 ppm | Low cytotoxicity. | [70] |
| 21 | CdS NPs | Antimicrobial agents. | ↑ | 4 μg/mL | Inhibition proper cell septum formation, change morphology, fragment nuclei. | [71] |
| 22 | CdTe | Antimicrobial agents. | ↑ | 0.4 mg/L | Morphological damages, apoptosis, genotoxicity. | [72] |
| 23 | ZnO@APTMS/Cu QDs | Antibacterial agents. | ↑ | 1.4 × 10-4 M | Inhibition proliferation. | [73] |
| 24 | CuO | Antimicrobial agents. | ↑ | 5 mg/L | Increase cell permeability, lipid peroxidation, DNA damage, morphological alterations, mitochondrial dysfunction, interruption ATP synthesis. | [74–76] |
| 25 | Mn3O4 | Antioxidant. | ↓ | 20 ng/μL | Protect biomolecules against ROS. | [77] |
| 26 | PEGylated nanoceria | Antioxidant. | ↓ | 10 μM | Cell protection, radical scavenger. | [78] |
| 27 | CeO2 | Against oxidative damage. | ↓ | 2.5 μg/mL | Suppressed ROS production, protect cells, and tissues. | [79] |
| 28 | AuNPs-rGO-NC | Anticancer agents, antimicrobial agents. | ↑ | 50 μg/mL | Reduction cell activity, | [80] |
| 29 | CONPs | Anticancer agents. | ↑ | 10 μM | DNA damage. | [81] |
| 30 | Graphene | Cancer theotherapy, bioimaging, biosensing. | ↑ | 25 μg/mL | DNA damage, mutagenesis. | [82, 83] |
| 31 | Fe2O3 | Antibacterial agents. | ↑ | 80 μg/mL | DNA damage. | [84] |
| 32 | NiO | Antibacterial agents. | ↑ | 10 mg/L | DNA damage. | [85, 86] |
| 33 | PtAuNRs | Anticancer agents. | ↓ | OD at 0.5 | Induce hyperthermia. | [87] |
| 34 | SWCNTs | Clinical diagnostic agent, bioengineered research. | ↑ | 50 μg/cm2 | DNA damage. | [88] |
| 35 | bsCdS | Anticancer agents. | ↑ | 15 μg/mL | Apoptosis, depletion ATP, DNA damage. | [89] |
| 36 | Ag@OTV | Against H1N1 infection. | ↓ | Unknown | Less cytotoxicity. | [90] |
| 37 | PATA3-C4@CuS | Antibacterial agents. | ↑ | 5.5 μg/mL | Less cytotoxicity. | [91] |
The Mechanisms of Increased ROS Induced by NPs in Cells
ROS are chemically reactive particles that contain oxygen, including hydrogen peroxide (H2O2), reactive superoxide anion radicals (O2-), and hydroxyl radicals (•OH) [92, 93]. ROS are predominantly generated in organelles such as the endoplasmic reticulum (ER), in peroxisomes, and most notably in the mitochondria [94]. During oxidative phosphorylation, oxygen is used for the synthesis of water by the addition of electrons through the mitochondrial electron transport chain (ETC). Some of these electrons are accepted by molecular oxygen to form O2-, which can further transform H2O2 and •OH [93].
In a physiological context, ROS are produced as a natural response to the normal metabolism of oxygen [95] and serve a vital role in various cellular signaling pathways [96, 97]. Dröge and Holmstrom et al. reported that ROS could activate numerous signaling cascades, including the epidermal growth factor (EGF) receptor, the mitogen-activated protein kinase (MAPK) cascades, the transcription factor activator protein-1 (AP-1), and the nuclear factor-KB (NF-κB), and further participated in the process of mammalian growth, proliferation, and differentiation [98, 99]. Further studies showed that ROS also regulated wound repair [100], survival after hypoxia [101], intracellular pH homeostasis [102], and innate immunity [103].
Nevertheless, following exposure to NPs, the intracellular generation of ROS may sharply increase by inducing ROS bursts in cells [20] (Table 1). The main mechanistic explanations for ROS bursts are that metal ions released by NPs promote ROS overexpression by impairing mitochondrial respiration [30, 104].
The metal ions released by NPs have been shown to mix into redox cycling and chemocatalysis via the Fenton reaction [H2O2 + Fe2+ → Fe3+ + HO− + •OH] or Fenton-like reaction [Ag+ H2O2+H+ = Ag+ + •OH + H2O] [23, 105, 106]. The dissociated metal ion (i.e., Ag+) also causes cellular enzyme deactivation, membrane structure disruption [31, 107], disturbed electron-shuttling process [108], depleted redox potential levels, reduced mitochondrial membrane potentials (MMP) [109], and further enhances the accumulation of intracellular ROS. NPs have been also reported to promote the intracellular ROS accumulation by disturbing the electron transfer process [32, 110], increasing the NADP+/NADPH ratio [30], and interfering mitochondrial function [18]. NPs further interfere with the expression of oxidative stress-related genes, such as soxS, soxR, oxyR, and ahpC [58]; antioxidant genes, like sod1 and gpx 1[111, 112]; and the NADPH production-related gene met9 [30]. The instability in the expression of oxidative and antioxidant genes caused by NPs accelerates intracellular ROS accumulation.
Interestingly, increased ROS production has been strongly associated with particular sizes and shapes of NPs [113, 114]. For example, TiO2 NPs contributed to intracellular ROS generation, which led to nucleic acid and protein damage [10]. Liao et al. found that 10 nm TiO2 NPs had higher genotoxicity than other sizes tested and therefore could induce more ROS generation [115]. In another case, Se NPs promoted the production of ROS in cells, and the yield of intracellular ROS was highly associated with the diameter of Se NPs. In this case, a diameter of 81 nm induced more ROS production than other sizes tested [113]. Cho et al. further showed that the shape of NPs strongly affected their capacity to induce ROS production. Day flower-mimicking metallic nanoparticles (D-NP) lead to a significantly higher production of ROS than night flower-mimicking metallic nanoparticles (N-NP), resulting in an enhanced cell killing effect [114] (Fig. 1).
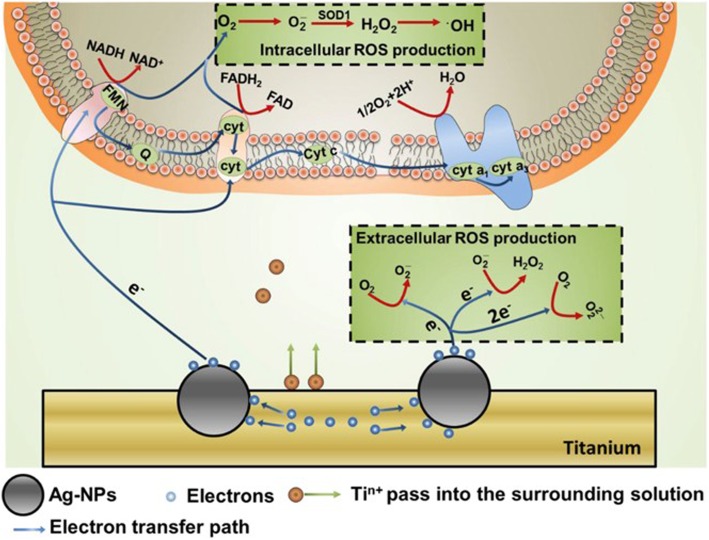
Fig. 1.
The production of ROS induced by NPs in surrounding solution and cells [32]. The electrons generated from NPs could enter into cells and disturb the functions of respiratory chain, then enhance the intracellular ROS production. Electrons also could react with O2 directly and increased the generation of extracellular ROS
NPs can induce intracellular ROS bursts at a very low concentration (showed in Table 1), for example, Nano-C60 at 1 μg/mL can significantly increase cell apoptosis by inducing oxidative stress [26, 27]. Notably, most NPs have a dose-dependent effect, as has been reported for VO2 NPs [60, 61] and CuO NPs [74, 75].
