Abstract
Background
Differentiating malignancy from benign diseases is the key to successful management of adnexal masses. Risk of malignancy algorithm (ROMA) has been used for this purpose. We have prospectively studied the diagnostic value of ROMA in patients presented with adnexal masses.
Methods
We prospective calculated ROMA values prior to surgery for adnexal masses. The risk calculated was then correlated with the histological findings, and results were analyzed according to menopausal status. ROMA cutoff value was determined using ROC curve, and sensitivity, specificity and predictive values were calculated. Statistics were performed on SPSS software (version 20.0).
Results
There were 94 patients with adnexal masses included in the study, 65 (69.1%) had epithelial ovarian cancer and 29 (30.9%) were diagnosed benign on histopathology. In both pre- and postmenopausal patients, ROMA values were significantly higher in patients with malignancy compared to those with benign disease (p < 0.05). ROMA score was of a significant diagnostic value in both premenopausal (AUC = 0.914, Z = 10.81, p < 0.001) and postmenopausal patients (AUC = 0.975, Z = 21.51, p < 0.001). In premenopausal females, ROMA > 13.3% was able to discriminate malignant from benign patients with 97.06% sensitivity and 85.00% specificity. The positive and negative predictive values were 91.7% and 94.4%. Similarly, in postmenopausal females, ROMA value of > 76% achieved 87.10% sensitivity and 100.00% specificity in discriminating malignant from benign patients with 100% positive and 69.2% negative predictive value. The overall accuracy of ROMA in pre- and postmenopausal patients was 87.0% and 85%, respectively.
Conclusions
ROMA is a useful and accurate test for differentiating epithelial ovarian cancer from benign ovarian masses. Further studies are needed to compare performance of ROMA with the Risk of Malignancy Index (RMI), CA 125 and HE4. Such comparative studies will be helpful to the clinician in deciding the best diagnostic tool for women with adnexal masses.
Keywords: ROMA, HE4, CA 125, Adnexal mass
Introduction
Adnexal mass is a common clinical problem encountered in daily practice. Both benign and early ovarian cancer can present with the adnexal masses that are seen on imaging performed for other reasons [1, 2]. There are no specific symptoms which clinch the diagnosis of ovarian cancer. A woman’s lifetime risk of developing ovarian cancer is approximately 1 in 70 [3]. The presence of an adnexal mass raises anxiety of patients. Multiple resources and money are spent in diagnosing these lesions. Many of them result in unnecessary surgery. It is important to correctly characterize the true nature of these masses for their appropriate and timely management [4]. According to a systematic review published in 2009, patients with ovarian cancer who are managed in specialized centers by oncologist have improved outcomes [5]. No screening protocol has yet been shown to reduce the number of advanced-stage diagnoses or the number of ovarian cancer deaths [6].
CA 125 is a widely utilized tumor marker for evaluation of adnexal masses but lacks sensitivity and specificity [7]. A risk of malignancy algorithm (ROMA) is a recent test, which combines the serum CA 125 and HE4 with menopausal status into a numerical score [8]. FDA has approved ROMA for distinguishing malignant from benign pelvic masses recently in 2011 [9]. There are few studies which had looked into the value of this algorithm, but none has been carried out so far in India. So, we undertook this study in north Indian subset of females to know how well ROMA differentiates malignant from benign ovarian masses.
Materials and Methods
This prospective study was conducted, in the Department of Surgical Oncology at a tertiary care teaching hospital, after the approval by institutional ethical committee. Patients were enrolled from January 2014 to January 2016 after informed written consent. At the time of presentation, complete clinical data were recorded. Patients aged above 18 years presenting with adnexal masses documented by imaging (USG/CT/MRI) without any other suspicious clinical finding were included in this study. Exclusion criteria involved pregnant females, those with prior personal history of other cancer in the past or history of gynecological surgery and patients with concurrent heart/renal/liver failure.
ROMA Algorithm (Risk of Malignancy Algorithm)
ROMA uses serum CA 125 and HE4 values along with menopausal status to generate a predictive index for epithelial ovarian cancer. Before surgery, serum levels of CA 125 and HE4 were measured by fully automated Abbott/ARCHITECT system utilizing the most sensitive Chemiluminescent Microparticle Immunoassay (CMIA) technology. ROMA score was calculated by the following formulas where LN is the natural logarithm.
