Abstract
The circadian clock broadly governs immune cell function, leading to time-of-day differences in inflammatory responses and subsequently, pathogen clearance. However, the effect of inflammatory signals on circadian machinery is poorly understood. We found that in bone marrow-derived macrophages, some host-derived pro-inflammatory cytokines, e.g., IFN-γ or TNF-α, and pathogen-associated molecular patterns, e.g., LPS or Pam3Csk4, suppress the amplitude in oscillations of circadian negative feedback arm clock components such as PER2, and when examined, specific combinations of these immune-related signals suppressed the amplitude of these oscillations to a greater degree in both bone marrow-derived and peritoneal macrophages. At the transcript level, multiple components of the circadian clock were affected in different ways by pro-inflammatory stimulus, including Per2 and Nr1d1. This suppressive effect on PER2 did not arise from nor correlate with cell death or clock resetting. Suppression of the clock by IFN-γ was dependent on its cognate receptor; however, pharmacological inhibition of the canonical JAK/STAT and MEK pathways did not hinder suppression, suggesting a mechanism involving a non-canonical pathway. In contrast, anti-inflammatory signals such as IL-4 and dexamethasone enhanced the expression of PER2 protein and Per2 mRNA. Our results suggest that the circadian system in macrophages can differentially respond to pro- and anti-inflammatory signals in their microenvironments.
Keywords: circadian, macrophage, pro-inflammatory, anti-inflammatory, cytokine, PAMPs
Introduction
The solar day/night cycle imposes a 24-h environment on all kingdoms of life, from prokaryotes to humans, and in response, members of all these groups of organisms have evolved ~24-h circadian clocks that can be entrained by the environmental cycles (1). Organisms use circadian rhythms to predict timing of environmental pressures and elicit physiological processes including, in multicellular organisms, time-of-day variations in immune responses (2–5). In mammals, receptors in the retina become activated on light exposure and then transmit information to the suprachiasmatic nucleus (SCN), where the master clock that governs behavior resides (6). Subsequently, the master clock helps to synchronize cells in the periphery (7, 8), including monocytes and macrophages, which exhibit robust cell-autonomous circadian rhythms (2, 9–21). These rhythms are entrained and affected by a range of signals and stimuli, including hormones and nutrients (22, 23); however, many of the important immune signals in circadian biology are uncharacterized.
At the cellular level, the circadian oscillator originates from a transcription-translation feedback loop (TTFL) comprised of several core clock components. A positive arm, consisting of the transcription factors BMAL1 and CLOCK (24–27), drives the expression of clock-controlled genes (CCGs) and also the expression of a negative arm encompassing the protein products of Per1/Per2/Per3 and Cry1/Cry2 (28–31). The negative arm represses the activity of the positive arm and thereby closes the feedback loop. An additional interlocking loop is comprised of positive elements encoded by Rora/Rorb/Rorc and negative elements encoded by Nr1d1/Nr1d2 (also known as Rev-erbα/β) (32– 34). This circadian oscillator is cell-autonomous and drives cyclic gene expression in many immune cells including T cells, B cells, macrophages, and more (9, 12, 35–38). Under homeostatic conditions, macrophages rhythmically express ~1,400 CCGs (9), of which a significant portion have immune function (e.g., toll-like receptor expression and others), suggesting that a macrophage can better prime itself in anticipation of pathogen encounter during some times of the day.
During an active infection, pathogen-associated molecular patterns (PAMPs) trigger these receptors on macrophages. Macrophages subsequently release pro-inflammatory cytokines to initiate pathogen clearance. Rhythmic cellular responses may therefore contribute to time-of-day differences in the in vivo clearance of microbes, including bacteria (Salmonella), viruses (herpesvirus, influenza virus), parasites (Leishmania), and fungi (Aspergillus) (39–42). However, after the initiation of microbe clearance, little is currently known about how PAMPs or pro-inflammatory cytokines affect the molecular circadian oscillator.