Catastrophic Consequences of NPs on Cells by Increased ROS Production
NPs which enter the cell often have adverse effects on it. The most supported explanation for the cytotoxicity of NPs is that oxidative stress is induced by a ROS burst. ROS bursts caused by NPs have resulted in the oxidative modification of biomacromolecules, in the damage of cellular structures, in the developing drug resistance, in gene mutation, and in carcinogenesis [116, 117]. Furthermore, ROS bursts have altered the normal physiological functions of cells, as in is the case with trigger inflammation, which ultimately blocks cell functions and damages the organism [23, 118, 119]. Generally, NPs are first adsorbed on the cell surface, and then passed through the membrane into the cell, where they induce ROS generation [36]. Due to its strong oxidative potential, ROS is highly stressful to cell [46] and attacks nearly all types of biomolecules in the cell, including carbohydrates, nucleic acids, unsaturated fatty acids, proteins and amino acids, and vitamins [36, 120, 121] (Fig. 2).
Fig. 2.
The crucial role of ROS in the cytotoxicity induced by NPs [33]. The possible cellular events taking place after NPs interact with intracellular systems
ROS Results in Lipid Peroxidate and Membrane Structure Damage
Lipids, especially unsaturated fatty acids, are important intracellular macromolecules, which play key roles in the structure and functioning of the cell membrane. NPs are strongly attracted to the cell membrane, where they can generate ROS and lead to outer membrane lipid peroxidation. The altered fatty acid content of the cell membrane may result in increased cell permeability, which results in the uncontrolled transport of NPs from the extracellular environment into the cytoplasm, where cellular damage may progress further [76, 122].
Intracellular NPs induce the next round of ROS bursts. Overburdened ROS lead to the rupturing of the membranes of organelles, the leakage of the organelles’ contents [52, 123], the inactivation of cell receptors [124], the release of lactate dehydrogenase (LDH), and further irreversible cell damage [125].
ROS Attacks Proteins and Results in Functional Inactivation
ROS attacks the hydrophobic residues of amino acids, contributing to the breakage of peptide bonds and interfering with the function of these proteins [126–128]. Carbonylation is another feature of proteins subjected to oxidative damage [129]. Carbonylated proteins form aggregates that are chemically irreversible and cannot be degraded via proteasomes, leading to the permanent loss of function in these proteins [130, 131]. Gurunathan et al. [132] showed that PtNPs could enhance the generation of ROS and increase carbonylated protein levels, which inhibited osteosarcoma proliferation and contributed to apoptosis. In one case, combustion and friction-derived nanoparticles (CFDNPs) had accumulated in the brain of young adults with Alzheimer’s disease, which likely promoted ROS generation, resulting in protein misfolding, aggregation, and fibrillation [133]. Furthermore, Pelgrift et al. showed that Mg NPs may inhibit gene transcription or damage proteins directly [10].
ROS-Induced Gene Mutation
Nucleic acids, including DNA and RNA, are essential to cell function, growth, and development, and their component nucleotides are vulnerable targets of ROS [134–136]. Due to their low redox potential, ROS can directly react with nucleobases and modify them [137]. For example, ROS could oxidize guanine to 8-oxo-7,8 dihydroguanine (8-oxoG) [138] and adenine to 1,2-dihydro-2-oxoadenine (2-oxoA) [139]. These base modifications lead to DNA damage [140]. Because of their genotoxic potential and their capacity to induce ROS formation [141], NPs significantly induce single- and double-strand DNA breakages [142, 143], chromosome damage, and aneuploid genic events [144].
The increased production of ROS is the main cause of gene miscoding, aneuploidy, polyploidy, and the activation of mutagenesis in cells exposed to NPs [145–148]. Among the nucleotide pools, guanine is the most vulnerable and is easily oxidized to 8-oxoG by ROS [149]. The increased level of 8-oxo-dG in DNA results in the mismatch of DNA bases [150]. Similarly, the incorporation of A:8-oxoG causes an increased rate of G:C > T:A deleterious transversion mutations [151, 152]. The ratio of G:C > T:A transversion to G:C > A:T transition mutation has also been used as an index to quantify the oxidative DNA damage [153].
The generation of ROS induced by NPs resulted in the accumulation of DNA damage, which drives the development of mutagenicity [154], oncogenesis [155], multidrug resistance [156, 157], aging, and immune escape [158]. Jin et al. showed that the overproduction of ROS dramatically increased mutagenesis of DNA-binding transcriptional regulator genes, which resulted in an expedited antibiotic efflux [159], which in turn promotes the multiple-antibiotic resistance of bacteria [34]. Giannoni et al. reported that mitochondrial DNA mutations occurred with increasing intracellular ROS and further damaged the activity of ETC complex I and resulted in mitochondrial dysfunction [160, 161].
DNA damage induced by NPs has been shown to inhibit amino acid synthesis, replication [162], and cause the aberrant accumulation of p53 [163] and Rab51 proteins [82, 142]. DNA damage may also delay or fully arrest the cell [164]. Cells with damaged DNA lose the capacity for growth and proliferation [165] and may eventually result in cell death [166] (Fig. 3).
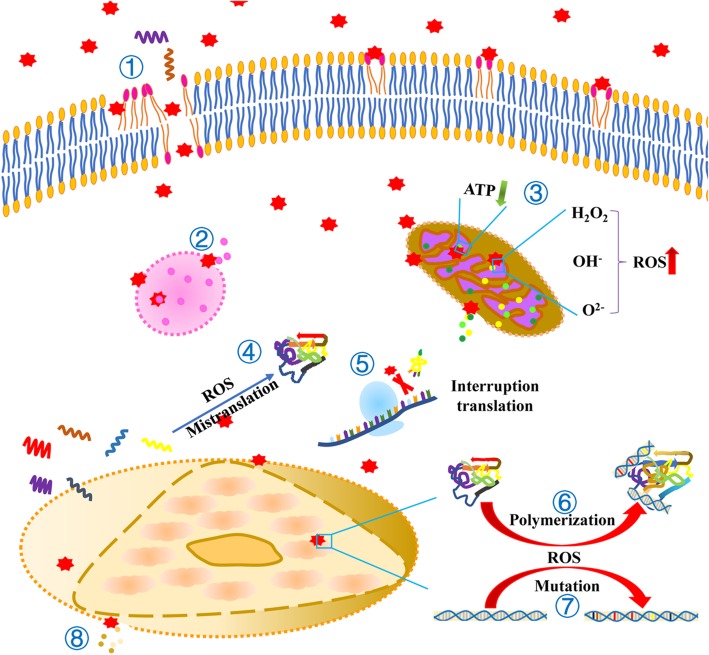
Fig. 3.
Cellular events induced by NPs. ① NPs contribute to the destruction of the cell membrane and to lipid peroxidation. ② The lysosomal membrane is destroyed by NPs and results in the release of their contents. ③ The mitochondrial membrane is damaged by NPs, leading to content release. NPs reduce the generation of ATP and increase the production of ROS. ④ The ROS induced by NPs results in the mistranslation of RNA. ⑤ NPs prevent the binding of tRNA to the ribosome. ⑥ The ROS induced by NPs result in the polymerization of proteins and DNA. ⑦ The ROS induced by NPs leads to DNA mutations ⑧ The nuclear membrane is destroyed by NPs, resulting in the release of its contents
Increased Production of ROS Induces Cell Damage and Disease Occurrence
NP cytotoxicity is associated with oxidative stress, endogenous ROS production, and the depletion of the intracellular antioxidant pools. The increased oxidative stress leads to oxidative damage to biomacromolecules, which further affects the normal functioning of the cell and contributes to the occurrence and development of various diseases [167].
NPs induce membrane damage and enhance the transport of NPs into the cytoplasm. NPs concentrate in lysosomes, mitochondria, and the nucleus, which results in catastrophic consequences for the cell [168, 169]. It has been reported that NPs can reduce adenosine triphosphate (ATP) generation [89], deplete glutathione, induce protein mistranslation [170], rupture lysosomes [171], and inhibit the ribosomal subunit from binding transfer RNA (tRNA). These cellular events indicate a collapse of the fundamental biological process in the cell and lead to a significant decrease in cell viability [47]. Singh and Scherz-Shouval et al. reported that NPs could disturb cytoskeletal functions by inducing ROS generation and activate the process of autophagic and apoptosis in cells [89].