Premenopausal woman:
Predictive index (PI) = − 12.0 + 2.38 * LN [HE4] + 0.0626 * LN [CA 125]
Postmenopausal woman:
Predictive index (PI) = − 8.09 + 1.04 * LN [HE4] + 0.732*LN [CA 125]
ROMA score (%) = exp(PI)/[1 + exp(PI)] * 100
According to the CA 125 and HE4 immunoassay manufacturer instructions, ROMA cutoff of 7.4% was taken for premenopausal females and 25.3% was taken for postmenopausal females.
Surgery
Patients operated for undiagnosed ovarian mass underwent oophorectomy. Decision for the extent of surgery was dependent on the intraoperative findings. For suspicious findings of ovarian cancer, patient was surgically staged and optimally cytoreduction was performed. In doubtful intraoperative situations, frozen section diagnosis guided the extent of surgery.
Statistical Analysis
Analyses were performed on SPSS software (version 20.0). Continuous groups were compared by independent/paired t test and categorical groups by Chi-square (χ2) test. Receiver operating characteristics (ROC) curve analysis was performed to assess diagnostic accuracy, sensitivity and specificity of the ROMA values. A two-tailed p < 0.05 was considered statistically significant.
Results
Among 94 patients, 29 (30.9%) were benign and 65 (69.1%) were epithelial ovarian cancer patients. The mean age among benign cases was 39.86 ± 2.45 years (range 18–70), while in malignant cases it was 44.40 ± 1.29 years (range 19–80) (p = 0.075). Among benign cases, 20 (69%) were premenopausal and 9 (31%) were postmenopausal. Similarly, in the malignant group, 34 (52.3%) were premenopausal and 31 (47.7%) were postmenopausal. This distribution of cases between both subgroups according to menopausal status was similar (p = 0.131) and hence not a confounding factor.
In benign cases, the most common histological subtypes were adenomas (27.6%) followed by others (24.1%) which included simple cyst and fibroma, tuberculosis (20.8%), endometriosis (16.6%) and dermoid (10.9%). In contrast, the most common malignant histological subtype was serous adenocarcinoma (84.6%) followed by mucinous adenocarcinomas (15.4%) only.
Among malignant cases, 3 (4.6%) patients were having FIGO stage I disease, 9 (13.8%) stage II, 44 (67.7%) stage III and 9 (13.8%) were having stage IV disease.
The values of ROMA were compared according to their menopausal status between two subgroups of benign and malignant cases. In premenopausal females, ROMA percentage values were 16.83 ± 6.48 (range 0.2–98.7) in benign and 78.25 ± 5.25 (range 5.6–99.9) in the malignant subgroup. In postmenopausal females, ROMA percentage values were 39.34 ± 8.19 (range 7–76) in benign and 92.26 ± 2.08 (range 57–100) in the malignant subgroup. On comparing, the values of ROMA in both pre- and postmenopausal malignant cases were found significantly (p < 0.05) higher as compared to benign cases.
In premenopausal females, ROMA (AUC = 0.914, Z = 10.81, p < 0.001) showed a significant diagnostic value. ROMA value of > 13.3% was able to discriminate both subgroups of patients with 97.06% sensitivity (95% CI 84.6–99.5) and 85.00% specificity (95% CI 62.1–96.6). The positive and negative predictive values at this cutoff were 91.7% and 94.4%, respectively. Overall accuracy in premenopausal females was 87.0% (Table 1, Fig. 1).
Table 1.
Sensitivity and specificity of ROMA to discriminate benign and malignant cases using ROC curve analysis according to menopausal status
| Menopausal status | Parameters | Cutoff value | Sensitivity (95% CI) | Specificity (95% CI) | + PV | − PV |
|---|---|---|---|---|---|---|
| Premenopause | ROMA (%) | > 13.3 | 97.06 | 85.00 | 91.7 | 94.4 |
| (84.6–99.5) | (62.1–96.6) | |||||
| Postmenopause | ROMA (%) | > 76 | 87.10 | 100.00 | 100.0 | 69.2 |
| (70.1–96.3) | (66.2–100.0) |
+ PV: positive predictive value
− PV: negative predictive value
Fig. 1.