Studies suggest that pro-inflammatory cytokines may influence the clock in several tissue types and model organisms. Repetitive subcutaneous administration of pro-inflammatory cytokines such as interferon (IFN)-α, or to a less well-studied extent IFN-γ, suppresses expression of multiple clock components Per1/2/3, Clock, and Bmal1 mRNA in the mouse SCN (43). Similarly, the presence of IFN-γ in rat SCN cultures suppresses rhythmic PER1LUC protein levels and suppresses neuronal firing rates (44). PAMPs such as lipopolysaccharide (LPS) also appear to suppress Bmal1 mRNA levels in peritoneal macrophages while other stimuli such as Pam3CSK4 (P3C) do not affect Bmal1 levels (15). Of interest, recent studies revealed that host inflammatory status affects circadian rhythms: a drug screen targeting inflammation in zebrafish revealed amplitude differences (45), and parasite infection disrupted mouse circadian rhythms (46). In general, the clock responds to pro-inflammatory stimuli, but how the macrophage clock is affected by a range of different inflammatory signals is poorly understood.
To understand how pro- and anti-inflammatory immune modulators might impact the clock in macrophages, we utilized bone marrow-derived macrophages (BMDMs) because they have a robust circadian phenotype and exhibit diversity and plasticity in response to a multitude of activation signals (47, 48). Given that pro-inflammatory signals and anti-inflammatory signals independently act to polarize macrophages toward opposite phenotypes, we hypothesized that the molecular clock in macrophages would respond differently to these stimuli. In this study, we found that some pro-inflammatory signals strongly suppressed PER2 rhythmicity, and that the pathway from IFN-γ to the core oscillator followed a non-canonical pathway from the IFN-γ receptor. We also found that anti-inflammatory signals enhanced the amplitude of PER2 expression, suggesting that the clock responds to different immune-associated stimuli by strengthening or relaxing clock control of gene expression. The results uncover a novel pro- and anti-inflammatory axis in the molecular circadian clock and provide a new perspective on circadian immunobiology.
Results
Pro-inflammatory Stimuli Alter PER2 Rhythms in Macrophages
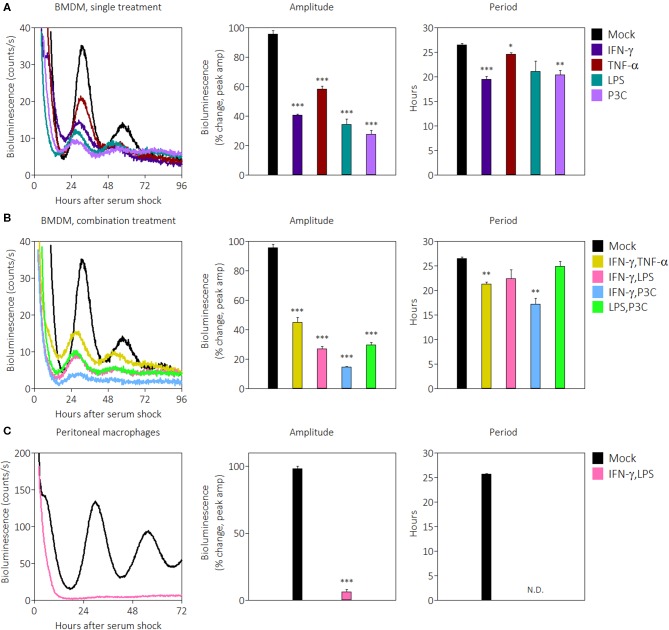
Previous work demonstrated that systemically administered IFN-γ suppresses Per1 mRNA levels in the mouse SCN (43). Since IFN-γ is the main cytokine associated with classical activation in macrophages, we investigated the influence of IFN-γ and other pro-inflammatory stimuli on the macrophage clock. The other stimuli included tumor necrosis factor (TNF)-α, a cytokine produced by macrophages, lipopolysaccharide (LPS), a pathogen-associated molecular pattern in gram-negative bacteria, and Pam3CSK4 (P3C), a synthetic triacylated lipopeptide. We generated bone marrow-derived macrophages (BMDMs) from male mPer2Luc reporter mice, in which the luciferase gene is fused to the endogenous Per2 gene allowing real-time tracking of PER2 protein (49). The purity of BMDMs was observed by flow cytometry to be over 95% based on cell surface stains for CD11b and F4/80 (Supplemental Figure 1A). After serum-synchronization of BMDMs by FBS, we observed the circadian clock via PER2LUC rhythms. BMDMs in the presence of pro-inflammatory signals exhibited significant reductions in expression of PER2LUC amplitude in the first peak: IFN-γ (40.6% ± 0.9), TNF-α (58.3% ± 2.2), LPS (34.3% ± 3.8), or P3C (27.7% ± 2.7), compared with mock treatments (±SEM; n = 3) (Figure 1A). The presence of three signals also shortened period: IFN-γ (19.5 h ± 0.6), TNF-α (24.6 h ± 0.3), and P3C (20.4 h ± 0.9) compared with mock (26.5 h ± 0.3) (Figure 1A). These data indicate that multiple pro-inflammatory stimuli were able to suppress the amplitude and shorten the period of the macrophage circadian clock.