NPs enter the body via different routes, for instance through the skin, lungs, or intestinal tract (Fig. 4a) and can have a wide variety of toxicological effects and induce biological responses such as inflammation and immune responses [172–174]. In one case, exposure of cells to silica NPs caused macrophages to secrete a large amounts of interleukin-1β (IL-1β), which ultimately resulted in cell death [175]. Gao and colleagues reported that pulmonary inflammation was considerably higher in mice after exposure to carbon nanotubes, which could activate alveolar macrophages and induce a strong inflammatory response [176]. In another study, guinea pigs exposed to ZnO NPs suffered pulmonary damage, which leads to a decrease in total lung capacity and vital capacity [177–179].
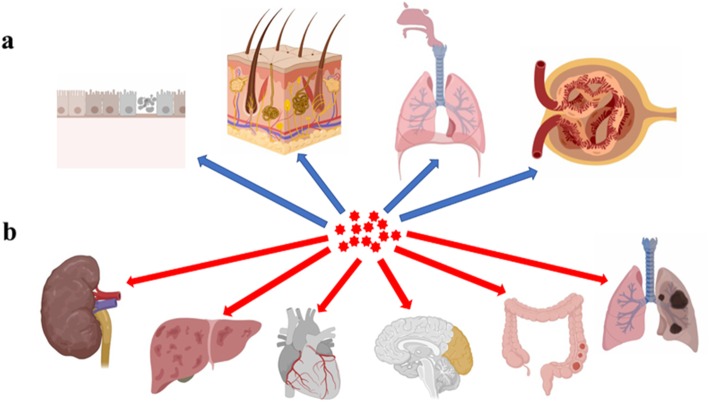
Fig. 4.
NP entrance into and damage of organs. a NPs could enter into the organisms through the oral cavity, nasal cavity, respiratory tract, kidneys, and intestinal tract; b NPs could spread by systemic circulation and accumulate in the kidneys, liver, heart, brain, intestinal tract, and lungs, leading to organ dysfunction (This figure was created in BioRender.com).
ZnO NPs also induced severe injuries in the alveolar epithelial barrier and caused inflammation in the human lungs [180]. In another case, NPs absorbed into the intestines caused the inflammation and degradation of the intestinal mucosa [181]. Shubayev et al. noted that Mg NPs enhanced the migration of macrophages to the nervous system by degrading the blood-brain and blood-nerve barriers in an MMP-dependent manner [182]. Furthermore, mice which inhaled carbon nanotubes exhibited immunosuppression and repressed antibody response in naive spleen cells [183]. Finally, Cd NPs caused a severe decrease in blood monocyte viability, ultimately resulting in immunodeficiency [184].
In addition to the above pathologies, the highly variable level of ROS has been identified as the main cause of the development of numerous human diseases. Tretyakova and Liou et al. showed that oxidized DNA tends to form DNA-protein conjugates, which accumulate in the heart and brain and contribute to the occurrence of cancer, aging-related diseases, and chronic inflammation [185, 186]. Andersen [187] concluded that diabetes, as well as cardiovascular and neurodegenerative diseases, were highly related to the imbalance of ROS. Additionally, Pérez-Rosés et al. showed that increased ROS promoted Alzheimer’s and Parkinson’s disease development [188].
It has been further reported that NPs promote the apoptosis of breast cancer cells [35] and destroy malignant tissues and pathogens by promoting the generation of ROS [189, 190]. However, ROS has also been found to induce the proliferation of both normal and cancerous cells, stimulating mutations, and initiating carcinogenesis in normal cells and multidrug resistance in cancerous cells [191, 192]. Handy et al. found that fish exposed to carbon nanotubes exhibited granulomas in their lungs and tumors in their livers with extended exposure times [193]. Some NPs have caused multiple organ failure, primarily affecting the heart, lung, kidneys, and liver. TiO2 NPs have been shown to promote reduced body weight, spleen lesions, blood clotting in the respiratory system, necrosis and fibrosis in liver cells, and in alveolar septal incrassation [194, 195]. In one study, NPs also prevented stem cell differentiation, which aggravated organ damage [196]. Further research has also reported that NPs decreased sperm quality [197] and that exposure of sperm to carbon NPs influenced their ability to fertilize eggs and impaired the development of the embryos in purple sea urchins [198]. Mounting evidence shows the toxicological effects of NPs on microorganisms, algae, nematode, plants, animals, and humans specifically [22, 199, 200] (Fig. 4b).
The New Type of NPs with Fewer or No Cytotoxicity
NPs possess a range of biomedical properties that make them valuable (e.g., as antibacterial and anticancer agents [26–28]). Their main mode of action is their ability to increase the production of ROS in cells; however, this property also makes these particles toxic, by causing gene mutation, apoptosis, and even carcinogenesis [45, 49, 58]. Consequently, there is an urgent need to develop new NPs which retain their required properties without leading to excessive ROS production. Recent studies have reported on novel types of NPs which could remove intracellular ROS. These types fall into two classes: (1) NPs which can scavenge ROS [77] and (2) NPs which are coated with additional materials to decrease their cytotoxicity [87].
Panikkanvalappil and colleagues showed that Pt NPs inhibit the double-strand breakage of DNA by degrading ROS [201]. In another case, Mn3O4 NPs modulated cellular redox resulting in the protection of biomacromolecules against oxidative stress [77]. Furthermore, the CeO2 NP is a novel agent that protects cells and tissues against oxidative damage with its free radical-scavenging capacity [79, 202].
H2O2 is the main by-product of NP-cell interactions. H2O2 destroys important biomolecules including proteins, lipids, and nucleic acids. However, when cells were treated with specialized MNPs coated with mercaptopropionic acid (MPA-NPs) or aminated silica (SiO2-MNPs), such damage was not observed [203, 204]. Similarly, GO coated with polyvinylpyrrolidone (PVP) has fewer toxic effects on dendritic cell (DCs), T-lymphocytes, and macrophages than without this coating. PVP-GO has been shown to reduce the apoptosis of T-lymphocytes and even increase the activity of macrophages [205]. Pt-coated AuNRs (PtAuNRs) retain the efficacy of traditional gold nanorods (AuNR) and can trigger cell death of desired cells while scavenging the ROS, thereby protecting healthy, untreated cells from the indirect death induced by ROS production [87].
Conclusions and Outlook
NPs that possess unique physicochemical properties (e.g., ultra-small size, large surface area to mass ratio, and high reactivity) make them highly desirable in different applications. Engineered NPs for commercial purposes have been rapidly increasing. For that reason, the biosafety of NPs has gained more attention in the public. In this review, we summarized the mechanisms and responsible for ROS formation by NPs at the cellular level as well as recent advances of ROS-related NP toxicity in the biomedical field and highlighted the emerging field of cell-friendly NPs. The generation of ROS induced by NPs associated with their size, morphology, surface area, and component. In addition, ROS has bio-multifunctional in cell biology and biomedicine as well as the key mediator of cellular signaling, including cell apoptosis, viability, and differentiation.
However, to improve the biosafety of NPs and accelerate their use in the biomedical field, some bottlenecks need to be overcome and much work is still required. First, it is expected that high-throughput methods (HTMs) are designed to efficiently detect the biotoxicity of NPs in vitro and in vivo. HTMs could save time and resources, combine multiple parameters on a single system, and minimize methodological or systematic errors. It also would offer a deep understanding of the relationship between NP properties and cell responses, which could help us identify the optimal condition.
Second, the molecular and cellular mechanisms related to the biotoxicity of NP-induced ROS are still unclear. There is a demand to further explore the mechanisms associated with the formation of ROS by NPs, which would provide more information to modify the chemico-physico features of NPs to control the ROS generation. This could help researchers develop novel strategies to reduce the hazards of engineered NPs for accelerating their clinical and commercial translation in the biomedical filed.
Finally, due to their structural characteristics, NPs may enter the body freely via multiple routes, and the accumulation of NPs in the body can induce inflammation and immune responses, which result in cell injury or death, organ dysfunction, and ultimately stimulate the occurrence of numerous diseases, such as Alzheimer’s, Parkinson’s, liver inflammation, and dysembryoplasia. These issues have become more pressing with the widespread use of NPs.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (Grant Nos. 31900957, 31671447, 31430041, 91849209), Shandong Provincial Natural Science Foundation (Grant No. ZR2019QC007), China Postdoctoral Science Foundation (Grant No. 2019M652326), Innovation and Technology Program for the Excellent Youth Scholars of Higher Education of Shandong Province (Grant No. 2019KJE015), and the Scientific Research Foundation of Qingdao University (Grant No. DC1900009689).