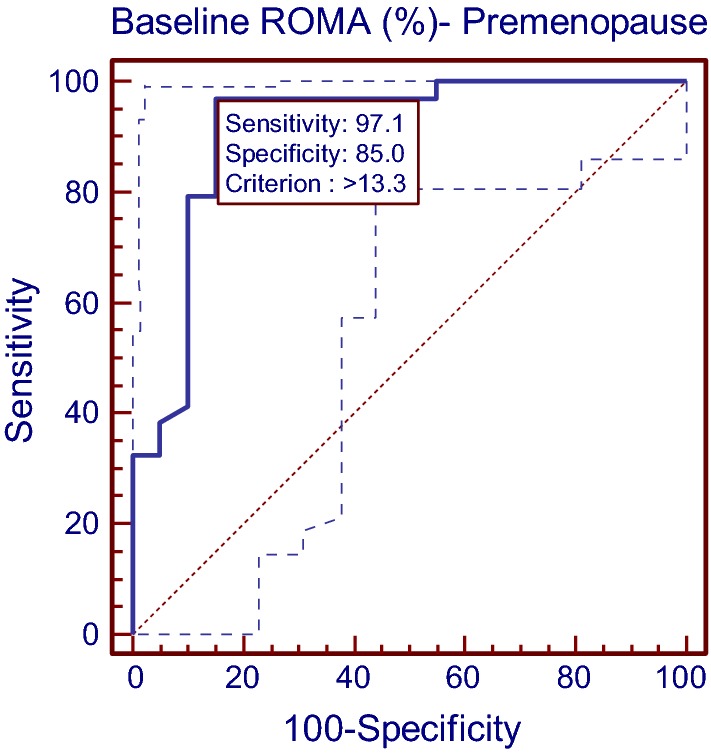
Diagnostics of ROMA to discriminate the benign and malignant premenopause cases using ROC curve analysis
In postmenopausal females, ROMA (AUC = 0.975, Z = 21.51, p < 0.001) showed a significant diagnostic value. ROMA value of > 76% achieved 87.10% sensitivity (95% CI 70.1–96.3) and 100.00% specificity (95% CI 66.2–100) in discriminating both the subgroups. Also the positive and negative predictive values were 100% and 69.2%, respectively. In postmenopausal female, ROMA was found to be 85% accurate (Table 1, Fig. 2).
Fig. 2.
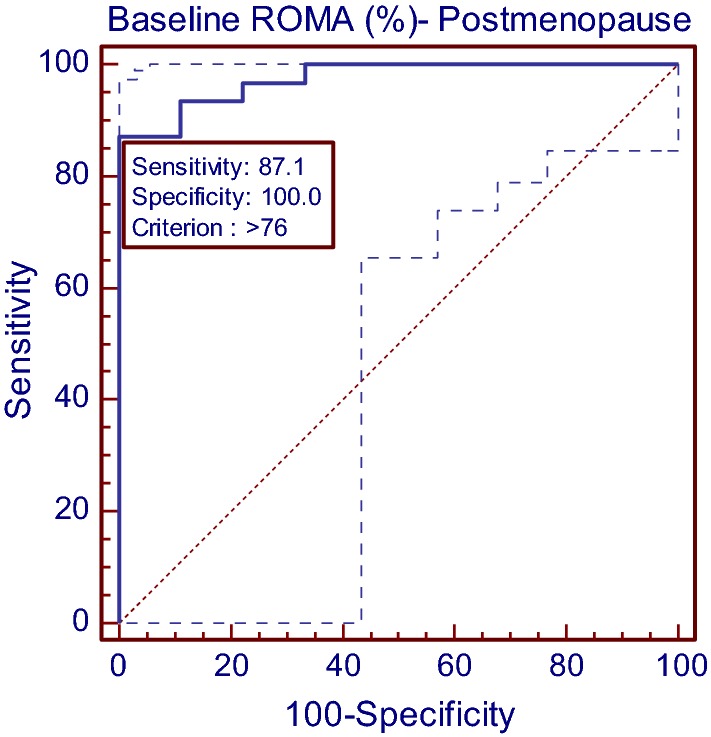
Diagnostics of ROMA to discriminate the benign and malignant postmenopause cases using ROC curve analysis
Discussion
In 2011, Moore et al. found that ROMA identified 94% of all epithelial ovarian cancers as high risk and 75% of all benign diseases as low risk. They found ROMA to be 100% sensitive in premenopausal patients. After successful completion of this community-based trial, ROMA was approved by the FDA for distinguishing malignant from benign pelvic masses in 2011 [9]. There are few studies which evaluated ROMA before and after its FDA approval (Table 2). But there are many issues related with these studies. Firstly, the patient population included in malignant cases not only included epithelial ovarian cancer pathology, but also included non-epithelial histology and Krukenberg tumors also. Secondly, the assays used for CA 125 and HE4 were different and hence likely to distort the diagnostics of the test. Thirdly, in some studies the interpretation of ROMA result was done without considering the menopausal status, while in others interpretation was done according to their menopausal status. Lastly, the accuracy of the ROMA was reported by only four studies [10–13] And only two of them actually reported the accuracy according to their menopausal status [10, 12]. All of these factors make it difficult to compare and interpret the results of these studies. Compared to these studies, the patient selection in our study was homogenous and included only EOC in malignant group and benign ovarian masses in the benign group. The assay utilized in our study was CMIA, which is the most sensitive assay currently available. Also, we have reported sensitivity, specificity, predictive values and AUC in our study. According to our study, the risk of having malignancy is high if the values for ROMA were > 13.3% in premenopausal females and > 76% in postmenopausal females presenting with adnexal masses.