Figure 1.
Pro-inflammatory stimuli suppress PER2LUC rhythms in macrophages. (A) Raw PER2LUC traces of synchronized mPer2Luc BMDMs stimulated with mock, IFN-γ, TNF-α, or P3C (50 ng/mL for 24 h), and amplitude and period analysis of rhythms from traces. (B) PER2LUC traces of synchronized BMDMs stimulated with mock, IFN-γ plus TNF-α, IFN-γ plus LPS, IFN-γ plus P3C, or LPS plus P3C (50 ng/mL each for 24 h), and amplitude and period analysis of rhythms. (C) PER2LUC traces of synchronized peritoneal macrophages stimulated with mock, or IFN-γ plus LPS, and amplitude and period analysis of rhythms. Data are represented as mean ± SE (n = 3). P-values are calculated from one-way ANOVA with post-hoc Tukey tests and are considered to be significantly different with *P < 0.05, **P < 0.005, or ***P < 0.0005.
During an active immune response, macrophages may encounter several pro-inflammatory signals in vivo simultaneously. In order to identify whether pro-inflammatory stimuli affect the clock additively or whether there is a dominant signal affecting the clock, we combined stimuli at the same concentrations as Figure 1A and observed their effect on PER2LUC rhythms. The results showed that some combinations of pro-inflammatory stimuli suppressed amplitudes to greater extents than their single treatment counterparts. BMDMs in the presence of pro-inflammatory stimuli combinations exhibited reductions in PER2LUC amplitude: IFN-γ plus TNF-α (45.1% ± 3.3), IFN-γ plus LPS (27.0% ± 1.7), IFN-γ plus P3C (14.7% ± 0.2), and LPS plus P3C (29.7% ± 1.9), compared with mock treatment conditions (Figure 1B). The presence of two combinations of stimuli also significantly shortened period: IFN-γ plus TNF-α (21.3 h ± 0.4), and IFN-γ plus P3C (17.2 h ± 1.2), compared with mock treatment conditions (26.5 h ± 0.3) (Figure 1B). These data indicate that a combination of pro-inflammatory stimuli suppressed the amplitude of the clock in an additive manner, dependent on stimulus used. However, this generalization does not extend to period shortening effects. These results suggested the possibility of a dosage effect; therefore, to determine whether increasing IFN-γ dose suppressed amplitude of rhythms to a greater degree, we tested a range of IFN-γ concentrations (from 5 to 200 ng/mL) on BMDMs and observed PER2LUC rhythms. IFN-γ at all concentrations affected PER2LUC rhythms in a similar manner (Supplemental Figure 1C). Similar results were observed when inflammatory signals were given to macrophages and washed out prior to synchronization (data not shown), suggesting the health of the macrophages was not impacted by constant exposure to pro-inflammatory signals. We can conclude that the clock in BMDMs responds to pro-inflammatory stimuli through different pathways to downregulate the expression of circadian clock component PER2. The results, however, do not exclude the possibility that PER2 suppression by pro-inflammatory cytokines is a unique response in BMDMs.
To investigate whether the clock suppressive effect was conserved in other macrophage types, we began by first focusing on the effect of IFN-γ plus LPS. We harvested peritoneal macrophages by thioglycolate elicitation, cultured them overnight, removed non-adherent cells by washing, and then synchronized the population using FBS. The purity of peritoneal macrophages was >80% based on cell surface stains for CD11b and F4/80 (Supplemental Figure 1B). Synchronized peritoneal macrophages were robustly rhythmic as previously reported (9), and in the presence of IFN-γ plus LPS, demonstrated such a remarkable reduction of PER2LUC amplitude (6.2% ± 2.0) that the cells appeared arrhythmic, and therefore the period estimate could not be accurately determined (Figure 1C).