Abbreviations
- •OH
Hydroxyl radical
- 2-oxoA
1,2-Dihydro-2-oxoadenine
- 8-oxoG
8-oxo-7,8 dihydroguanine
- Ag NPs
Silver nanoparticles
- AP-1
Transcription factor activator protein-1
- ATP
Adenosine triphosphate
- AuNR
Gold nanorods
- CFDNPs
Combustion and friction-derived nanoparticles
- DCs
Dendritic cells
- D-NP
Day flower-mimicking metallic nanoparticles
- EGF
Epidermal growth factor
- ER
Endoplasmic reticulum
- ETC
Mitochondrial electron transport chain
- Fe3O4-PEG-G5-MMP2@Ce6
Fe3O4-polyethylene glycol-polyamide-amine-matrix metalloproteinase2@ chlorin e6
- H2O2
Hydrogen peroxide
- HS-Fe-PEG-HER2 NPs
Hollow silica-Fe-polyethylene glycol-human epidermal growth factor receptor 2 nanoparticles
- LDH
Lactate dehydrogenase
- MMP
Mitochondrial membrane potentials
- MPA-NPs
MNPs coated with mercaptopropionic acid
- NADP+/NADPH
Nicotinamide adenine dinucleotide phosphate oxidized/reduced
- NF-κB
Nuclear factor-κB
- N-NP
Night flower-mimicking metallic nanoparticles
- NPs
Nanoparticles
- O2-
Reactive superoxide anion radical
- pDNA-PEI-CeO NPs
pDNA-polyethylenimine CeO nanoparticles
- PtAuNRs
Pt-coated AuNRs
- PVP
Polyvinylpyrrolidone
- ROS
Reactive oxygen species
- SiO2-MNPs
MNPs with aminated silica
- tRNA
Transfer RNA
Authors’ Contributions
Project administration, Zhongjie Yu and Peifeng Li; writing—original draft preparation, Zhongjie Yu, Qi Li, Jing Wang, and Yin Wang; writing—review and editing, Zhongjie Yu and Qihui Zhou; funding acquisition, Qihui Zhou, Yin Wang, and Peifeng Li. The authors have read and agreed to the published final version of the manuscript.
Funding
This review was supported by Prof. Yin Wang, Qihui Zhou, and Peifeng Li.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qihui Zhou, Email: qihuizhou@qdu.edu.cn.
Peifeng Li, Email: peifli@qdu.edu.cn.
References
- 1.Tang B, Wang J, Xu S, Afrin T, Xu W, Sun L, Xungai W. Application of anisotropic silver nanoparticles: multifunctionalization of wool fabric. J Colloid Interface Sci. 2011;356:513–518. doi: 10.1016/j.jcis.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Chaloupka K, Malam Y. AM. S. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Haiping T, Zhizhen Y, Zhu L, He H, Binghui Z, Yang Z, Mingjia Z, Zhixiang Y. Synthesis of radial ZnO nanostructures by a simple thermal evaporation method. Phys E. 2008;40:507–511. [Google Scholar]
- 4.Ghamsari MS, Vafaee M. Sol–gel derived zinc oxide buffer layer for use in random laser media. Mater Lett. 2008;62:1754–1756. [Google Scholar]
- 5.Lee S-H, Deshpande R, Benhammou D, Parilla PA, Mahan AH, Dillon AC. Metal oxide nanoparticles for advanced energy applications. Thin Solid Films. 2009;517:3591–3595. [Google Scholar]
- 6.Talapin DV, Lee J-S, Kovalenko MV, Shevchenko EV. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem Rev. 2010;110:389–458. doi: 10.1021/cr900137k. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Morrin A, Killard AJ, Smyth MR. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18:319–326. [Google Scholar]
- 8.Silva AT, Nguyen A, Ye C, Verchot J, Moon JH. Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 2010;10:291. doi: 10.1186/1471-2229-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 10.Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Mirkin CA, Thaxton CS, Rosi NL. Nanostructures in biodefense and molecular diagnostics. Expert Rev Mol Diagn. 2004;4:749–751. doi: 10.1586/14737159.4.6.749. [DOI] [PubMed] [Google Scholar]
- 12.Duan M, Xia F, Li T, Shapter JG, Yang S, Li Y, Gao G, Cui D. Matrix metalloproteinase-2-targeted superparamagnetic Fe3O4-PEG-G5-MMP2@Ce6 nanoprobes for dual-mode imaging and photodynamic therapy. Nanoscale. 2019;11:18426–18435. doi: 10.1039/c9nr06774d. [DOI] [PubMed] [Google Scholar]
- 13.Hasanzadeh L, Darroudi M, Ramezanian N, Zamani P, Aghaee-Bakhtiari SH, Nourmohammadi E, Kazemi OR. Polyethylenimine-associated cerium oxide nanoparticles: a novel promising gene delivery vector. Life Sci. 2019;232:116661. doi: 10.1016/j.lfs.2019.116661. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xia S, Zhou W, Ji R, Zhan W. Targeted Fe-doped silica nanoparticles as a novel ultrasound-magnetic resonance dual-mode imaging contrast agent for HER2-positive breast cancer. Int J Nanomedicine. 2019;14:2397–2413. doi: 10.2147/IJN.S189252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemer S, Westmeier D, Barz M, Eckrich J, Wunsch D, Seckert C, Thyssen C, Schilling O, Hasenberg M, Pang C, Docter D, Knauer SK, Stauber RH, Strieth S. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: implications for the design of improved nanoantibiotics. Biomaterials. 2019;192:551–559. doi: 10.1016/j.biomaterials.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Song MF, Li YS, Kasai H, Kawai K. Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J Clin Biochem Nutr. 2012;50:211–216. doi: 10.3164/jcbn.11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patlolla AK, Hackett D, Tchounwou PB. Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem. 2015;399:257–268. doi: 10.1007/s11010-014-2252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;24:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Cheng FY, Chiu HW, Tsai JC, Fang CY, Chen CW, Wang YJ. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35:4706–4715. doi: 10.1016/j.biomaterials.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Akter M, Sikder MT, Rahman MM, Ullah A, Hossain KFB, Banik S, Hosokawa T, Saito T, Kurasaki M. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajoria S, Rani S, Chaudhari D, Jain S, Gupta U. Glycine-poly-l-lactic acid copolymeric nanoparticles for the efficient delivery of bortezomib. Pharm Res. 2019;36:160. doi: 10.1007/s11095-019-2686-4. [DOI] [PubMed] [Google Scholar]
- 22.Asharani P, Sethu S, Lim HK, Balaji G, Valiyaveettil S, Hande MP. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome integrity. 2012;3:2. doi: 10.1186/2041-9414-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 24.Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, AI. B. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Yang W, Man N, Zheng F, Shen Y, Sun K, Li Y, Long-Ping W. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy. 2009;5:1107–1117. doi: 10.4161/auto.5.8.9842. [DOI] [PubMed] [Google Scholar]
- 27.Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem. 2003;38:913–923. doi: 10.1016/j.ejmech.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Isakovic A, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Mirkovic M, Dramicanin M, Harhaji L, Raicevic N, Nikolic Z, Trajkovic V. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicological sciences: an official journal of the Society of Toxicology. 2006;91:173–183. doi: 10.1093/toxsci/kfj127. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, Lee CY, An HR, Kim H, Lee YC, Park EC, Chun HS, Yang HY, Choi SH, Kim HS, Kang KS, Park HG, Kim JP, Choi Y, Lee J, Lee HU. Advanced carbon dots via plasma-induced surface functionalization for fluorescent and bio-medical applications. Nanoscale. 2017;9:9210–9217. doi: 10.1039/c7nr03026f. [DOI] [PubMed] [Google Scholar]
- 30.Lee AR, Lee SJ, Lee M, Nam M, Lee S, Choi J, Lee HJ, Kim DU, Hoe KL. Editor’s highlight: a genome-wide screening of target genes against silver nanoparticles in fission yeast. Toxicological sciences: an official journal of the Society of Toxicology. 2018;161:171–185. doi: 10.1093/toxsci/kfx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KLJR, JJ. S. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Jin W, Qasim AM, Gao A, Peng X, Li W, Feng H, Chu PK. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25–34. doi: 10.1016/j.biomaterials.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Tomankova K, Horakova J, Harvanova M, Malina L, Soukupova J, Hradilova S, Kejlova K, Malohlava J, Licman L, Dvorakova M, Jirova D, Kolarova H. Reprint of: cytotoxicity, cell uptake and microscopic analysis of titanium dioxide and silver nanoparticles in vitro. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2015;85:20–30. doi: 10.1016/j.fct.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Kaweeteerawat C, Na Ubol P, Sangmuang S, Aueviriyavit S, Maniratanachote R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. Journal of toxicology and environmental health Part A. 2017;80:1276–1289. doi: 10.1080/15287394.2017.1376727. [DOI] [PubMed] [Google Scholar]