Table 2.
Studies of ROMA in the literature
| S. no. | Study | Year | EOC | ROMA cutoff (%) | CA 125 and HE4 test methods | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|---|---|---|---|
| 1. | Moore et al. [8] | 2009 | 129 | 13.1*, 27.7** |
CMIA EIA |
94 | 75 | ND |
| 2. | Bandiera et al. [14] | 2011 | 113 | 7.4*, 25.3** |
CMIA CMIA |
84.6*, 93.1** | 81.2*, 84.4** | ND |
| 3. | Van Gorp et al. [10] | 2011 | 161 | 12.5*, 14.4** |
EIA EIA |
84.9, 67.5*, 90.8** | 79.7, 87.9*, 66.3** | 0.898, 0.846*, 0.891** |
| 4. | Montagnana et al. [15] | 2011 | 55 | 12.5*, 14.4** |
CLEIA EIA |
75 | 82 | ND |
| 5. | Kim et al. [16] | 2011 | 72 | 7.6*, 10.9** |
CMIA CMIA |
88 | 94 | ND |
| 6. | Jacob et al. [17] | 2011 | 29 | 13.1 |
ELISA ELISA |
90 | 87 | ND |
| 7. | Moore et al. [9] | 2011 | 48 | 13.1*, 27.7** |
CMIA ELISA |
94 | 75 | ND |
| 8. | Chan et al. [11] | 2013 | 65 | 7.4*, 25.3** |
CMIA CMIA |
89.2 | 87.3 | 0.95 |
| 9. | Sandri et al. [12] | 2013 | 153 | 7.4*, 25.3** |
CMIA CMIA |
ND | ND | 0.93, 0.91*, 0.93** |
| 10. | Karlsen et al. [13] | 2015 | 550 | ND | ND | ND | ND | 0.920 |
| 11. | Present study | 2016 | 65 | 7.4*, 25.3** |
CMIA CMIA |
89.2, 97.1*, 87.1** | 92.1, 85*, 100** | 0.918, 0.914*, 0.975** |
*Premenopausal value
**Postmenopausal value
EIA—enzyme immunoassay
ELISA—enzyme linked immunoadsorbent assay
CLEIA—chemiluminescence enzyme immunoassay
CMIA—chemiluminescent microparticle immunoassay
ND—not defined
Few studies have been carried out to compare ROMA, CA 125 and HE4 according to the menopausal status of the females. Among premenopausal group, ROMA and HE4 had similar sensitivity to diagnose epithelial ovarian cancer but sensitivity of ROMA was less than CA 125. In terms of specificity, both these studies had found ROMA to be more specific than CA 125 but less specific than HE4 [10, 14].
Studies had found different results in the postmenopausal group. Bandiera et al. found ROMA to be less sensitive than CA 125 but more sensitive than HE4, while ROMA was more specific than CA 125 [14]. In another study, ROMA was found to be more sensitive than both CA 125 and HE4. But, specificity of ROMA was more than CA 125 [10]. Well-designed studies, appropriate patients selection and methodology are essential before concluding the superiority of these diagnostic tests among them.