To more systematically determine the effects of singular pro-inflammatory stimuli, PER2LUC rhythms from peritoneal macrophages were observed after treatment with IFN-γ, TNF-α, LPS, or IFN-γ plus LPS (Supplemental Figure 1D). Peritoneal macrophages in the presence of IFN-γ plus LPS again exhibited reductions in PER2LUC amplitude (24.0% ± 2.0) compared with mock (100% ± 17.3) whereas no single signal elicited a significant change in rhythm amplitude: IFN-γ (86.5% ± 11.5), TNF-α (116.7% ± 4.0), or LPS (136.2% ± 20.0) (Supplemental Figure 1D), although LPS alone did increase the overall level of bioluminescence. The presence of IFN-γ plus LPS again promoted arrhythmicity, i.e., no second peak and without an increase in bioluminescence, and IFN-γ alone modestly reduced period (23.5 h ± 0.3) compared with mock (25.7 h ± 0.3) (Supplemental Figure 1D), suggesting that unlike BMDMs, peritoneal macrophages require both activation signals to suppress circadian clock PER2 amplitude. Together, the results indicate that PER2LUC amplitude suppression by some combinations of pro-inflammatory stimuli is not limited to BMDMs and is possibly a pan-macrophage effect.
Pro-inflammatory Stimuli Alter Core Clock Transcript Rhythms
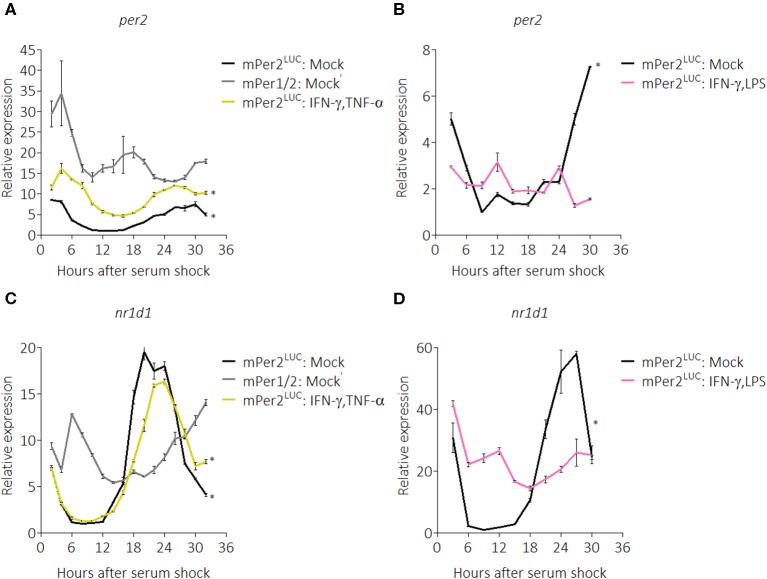
To ascertain the breadth of the suppressive effect on clock components, we next used quantitative RT-PCR to probe the circadian expression profile of core clock genes in the presence of pro-inflammatory cytokines. We chose IFN-γ plus TNF-α as stimuli because the original characterization of activated macrophages was described in the context of these two cytokines (50). mRNA was harvested from BMDMs over a 32-h time-course with a 2-h resolution and quantified. Gene expression was normalized to a non-circadian housekeeping gene, Eif2a (51), and then all data were normalized to mock treatment conditions in mPer2Luc BMDMs. mPer1Brdm1mPer2Brdm1 (mPer1/2) mice harbor deletions of regions in the Per1 and Per2 genes essential for proper circadian function (52) and were used as circadian clock-deficient controls. Per2 mRNA from mPer2Luc BMDMs exhibited significant circadian rhythms (p = 0.0013, robustness = 88.4%, significance determined by a Cosinor regression model) while those from mPer1/2 were non-circadian (p = 1.000, robustness = 22.6%) (Figure 2A). Surprisingly, we found that contrary to the PER2LUC results in Figure 1, Per2 mRNA levels were elevated in BMDMs stimulated with IFN-γ plus TNF-α (Figure 2A), though, the peak-to-trough amplitude was comparable to mock; these rhythms were determined to be circadian (p = 0.0013, robustness = 88.5%), suggesting a role for post-transcriptional control in effecting the cytokine-induced suppression of PER2 protein levels seen in Figure 1.
Figure 2.