- 35.Farah MA, Ali MA, Chen SM, Li Y, Al-Hemaid FM, Abou-Tarboush FM, Al-Anazi KM, Lee J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf B: Biointerfaces. 2016;141:158–169. doi: 10.1016/j.colsurfb.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed B, Hashmi A, Khan MS, Musarrat J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv Powder Technol. 2018;29:1601–1616. [Google Scholar]
- 37.Wang Y, Wan J, Miron RJ, Zhao Y, Zhang Y. Antibacterial property and mechanisms of gold-silver nanocage. Nanoscale. 2016;8:11143–11152. doi: 10.1039/c6nr01114d. [DOI] [PubMed] [Google Scholar]
- 38.Memarzadeh K, Sharili AS, Huang J, Rawlinson SC, Allaker RP. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J Biomed Mater Res A. 2015;103:981–989. doi: 10.1002/jbm.a.35241. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Wu R, Chen Y. Effects of ZnO nanoparticles on wastewater biological nitrogen and phosphorus removal. Environ Sci Technol. 2011;45:2826–2832. doi: 10.1021/es2000744. [DOI] [PubMed] [Google Scholar]
- 40.Verma SK, Jha E, Panda PK, Das JK, Thirumurugan A, Suar M, Parashar S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine (London) 2018;13:43–68. doi: 10.2217/nnm-2017-0237. [DOI] [PubMed] [Google Scholar]
- 41.Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krol A, Pomastowski P, Rafinska K, Railean-Plugaru V, Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv Colloid Interf Sci. 2017;249:37–52. doi: 10.1016/j.cis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 43.Valdiglesias V, Costa C, Kilic G, Costa S, Pasaro E, Laffon B, Teixeira JP. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ Int. 2013;55:92–100. doi: 10.1016/j.envint.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;50:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 45.Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Li F, Xiang W, Yi Y, Chen Y, Cheng L, Liu Z, Xu H. Selenium-containing amphiphiles reduced and stabilized gold nanoparticles: kill cancer cells via reactive oxygen species. ACS Appl Mater Interfaces. 2016;8:22106–22112. doi: 10.1021/acsami.6b08282. [DOI] [PubMed] [Google Scholar]
- 47.Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 48.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Krishnamoorthy K, Moon JY, Hyun HB, Cho SK, Kim SJ. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J Mater Chem. 2012;22:24610. [Google Scholar]
- 50.Al-Shabib NA, Husain FM, Ahmed F, Khan RA, Khan MS, Ansari FA, Alam MZ, Ahmed MA, Khan MS, Baig MH, Khan JM, Shahzad SA, Arshad M, Alyousef A, Ahmad I. Low temperature synthesis of superparamagnetic iron oxide (Fe3O4) nanoparticles and their ROS mediated inhibition of biofilm formed by food-associated bacteria. Front Microbiol. 2018;9:2567. doi: 10.3389/fmicb.2018.02567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kauffer FA, Merlin C, Balan L, Schneider R. Incidence of the core composition on the stability, the ROS production and the toxicity of CdSe quantum dots. J Hazard Mater. 2014;268:246–255. doi: 10.1016/j.jhazmat.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 52.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, WA. J. Bactericidal activity of photocatalytic TiO(2) reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- 54.Skocaj M, Filipic M, Petkovic J, Novak S. Titanium dioxide in our everyday life; is it safe? Radiol Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botelho MC, Costa C, Silva S, Costa S, Dhawan A, Oliveira PA, Teixeira JP. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014;68:59–64. doi: 10.1016/j.biopha.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Huang S, Chueh PJ, Lin YW, Shih TS, Chuang SM. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol Appl Pharmacol. 2009;241:182–194. doi: 10.1016/j.taap.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Freyre-Fonseca V, Delgado-Buenrostro NL, Gutierrez-Cirlos EB, Calderon-Torres CM, Cabellos-Avelar T, Sanchez-Perez Y, Pinzon E, Torres I, Molina-Jijon E, Zazueta C, Pedraza-Chaverri J, Garcia-Cuellar CM, Chirino YI. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol Lett. 2011;202:111–119. doi: 10.1016/j.toxlet.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Gu AZ, Xie S, Li X, Cen T, Li D, Chen J. Nano-metal oxides induce antimicrobial resistance via radical-mediated mutagenesis. Environ Int. 2018;121:1162–1171. doi: 10.1016/j.envint.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 59.Xu B, Liu Y, Yuan J, Wang P, Wang Q (2018) Synthesis, characterization, and antifogging application of polymer/Al(2)O(3) nanocomposite hydrogels with high strength and self-healing capacity. Polymers. 10 [DOI] [PMC free article] [PubMed]
- 60.Li J, Zhou H, Wang J, Wang D, Shen R, Zhang X, Jin P, Liu X. Oxidative stress-mediated selective antimicrobial ability of nano-VO2 against Gram-positive bacteria for environmental and biomedical applications. Nanoscale. 2016;8:11907–11923. doi: 10.1039/c6nr02844f. [DOI] [PubMed] [Google Scholar]
- 61.WS Xi, H Tang, YY Liu, CY Liu, YF Gao , A Cao, YF Liu, Z Chen, HF Wang. Cytotoxicity of vanadium oxide nanoparticles and titanium dioxide-coated vanadium oxide nanoparticles to human lung cells. Journal of applied toxicology: JAT. 2019. [DOI] [PubMed]
- 62.Raj S, Kumar S, Chatterjee K. Facile synthesis of vanadia nanoparticles and assessment of antibacterial activity and cytotoxicity. Mater Technol. 2016;31:562–573. [Google Scholar]
- 63.Wang D, Zhao L, Ma H, Zhang H, Guo L-H. Quantitative analysis of reactive oxygen species photogenerated on metal oxide nanoparticles and their bacteria toxicity: the role of superoxide radicals. Environ Sci Technol. 2017;51:10137–10145. doi: 10.1021/acs.est.7b00473. [DOI] [PubMed] [Google Scholar]
- 64.Park SC, Kim NH, Yang W, Nah JW, Jang MK, Lee D. Polymeric micellar nanoplatforms for Fenton reaction as a new class of antibacterial agents. Journal of controlled release: official journal of the Controlled Release Society. 2016;221:37–47. doi: 10.1016/j.jconrel.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 65.Iqbal G, Faisal S, Khan S, Shams DF, Nadhman A. Photo-inactivation and efflux pump inhibition of methicillin resistant Staphylococcus aureus using thiolated cobalt doped ZnO nanoparticles. J Photochem Photobiol B. 2019;192:141–146. doi: 10.1016/j.jphotobiol.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Wang C, Cui Q, Wang X, Li L. Preparation of hybrid gold/polymer nanocomposites and their application in a controlled antibacterial assay. ACS Appl Mater Interfaces. 2016;8:29101–29109. doi: 10.1021/acsami.6b12487. [DOI] [PubMed] [Google Scholar]
- 67.Yazdanbakhsh AR, Rafiee M, Daraei H, Amoozegar MA. Responses of flocculated activated sludge to bimetallic Ag-Fe nanoparticles toxicity: performance, activity enzymatic, and bacterial community shift. J Hazard Mater. 2019;366:114–123. doi: 10.1016/j.jhazmat.2018.11.098. [DOI] [PubMed] [Google Scholar]
- 68.Davaeifar S, Modarresi MH, Mohammadi M, Hashemi E, Shafiei M, Maleki H, Vali H, Zahiri HS, Noghabi KA. Synthesizing, characterizing, and toxicity evaluating of phycocyanin-ZnO nanorod composites: a back to nature approaches. Colloids Surf B: Biointerfaces. 2019;175:221–230. doi: 10.1016/j.colsurfb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Jiang J, Zhang C, Zeng GM, Gong JL, Chang YN, Song B, Deng CH, Liu HY. The disinfection performance and mechanisms of Ag/lysozyme nanoparticles supported with montmorillonite clay. J Hazard Mater. 2016;317:416–429. doi: 10.1016/j.jhazmat.2016.05.089. [DOI] [PubMed] [Google Scholar]
- 70.Hsu SH, Lin YY, Huang S, Lem KW, Nguyen DH, Lee DS. Synthesis of water-dispersible zinc oxide quantum dots with antibacterial activity and low cytotoxicity for cell labeling. Nanotechnology. 2013;24:475102. doi: 10.1088/0957-4484/24/47/475102. [DOI] [PubMed] [Google Scholar]