Conclusions
ROMA is a useful and accurate test to differentiate EOC from benign ovarian masses. More uniform studies are needed to compare ROMA, RMI, CA 125 and HE4. Such comparative studies in future will be helpful to the clinician to order the best diagnostic test for optimizing patient management.
Acknowledgements
No financial support or sponsorship was utilized in terms of grant or funds. We would like to thank our Vice Chancellor, Professor ML Bhatt, for encouraging and guiding us for this study.
Dr. Vijay Kumar
is a senior Surgical Oncologist working as an Additional Professor at Department of Surgical Oncology, King George’s Medical University, Lucknow. He has numerous publications to his credits and is actively involved in academic and research activities. Although he operates on all solid tumors, he has a keen interest in Gynecological oncology and Head & Neck oncology. He is a dedicated teacher and takes interest in training his students. He is also a guide and co-guide for many Ph.D. and PG students.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest in the present study.
Ethical Approval
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Vijay Kumar is the Prof. Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh. Shiv Rajan is the Assistant Prof. Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh. Sameer Gupta is the Associate Prof. Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh. Naseem Akhtar is the Assistant Prof. Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh. Sonali Sharma is the Senior resident, Department of Gynecology and Obstetrics, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, Punjab. Punnet Sinha is the Senior resident, Department of Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh. Sanjeev Misra is the Director and CEO, All India Institute of Medical Sciences, Jodhpur, Rajasthan. Arun Chaturvedi is the Professor and Head, Surgical Oncology, King George’s Medical University, Lucknow, Uttar Pradesh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122:210–217. doi: 10.1097/AOG.0b013e318298def5. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol. 2010;202:373.e1–373.e9. doi: 10.1016/j.ajog.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. Available at http://seer.cancer.gov/csr/1975_2011/.
- 4.American College of Obstetricians and Gynecologists. Management of adnexal masses. ACOG Practice Bulletin No. 83. Obstet Gynecol. 2007; 110:201–214. [DOI] [PubMed]
- 5.du Bois A, Rochon J, Pfisterer J, et al. Variations in institutional infrastructure, physician specialization and experience, and outcome in ovarian cancer: a systematic review. GynecolOncol. 2009;112:422–436. doi: 10.1016/j.ygyno.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 7.Sturgeon CM, Duffy MJ, Stenman UH, et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast and ovarian cancer. Clin Chem. 2008;54:e11–e79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 8.Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA-125 for the prediction of ovarian cancer in patients with a pelvic mass. GynecolOncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore RG, Miller MC, Disilvestro P, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. ObstetGynecol. 2011;118:280–288. doi: 10.1097/AOG.0b013e318224fce2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanGorp T, Cadron I, Despierre E, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the risk of ovarian malignancy algorithm. Br J Cancer. 2011;104:863–870. doi: 10.1038/sj.bjc.6606092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan KK, Chen CA, Nam JH, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. GynecolOncol. 2013;28:239–244. doi: 10.1016/j.ygyno.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Sandri MT, Bottari F, Franchi D, et al. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. GynecolOncol. 2013;128:233–238. doi: 10.1016/j.ygyno.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Karlsen MA, Høgdall EV, Christensen IJ, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer—an international multicenter study in women with an ovarian mass. GynecolOncol. 2015;138:640–646. doi: 10.1016/j.ygyno.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Bandiera E, Romani C, Specchia C, et al. Serum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnostic and prognostic tools for epithelial ovarian cancer management. Cancer Epidemiol Biomarkers Prev. 2011;20:2496–2506. doi: 10.1158/1055-9965.EPI-11-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montagnana M, Danese E, Ruzzenente O, et al. The ROMA (risk of ovarian malignancy algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? ClinChem Lab Med. 2011;49:521–525. doi: 10.1515/CCLM.2011.075. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Whang DH, Park J, et al. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. ClinChem Lab Med. 2011;49:527–534. doi: 10.1515/CCLM.2011.085. [DOI] [PubMed] [Google Scholar]
- 17.Jacob F, Meier M, Caduff R, et al. No benefit from combining HE4 and CA-125 as ovarian tumor markers in a clinical setting. GynecolOncol. 2011;121:487–491. doi: 10.1016/j.ygyno.2011.02.022. [DOI] [PubMed] [Google Scholar]