Pro-inflammatory stimuli affect Per2 and Nr1d1 mRNA rhythms. qRT-PCR data showing (A) Per2 mRNA expression over a 32 h circadian time course (2 h resolution) from mPer2Luc BMDMs or mPer1/2 BMDMs. Cells were stimulated with mock or IFN-γ plus TNF-α (50 ng/mL each for 24 h). (B) Per2 mRNA expression over a 30 h circadian time course (3 h resolution) from mPer2Luc BMDMs. Cells were stimulated with mock or IFN-γ plus LPS (50 ng/mL each for 24 h). (C) Nr1d1 mRNA expression in exact conditions as in (A). (D) Nr1d1 mRNA expression in exact conditions as in (B). Genes here were normalized to non-rhythmic Eif2a expression. Data are represented as mean ± SE (n = 3). P–values (*) are calculated from Cosinor analysis and significance (at P < 0.05) is considered to be rhythmic circadianly.
To investigate whether the same effect on the clock resulted from stimulation with other pro-inflammatory conditions, we performed another time-course (30-h with 3-h resolution) in the presence of IFN-γ plus LPS. We found that in these conditions, Per2 mRNA levels were suppressed and no longer exhibited circadian rhythmicity (p = 1.000, robustness = 11.5%) (Figure 2B). We also explored regulation of the core clock component, Nr1d1 (also known as Rev-erbα), confirming that Nr1d1 mRNA levels in mPer1/2 controls did not oscillate, whereas mock-treated mPer2Luc controls oscillated circadianly (p = 0.0005, robustness = 91.8%), as did the IFN-γ plus TNF-α stimulated group (p = 0.0003, robustness = 93.5%) (Figure 2C). In contrast, in the IFN-γ plus LPS stimulated group, Nr1d1 levels were suppressed and lost circadian rhythmicity (p = 0.989, robustness = 57.7%) (Figure 2D), suggesting that different inflammatory stimuli impart diverse effects on the clock.
This work was extended to see the effects of pro-inflammatory stimulation by IFN-γ plus TNF-α on expression of other clock components (Supplemental Figure 2). The results revealed that in the presence of IFN-γ plus TNF-α, Bmal1, Rora, and Cry2 mRNA transcripts were differentially expressed and modestly suppressed, despite functional Per2 and Nr1d1 rhythmicity (Supplemental Figure 2). Pro-inflammatory stimuli IFN-γ and TNF-α can influence the expression of circadian clock components while maintaining circadian rhythmicity of the cell.
PER2 Amplitude Suppression Is Not Mediated Through Cell Death or Clock Resetting
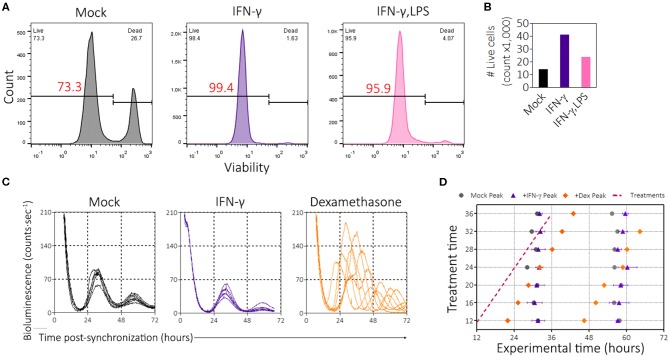
In the previous experiments, we noticed that the presence of IFN-γ promoted growth of BMDMs in culture. However, because some pro-inflammatory stimuli such as LPS induce apoptosis (53), we wondered whether the suppression of PER2LUC amplitude by IFN-γ was simply the result of cell death leading to a smaller population of viable cells. To investigate this possibility, we seeded mPer2Luc BMDMs at a low density (5 × 105 cells per 35 mm dish) to minimize the effect of overcrowding and then treated with inflammatory signals (IFN-γ or IFN-γ plus LPS) for 24 h. The cells were synchronized, and then harvested and stained with a viability dye (Zombie Red, BioLegend) 46 h post-synchronization. Cells were analyzed by flow cytometry, first gating for single cells using FSC-A vs. FSC-H and then by forward- & side-scatter (Figure S3A), then those cell populations were examined for viability (Figure 3A). Mock treatment conditions established a baseline of ~73% live cells (Figure 3A). However, IFN-γ treatment and IFN-γ plus LPS treatments produced cultures that had ~99% and ~96% live cells, respectively (Figure 3A). In culture, IFN-γ treatment resulted in preservation and/or proliferation of BMDMs, as the total number of gated single cells in culture was more than double compared with mock treatment conditions (Figure 3B). The presence of IFN-γ plus LPS stimuli also mildly increased the relative number of cells in culture (Figure 3B). Although BMDMs were seeded at a lower cell density, PER2LUC bioluminescence was still detectable, and suppression by pro-inflammatory stimuli was observed (Supplemental Figure 3). Because IFN-γ can induce metabolic changes in macrophages and because the macrophages were recorded under the LumiCycle in hermetically sealed dishes, we determined that the pH levels in similar cultures were not affected by the presence of IFN-γ after 48 h in culture (Supplemental Figure 3). The results suggest that cell death was not a cause of the reduced PER2LUC signal from the plates. Moreover, PER2 suppression by IFN-γ occurred in spite of an increase in number of cells.