- 71.Hossain ST, Mukherjee SK. Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J Hazard Mater. 2013;260:1073–1082. doi: 10.1016/j.jhazmat.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Ambrosone A, Mattera L, Marchesano V, Quarta A, Susha AS, Tino A, Rogach AL, Tortiglione C. Mechanisms underlying toxicity induced by CdTe quantum dots determined in an invertebrate model organism. Biomaterials. 2012;33:1991–2000. doi: 10.1016/j.biomaterials.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 73.Moussa H, Merlin C, Dezanet C, Balan L, Medjahdi G, Ben-Attia M, Schneider R. Trace amounts of Cu(2)(+) ions influence ROS production and cytotoxicity of ZnO quantum dots. J Hazard Mater. 2016;304:532–542. doi: 10.1016/j.jhazmat.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Gallo A, Manfra L, Boni R, Rotini A, Migliore L, Tosti E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ Int. 2018;118:325–333. doi: 10.1016/j.envint.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 75.Buffet PE, Richard M, Caupos F, Vergnoux A, Perrein-Ettajani H, Luna-Acosta A, Akcha F, Amiard JC, Amiard-Triquet C, Guibbolini M, Risso-De Faverney C, Thomas-Guyon H, Reip P, Dybowska A, Berhanu D, Valsami-Jones E, Mouneyrac C. A mesocosm study of fate and effects of CuO nanoparticles on endobenthic species (Scrobicularia plana, Hediste diversicolor) Environ Sci Technol. 2013;47:1620–1628. doi: 10.1021/es303513r. [DOI] [PubMed] [Google Scholar]
- 76.Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, Banin E. Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small. 2012;8:3326–3337. doi: 10.1002/smll.201200772. [DOI] [PubMed] [Google Scholar]
- 77.Singh N, Savanur MA, Srivastava S, D'Silva P, Govindasamy M. A manganese oxide nanozyme prevents oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11:3855–3863. doi: 10.1039/c8nr09397k. [DOI] [PubMed] [Google Scholar]
- 78.Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla SV, Wozniak K, Self WT, Seal S. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc. 2009;131:14144–14145. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rubio L, Annangi B, Vila L, Hernandez A, Marcos R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch Toxicol. 2016;90:269–278. doi: 10.1007/s00204-015-1468-y. [DOI] [PubMed] [Google Scholar]
- 80.Kadiyala NK, Mandal BK, Ranjan S, Dasgupta N. Bioinspired gold nanoparticles decorated reduced graphene oxide nanocomposite using Syzygium cumini seed extract: evaluation of its biological applications. Mater Sci Eng C Mater Biol Appl. 2018;93:191–205. doi: 10.1016/j.msec.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 81.Wason MS, Lu H, Yu L, Lahiri SK, Mukherjee D, Shen C, Das S, Seal S, Zhao J (2018) Cerium oxide nanoparticles sensitize pancreatic cancer to radiation therapy through oxidative activation of the JNK apoptotic pathway. Cancers. 10 [DOI] [PMC free article] [PubMed]
- 82.Wang D, Zhu L, Chen JF, Dai L. Can graphene quantum dots cause DNA damage in cells? Nanoscale. 2015;7:9894–9901. doi: 10.1039/c5nr01734c. [DOI] [PubMed] [Google Scholar]
- 83.Nurunnabi M, Khatun Z, Reeck GR, Lee DY, Lee YK. Photoluminescent graphene nanoparticles for cancer phototherapy and imaging. ACS Appl Mater Interfaces. 2014;6:12413–12421. doi: 10.1021/am504071z. [DOI] [PubMed] [Google Scholar]
- 84.Karlsson HL, Cronholm P, Gustafsson J, Lennart M. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 85.Manna I, Bandyopadhyay M. Engineered nickel oxide nanoparticles affect genome stability in Allium cepa (L.) Plant physiology and biochemistry: PPB. 2017;121:206–215. doi: 10.1016/j.plaphy.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Angel Ezhilarasi A, Judith Vijaya J, Kaviyarasu K, John Kennedy L, Ramalingam RJ, Al-Lohedan HA. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J Photochem Photobiol B. 2018;180:39–50. doi: 10.1016/j.jphotobiol.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 87.Aioub M, Panikkanvalappil SR, El-Sayed MA. Platinum-coated gold nanorods: efficient reactive oxygen scavengers that prevent oxidative damage toward healthy, untreated cells during plasmonic photothermal therapy. ACS Nano. 2017;11:579–586. doi: 10.1021/acsnano.6b06651. [DOI] [PubMed] [Google Scholar]
- 88.Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, Vallyathan V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh BR, Singh BN, Khan W, Singh HB, Naqvi AH. ROS-mediated apoptotic cell death in prostate cancer LNCaP cells induced by biosurfactant stabilized CdS quantum dots. Biomaterials. 2012;33:5753–5767. doi: 10.1016/j.biomaterials.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Lin Z, Zhu B. Silver nanoparticles based codelivery of Oseltamivir to inhibit the activity of H1N1 influenza virus through ROS mediated signaling pathways. Workshop for Control of EID. 2017;2017:41. doi: 10.1021/acsami.6b06613. [DOI] [PubMed] [Google Scholar]
- 91.Dai X, Zhao Y, Yu Y, Chen X, Wei X, Zhang X, Li C. Single continuous near-infrared laser-triggered photodynamic and photothermal ablation of antibiotic-resistant bacteria using effective targeted copper sulfide nanoclusters. ACS Appl Mater Interfaces. 2017;9:30470–30479. doi: 10.1021/acsami.7b09638. [DOI] [PubMed] [Google Scholar]
- 92.Keren I, Wu Y, Inocencio J, Mulcahy LR, Kim L. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 93.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 94.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 99.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 100.Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schieber M, Chandel NS. TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep. 2014;9:9–15. doi: 10.1016/j.celrep.2014.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson D, Allman E, Keith N. Regulation of acid-base transporters by reactive oxygen species following mitochondrial fragmentation. Am J Phys Cell Physiol. 2012;302:1045–1054. doi: 10.1152/ajpcell.00411.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gaetke LM, Ching Kuang C. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 105.He W, Zhou YT, Wamer WG, Boudreau MD, Yin JJ. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials. 2012;33:7547–7555. doi: 10.1016/j.biomaterials.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 106.Li Y, Qin T, Ingle T, Yan J, He W, Yin JJ, Chen T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch Toxicol. 2017;91:509–519. doi: 10.1007/s00204-016-1730-y. [DOI] [PubMed] [Google Scholar]
- 107.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 108.Holt KB, AJ. B. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ Biochemistry. 2005;44:13214–13223. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- 109.Kang SJ, Lee YJ, Lee E-K, Kwak M-K. Silver nanoparticles-mediated G2/M cycle arrest of renal epithelial cells is associated with NRF2-GSH signaling. Toxicol Lett. 2012;211:334–341. doi: 10.1016/j.toxlet.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 110.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 111.Lee J, Kwon ES, Kim DW, Cha J, Jung-Hye R. Regulation and the role of Cu,Zn-containing superoxide dismutase in cell cycle progression of Schizosaccharomyces pombe. Biochem Biophys Res Commun. 2002;297:854–862. doi: 10.1016/s0006-291x(02)02290-8. [DOI] [PubMed] [Google Scholar]
- 112.Yamada K, Nakagawa CW, Mutoh N. Schizosaccharomyces pombe homologue of glutathione peroxidase, which does not contain selenocysteine, is induced by several stresses and works as an antioxidant. Yeast. 1999;15:1125–1132. doi: 10.1002/(SICI)1097-0061(199908)15:11<1125::AID-YEA442>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 113.Huang T, Holden JA, Heath DE, O'Brien-Simpson NM, O'Connor AJ. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale. 2019;11:14937–14951. doi: 10.1039/c9nr04424h. [DOI] [PubMed] [Google Scholar]