Figure 3.
IFN-γ promotes cell survival and proliferation and is not a clock-resetter. (A) Histograms of BMDMs stained with viability dye (zombie red, BioLegend). Gating strategy in Supplemental Figure 3A. (B) Enumeration of the number of events per group in gated flow plots in (A) to determine proliferation. (C) Single, averaged, raw bioluminescence traces of BMDMs treated with IFN-γ (50 ng/mL) or dexamethasone (1 μM) between the times of 12 and 36 h, each sample at every 4 h interval. (D) Circadian wedge diagram depicting time of treatment (magenta dotted line) and time of resultant peaks from each sample group: mock (black circle), IFN-γ (purple triangle), or Dex (orange diamond). Data are represented as mean ± SE (n = 2–3).
From the limit cycle theory of rhythms, inducing a change in one component of the feedback loop (e.g., a stimulus leading to resetting) can result in a temporarily reduced amplitude of an oscillator such as the circadian clock (54, 55). We therefore wanted to explore the possibility that IFN-γ could reset the clock. To this end, we performed a phase-response experiment in which we treated synchronized mPer2Luc BMDMs with mock, IFN-γ, or dexamethasone (a known circadian phase-resetter) during live recording of PER2LUC bioluminescence. Treatments were administered between 12 and 36 h after synchronization, every 4 h. Bioluminescence traces showed that dexamethasone enhanced the amplitude and reset the clock (Figure 3C). Consistent with previous results and despite stimulation during the circadian cycle, IFN-γ suppressed the amplitudes of PER2 expression (Figure 3C), suggesting that the mechanism of action was rapid. Peak times were quantified and displayed in a circadian wedge diagram, revealing that IFN-γ did not reset the circadian clock; i.e., the peak expression of PER2 was not set by the time of IFN-γ treatment (Figure 3D). Thus, IFN-γ did not suppress PER2 through clock resetting mechanisms.
Suppression Is Initiated Through the Interferon Receptor but Is Likely Not Signaled via a Canonical Pathway
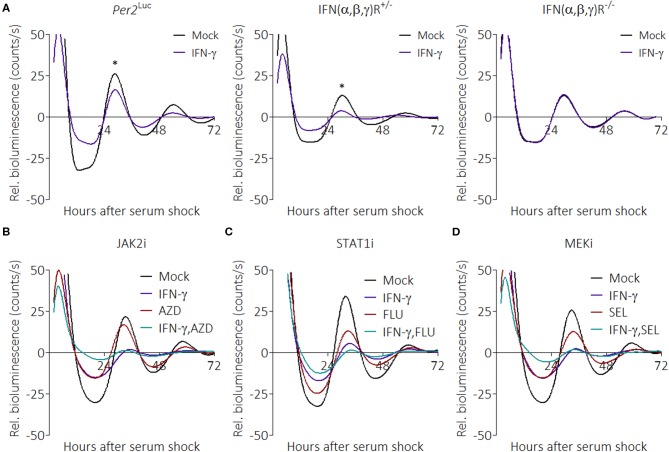
Little is known about how pro-inflammatory stimuli affect the circadian clock; therefore, we interrogated the pathways that might be involved. IFN-γ canonically binds to the IFN-γ receptor (IFNGR) to activate inflammatory pathways; however, because IFN-γ can also bind glycosaminoglycan heparan sulfate at the cell surface to induce effects (56), we sought to determine whether the IFN receptor was required for suppression by IFN-γ. To test this, we crossed interferon (α/β,γ) receptor knockout mice to our mPer2Luc mouse line and generated homozygous and heterozygous offspring [notated IFN(α,β,γ)R−/− and IFN(α,β,γ)R+/−]. BMDMs were generated from these mice, and synchronized macrophages were treated with IFN-γ. Parental mPer2Luc BMDMs showed suppressed PER2 rhythms in the presence of IFN-γ, as did IFN(α,β,γ)R+/− BMDMs (Figure 4A). In contrast, PER2 rhythms were not suppressed in IFN(α,β,γ)R−/− BMDMs, suggesting the suppression pathway is initiated directly through the IFN receptor.
Figure 4.
IFN-γ suppresses PER2 through its cognate receptor but does not act through canonical pathways. (A) Detrended PER2LUC traces from mPer2Luc, mPer2LucIFN(α/β,γ)R+/−, or mPer2LucIFN(α/β,γ)R−/− BMDMs treated with mock or IFN-γ (50 ng/mL for 24 h). (B) PER2LUC traces from mPer2Luc BMDMs treated with mock, IFN-γ, JAK2 inhibitor, or IFN-γ plus JAK2 inhibitor. (C) PER2LUC traces from mPer2Luc BMDMs treated with mock, IFN-γ, STAT1 inhibitor, or IFN-γ plus STAT1 inhibitor. (D) PER2LUC traces from mPer2Luc BMDMs treated with mock, IFN-γ, MEK inhibitor, or IFN-γ plus MEK inhibitor. Data are represented as mean (n = 3–4). P-values (*) are calculated from one-way ANOVA with post-hoc Tukey tests at the acrophase and are considered to be significantly different with P < 0.05.
The canonical IFNGR signaling pathways are JAK/STAT and MEK, therefore we tested their influence on the clock over time with specific inhibitors (57). Synchronized mPer2Luc BMDMs were given combinations of IFN-γ, AZD1480 (AZD, an ATP-competitive JAK2 inhibitor) (Figure 4B), Fludarabine (FLU, a STAT1 activation inhibitor) (Figure 4C), or Selumetinib (SEL, a highly selective MEK1 and MEK2 inhibitor) (Figure 4D), to see whether inhibition of these pathways would mitigate IFN-γ-mediated suppression of PER2. To our surprise, none of the inhibitors prevented the effect of IFN-γ on the clock. The inhibitors alone were able to influence the clock, suggesting that the results were not due to a deficiency in dosage. These data suggest that suppression of the clock by IFN-γ may occur through one or more non-canonical pathways.
Anti-inflammatory Stimuli IL-4 and Dexamethasone Enhance PER2 Rhythms
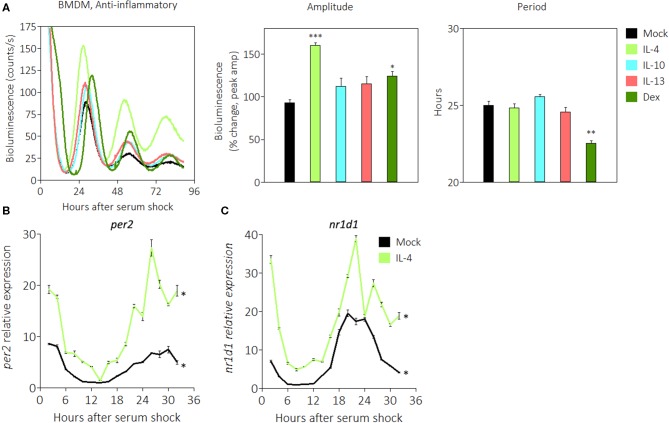
Macrophage activation can polarize in multiple directions, and anti-inflammatory stimuli skew macrophages toward a phenotype associated with helminth infection or wound healing (48). Glucocorticoids such as dexamethasone (Dex) are anti-inflammatory stimuli that bind to cognate receptors to suppress expression of pro-inflammatory genes (58). We previously observed that PER2 amplitudes were enhanced upon stimulation with Dex (Figure 3C), and this led us to investigate whether other anti-inflammatory signals would similarly affect the circadian clock. Other stimuli tested include IL-4, a cytokine that promotes the alternative activation of macrophages (48), IL-10, a cytokine that inhibits pro-inflammatory cytokine production (59), and IL-13, a cytokine that inhibits pro-inflammatory responses in airway maladies (60). The results revealed that BMDMs in the presence of two anti-inflammatory signals exhibited significant enhancements in PER2LUC amplitude of the first peak: IL-4 (160.0% ± 3.5) or Dex (124.5% ± 8.6), compared with mock treatments (Figure 5A). The presence of only Dex significantly shortened period (22.6 h ± 0.1) compared with mock (25.0 h ± 0.3) (Figure 5A).