- 114.Cho S, Lee B, Park W, Huang X, Kim DH. Photoperiodic flower mimicking metallic nanoparticles for image-guided medicine applications. ACS Appl Mater Interfaces. 2018;10:27570–27577. doi: 10.1021/acsami.8b09596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liao F, Chen L, Liu Y, Zhao D, Peng W, Wang W, Feng S. The size-dependent genotoxic potentials of titanium dioxide nanoparticles to endothelial cells. Environ Toxicol. 2019;34:1199–1207. doi: 10.1002/tox.22821. [DOI] [PubMed] [Google Scholar]
- 116.Green M, Howman E (2005) Semiconductor quantum dots and free radical induced DNA nicking. Chem Commun:121–123 [DOI] [PubMed]
- 117.Chen N, He Y, Su Y, Li X, Huang Q, Wang H, Zhang X, Tai R, Fan C. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–1244. doi: 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- 118.Roesslein M, Hirsch C, Kaiser JP, Krug HF, Wick P. Comparability of in vitro tests for bioactive nanoparticles: a common assay to detect reactive oxygen species as an example. Int J Mol Sci. 2013;14:24320–24337. doi: 10.3390/ijms141224320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164–5173. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- 121.Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R, Gedanken A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater. 2009;19:842–852. [Google Scholar]
- 122.Ma H, Williams PL, Diamond SA. Ecotoxicity of manufactured ZnO nanoparticles-a review. Environ Pollut. 2013;172:76–85. doi: 10.1016/j.envpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 123.Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232:244–251. doi: 10.1016/j.taap.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 125.Gu L, Li Q, Quan X, Cen Y, Jiang X. Comparison of nanosilver removal by flocculent and granular sludge and short- and long-term inhibition impacts. Water Res. 2014;58:62–70. doi: 10.1016/j.watres.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 126.Ahmad S, Khan H, Shahab U, Rehman S, Rafi Z, Khan MY, Ansari A, Siddiqui Z, Ashraf JM, Abdullah SM, Habib S, Moin U. Protein oxidation: an overview of metabolism of sulphur containing amino acid, cysteine. Front Biosci (Schol Ed) 2017;9:71–87. doi: 10.2741/s474. [DOI] [PubMed] [Google Scholar]
- 127.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 128.Murray Stewart T, Dunston TT, Woster PM, Casero RA., Jr Polyamine catabolism and oxidative damage. J Biol Chem. 2018;293:18736–18745. doi: 10.1074/jbc.TM118.003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264:3341–3346. [PubMed] [Google Scholar]
- 131.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gurunathan S, Jeyaraj M, Kang MH, Kim JH. Tangeretin-assisted platinum nanoparticles enhance the apoptotic properties of doxorubicin: combination therapy for osteosarcoma treatment. Nanomaterials (Basel) 2019;9:1089. doi: 10.3390/nano9081089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Calderon-Garciduenas L, Reynoso-Robles R, Gonzalez-Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson’s diseases. Environ Res. 2019;176:108574. doi: 10.1016/j.envres.2019.108574. [DOI] [PubMed] [Google Scholar]
- 134.Imlay JA, S. L. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 135.Maki HSM. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 136.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 137.Bjelland S, Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 138.Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 139.Nunoshiba T, Obata F, Boss AC, Oikawa S, Mori T, Kawanishi S, Yamamoto K. Role of iron and superoxide for generation of hydroxyl radical, oxidative DNA lesions, and mutagenesis in Escherichia coli. J Biol Chem. 1999;274:34832–34837. doi: 10.1074/jbc.274.49.34832. [DOI] [PubMed] [Google Scholar]
- 140.Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu ZJ, Arcaro KF, Rotello VM. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Proquin H, Rodríguez-Ibarra C, Moonen C, Urrutia Ortega IM, Briedé JJ, de Kok TM, van Loveren H, Chirino YI. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano-sized fractions. Mutagenesis. 2018;33:267–268. doi: 10.1093/mutage/gey011. [DOI] [PubMed] [Google Scholar]
- 142.Kang SJ, Kim BM, Lee YJ, Chung HW. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 143.Kawanishi S, Hiraku Y, Murata M, Shinji O. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Med. 2002;32:822–832. doi: 10.1016/s0891-5849(02)00779-7. [DOI] [PubMed] [Google Scholar]
- 144.Di Bucchianico S, Fabbrizi MR, Cirillo S, Uboldi C, Gilliland D, Valsami-Jones E, Migliore L. Aneuploidogenic effects and DNA oxidation induced in vitro by differently sized gold nanoparticles. Int J Nanomedicine. 2014;9:2191–2204. doi: 10.2147/IJN.S58397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levine AS, Sun L, Tan R, Gao Y, Yang L, Chen H, Teng Y, Lan L. The oxidative DNA damage response: a review of research undertaken with Tsinghua and Xiangya students at the University of Pittsburgh. Sci China Life Sci. 2017;60:1077–1080. doi: 10.1007/s11427-017-9184-6. [DOI] [PubMed] [Google Scholar]
- 146.Jena NR. DNA damage by reactive species: mechanisms, mutation and repair. J Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 147.Kirsch-Volders M, Vanhauwaert A, De Boeck M, Ilse D. Importance of detecting numerical versus structural chromosome aberrations. Mutat Res. 2002;504:137–148. doi: 10.1016/s0027-5107(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 148.Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie. 2006;88:1515–1531. doi: 10.1016/j.biochi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 149.Belenky P, Ye JD, Porter CB, Cohen NR, Lobritz MA, Ferrante T, Jain S, Korry BJ, Schwarz EG, Walker GC, Collins JJ. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015;13:968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bridge G, Rashid S, Martin SA. DNA mismatch repair and oxidative DNA damage: implications for cancer biology and treatment. Cancers. 2014;6:1597–1614. doi: 10.3390/cancers6031597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Avkin S, Zvi L. Efficiency, specificity and DNA polymerase-dependence of translesion replication across the oxidative DNA lesion 8-oxoguanine in human cells. Mutat Res. 2002;510:81–90. doi: 10.1016/s0027-5107(02)00254-3. [DOI] [PubMed] [Google Scholar]
- 152.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang ZY, Xiong M, Fu LY, Zhang HY. Oxidative DNA damage is important to the evolution of antibiotic resistance: evidence of mutation bias and its medicinal implications. J Biomol Struct Dyn. 2013;31:729–733. doi: 10.1080/07391102.2012.709457. [DOI] [PubMed] [Google Scholar]
- 154.Dufour EK, Kumaravel T, Nohynek GJ, Kirkland D, Toutain H. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells. Mutat Res. 2006;607:215–224. doi: 10.1016/j.mrgentox.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 155.Rusyn I, Asakura S, Pachkowski B, Bradford BU, Denissenko MF, Peters JM, Holland SM, Reddy JK, Cunningham ML, Swenberg JA. Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res. 2004;64:1050–1057. doi: 10.1158/0008-5472.can-03-3027. [DOI] [PubMed] [Google Scholar]
- 156.Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, Blasiak J, Skorski T. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 158.Smith KC. Spontaneous mutagenesis: experimental, genetic and other factors. Mutat Res. 1992;277:139–162. doi: 10.1016/0165-1110(92)90002-q. [DOI] [PubMed] [Google Scholar]
- 159.Jin M, Lu J, Chen Z, Nguyen SH, Mao L, Li J, Yuan Z, Guo J. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ Int. 2018;120:421–430. doi: 10.1016/j.envint.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 160.Giannoni E, Fiaschi T, Ramponi G, Chiarugi P. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene. 2009;28:2074–2086. doi: 10.1038/onc.2009.77. [DOI] [PubMed] [Google Scholar]
- 161.Martin LJ. DNA damage and repair: relevance to mechanisms of neurodegeneration. J Neuropathol Exp Neurol. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. Journal of controlled release: official journal of the Controlled Release Society. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Setyawati MI, Yuan X, Xie J, Leong DT. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials. 2014;35:6707–6715. doi: 10.1016/j.biomaterials.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 164.Ghosh M, Sinha S, Jothiramajayam M, Jana A, Nag A, Mukherjee A. Cyto-genotoxicity and oxidative stress induced by zinc oxide nanoparticle in human lymphocyte cells in vitro and Swiss albino male mice in vivo. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2016;97:286–296. doi: 10.1016/j.fct.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 165.Li JJ, Yung LY, Hartono D, Bay BH, Zou L, Ong CN. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv Mater. 2010;20:138–142. [Google Scholar]
- 166.Li J, Song Y, Vogt RD, Liu Y, Luo J, Li T. Bioavailability and cytotoxicity of cerium- (IV), copper- (II), and zinc oxide nanoparticles to human intestinal and liver cells through food. Sci Total Environ. 2019;702:134700. doi: 10.1016/j.scitotenv.2019.134700. [DOI] [PubMed] [Google Scholar]
- 167.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 168.Wang Z, Li N, Zhao J, White JC, Qu P, Xing B. CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol. 2012;25:1512–1521. doi: 10.1021/tx3002093. [DOI] [PubMed] [Google Scholar]
- 169.Derfus AM, Chan WCW, Bhatia SN. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv Mater. 2004;16:961–966. [Google Scholar]
- 170.Al Mamun AAM, Gautam S, Humayun MZ. Hypermutagenesis in mutA cells is mediated by mistranslational corruption of polymerase, and is accompanied by replication fork collapse. Mol Microbiol. 2006;62:1752–1763. doi: 10.1111/j.1365-2958.2006.05490.x. [DOI] [PubMed] [Google Scholar]
- 171.Canesi L, Ciacci C, Fabbri R, Marcomini A, Pojana G, Gallo G. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar Environ Res. 2012;76:16–21. doi: 10.1016/j.marenvres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 172.Tiwari DK, Jin T, Behari J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods. 2011;21:13–24. doi: 10.3109/15376516.2010.529184. [DOI] [PubMed] [Google Scholar]
- 173.Shi J, Sun X, Lin Y, Zou X, Li Z, Liao Y, Du M, Zhang H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-kappaB pathways. Biomaterials. 2014;35:6657–6666. doi: 10.1016/j.biomaterials.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 174.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Phys Lung Cell Mol Phys. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 175.Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K. Effect of silica particle size on macrophage inflammatory responses. PLoS One. 2014;9:e92634. doi: 10.1371/journal.pone.0092634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao N, Zhang Q, Mu Q, Bai Y, Li L, Zhou H, Butch ER, Powell TB, Snyder SE, Jiang G, Bing Y. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano. 2011;5:4581–4591. doi: 10.1021/nn200283g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lam HF, Chen LC, Ainsworth D, Peoples S, Amdur MO. Pulmonary function of guinea pigs exposed to freshly generated ultrafine zinc oxide with and without spike concentrations. Am Ind Hyg Assoc J. 1988;49:333–341. doi: 10.1080/15298668891379855. [DOI] [PubMed] [Google Scholar]
- 178.Lam HF, Conner MW, Rogers AE, Fitzgerald S, Amdur MO. Functional and morphologic changes in the lungs of guinea pigs exposed to freshly generated ultrafine zinc oxide. Toxicol Appl Pharmacol. 1985;78:29–38. doi: 10.1016/0041-008x(85)90301-1. [DOI] [PubMed] [Google Scholar]
- 179.Conner MW, Flood WH, Rogers AE, Amdur MO. Lung injury in guinea pigs caused by multiple exposures to ultrafine zinc oxide: changes in pulmonary lavage fluid. J Toxicol Environ Health. 1988;25:57–69. doi: 10.1080/15287398809531188. [DOI] [PubMed] [Google Scholar]
- 180.Kim YH, Fazlollahi F, Kennedy IM, Yacobi NR, Hamm-Alvarez SF, Borok Z, Kim KJ, Crandall ED. Alveolar epithelial cell injury due to zinc oxide nanoparticle exposure. Am J Respir Crit Care Med. 2010;182:1398–1409. doi: 10.1164/rccm.201002-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Smith CJ, Shaw BJ, Handy RD. Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol. 2007;82:94–109. doi: 10.1016/j.aquatox.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 182.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bruneau A, Fortier M, Gagne F, Gagnon C, Turcotte P, Tayabali A, Davis TA, Auffret M, Fournier M. In vitro immunotoxicology of quantum dots and comparison with dissolved cadmium and tellurium. Environ Toxicol. 2015;30:9–25. doi: 10.1002/tox.21890. [DOI] [PubMed] [Google Scholar]
- 185.Tretyakova NY, Groehler A, Ji S. DNA-protein cross-links: formation, structural identities, and biological outcomes. Acc Chem Res. 2015;48:1631–1644. doi: 10.1021/acs.accounts.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 188.Perez-Roses R, Risco E, Vila R, Penalver P, Canigueral S. Biological and nonbiological antioxidant activity of some essential oils. J Agric Food Chem. 2016;64:4716–4724. doi: 10.1021/acs.jafc.6b00986. [DOI] [PubMed] [Google Scholar]
- 189.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 190.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahalingaiah PK, Singh KP. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS One. 2014;9:e87371. doi: 10.1371/journal.pone.0087371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:163–175. doi: 10.1101/sqb.2016.81.030791. [DOI] [PubMed] [Google Scholar]
- 193.Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007;9:125–144. [Google Scholar]
- 194.Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of applied toxicology : JAT. 2009;29:330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 195.Hong J, Wang L, Zhao X, Yu X, Sheng L, Xu B, Liu D, Zhu Y, Long Y, Hong F. Th2 factors may be involved in TiO(2) NP-induced hepatic inflammation. J Agric Food Chem. 2014;62:6871–6878. doi: 10.1021/jf501428w. [DOI] [PubMed] [Google Scholar]
- 196.Park MV, Annema W, Salvati A, Lesniak A, Elsaesser A, Barnes C, McKerr G, Howard CV, Lynch I, Dawson KA, Piersma AH, de Jong WH. In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles. Toxicol Appl Pharmacol. 2009;240:108–116. doi: 10.1016/j.taap.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 197.Kadar E, Tarran GA, Jha AN, Al-Subiai SN. Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ Sci Technol. 2011;45:3245–3251. doi: 10.1021/es1029848. [DOI] [PubMed] [Google Scholar]
- 198.Mesaric T, Sepcic K, Drobne D, Makovec D, Faimali M, Morgana S, Falugi C, Gambardella C. Sperm exposure to carbon-based nanomaterials causes abnormalities in early development of purple sea urchin (Paracentrotus lividus) Aquat Toxicol. 2015;163:158–166. doi: 10.1016/j.aquatox.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 199.Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- 200.Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81:1253–1262. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 201.Panikkanvalappil SR, Mahmoud MA, Mackey MA, El-Sayed MA. Surface-enhanced Raman spectroscopy for real-time monitoring of reactive oxygen species-induced DNA damage and its prevention by platinum nanoparticles. ACS Nano. 2013;7:7524–7533. doi: 10.1021/nn403722x. [DOI] [PubMed] [Google Scholar]
- 202.Tarnuzzer RW, Colon J, Patil S, Sudipta S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 203.Rispail N, De Matteis L, Santos R, Miguel AS, Custardoy L, Testillano PS, Risueno MC, Perez-de-Luque A, Maycock C, Fevereiro P, Oliva A, Fernandez-Pacheco R, Ibarra MR, de la Fuente JM, Marquina C, Rubiales D, Prats E. Quantum dot and superparamagnetic nanoparticle interaction with pathogenic fungi: internalization and toxicity profile. ACS Appl Mater Interfaces. 2014;6:9100–9110. doi: 10.1021/am501029g. [DOI] [PubMed] [Google Scholar]
- 204.Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397–415. doi: 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhi X, Fang H, Bao C, Shen G, Zhang J, Wang K, Guo S, Wan T, Cui D. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34:5254–5261. doi: 10.1016/j.biomaterials.2013.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].