Figure 5.
Anti-inflammatory stimuli enhance PER2LUC rhythms in macrophages. (A) Detrended PER2LUC traces of synchronized mPer2Luc BMDMs stimulated with mock, IL-4, IL-10, IL-13, or dexamethasone (Dex), and amplitude and period analysis of rhythms from traces. (B) Per2 mRNA expression over a 32-h circadian time course (2 h resolution) from mPer2Luc BMDMs. Cells were stimulated with mock or IL-4. (C) Nr1d1 mRNA expression from cells stimulated with mock or IL-4. Data are represented as mean ± SE (n = 3). In (A), P-values are calculated from one-way ANOVA with post-hoc Tukey tests and are considered to be significantly different with *P < 0.05, **P < 0.005, or ***P < 0.0005. In (B,C), P–values (*) are calculated from Cosinor analysis and significance (P < 0.05) is considered to be rhythmic circadianly.
We then used quantitative RT-PCR to probe the expression of circadian genes in the presence of anti-inflammatory IL-4. Gene expression was normalized to Eif2a and to mock conditions as in Figure 2A. We found that Per2 mRNA in the presence of IL-4 exhibited significant circadian rhythms, and the expression level was 3–4 fold higher at the acrophase (Figure 5B). Similarly, Nr1d1 mRNA in the presence of IL-4 exhibited significant circadian rhythms, and the expression level was ~2 fold higher at the acrophase (Figure 5C). These data suggest that some anti-inflammatory stimuli such as IL-4 or Dex act in a manner opposite to pro-inflammatory stimuli to enhance the circadian clock in macrophages.
Discussion
The ability of macrophages to be activated and polarized into different subsets has been known for several decades (48), yet little is known about how polarization affects the cell autonomous circadian clock. We report here that, in macrophages, pro-inflammatory signals, in general, alter the circadian clock by reducing the amplitude in oscillations of the core clock component PER2, whereas anti-inflammatory signals enhance the amplitude of its oscillations. Although previous studies have shown that inflammatory cytokines could suppress the core circadian clock (43, 44, 61), these studies are largely described in non-immune tissue with the exception of studies showing endotoxin or other PAMPs affecting macrophage clocks (15, 17). We note that we see a strong effect of Pam3CSK4 on the circadian oscillator (Figure 1A) that was not previously observed (15). We also note that the strongly suppressive effect seen with LPS alone in the BMDM circadian clock was not seen in peritoneal macrophages (Supplemental Figure 1D), suggesting unique differences in macrophage clock responsiveness depending on tissue origin. To our knowledge, the work presented here is the first to describe the immune clock's response to pro-inflammatory stimuli such as IFN-γ or immunosuppressive stimuli such as IL-4.
We found evidence for overall perturbation of components of the circadian clock by pro-inflammatory stimuli; notably, in the presence of IFN-γ plus TNF-α, Per2 mRNA levels had an overall baseline increase (Figure 2A), which was paradoxical in conjunction with our PER2LUC results. However, we note that the fold-change between acrophase and trough in the presence of IFN-γ plus TNF-α was only 3.5 times compared with mock treatment conditions where expression range was 8.5-fold between lowest and highest expression levels. This suggested to us that although Per2 mRNA was expressing at an overall higher level during pro-inflammatory conditions, the range of oscillations was weaker than in homeostatic conditions. Furthermore, we noted that in the presence of IFN-γ plus TNF-α, Rora mRNA appeared to be arrhythmic (Supplemental Figure 2), raising the possibility that the dysfunction of these components of the circadian oscillator may be driving compensatory production of Per2 mRNA levels, and in turn may be post-transcriptionally regulated to lower protein levels. An emerging caveat is that not all circadianly rhythmic proteins correlate with rhythmic transcripts (62), suggesting the possibility that post-transcriptional regulation may act to integrate metabolic or environmental cues to the clock more rapidly than transcription may allow (63) which may fit in the case of pro- or anti-inflammatory stimuli on the macrophage clock.
In addition to PER2LUC amplitude suppression, macrophages in the presence of IFN-γ proliferate ≥2-fold more compared with mock treatment conditions (Figure 3B). To a lesser extent, the presence of IFN-γ plus LPS also encouraged proliferation (Figure 3B). In pilot experiments, we found that macrophages that were seeded at higher densities before assay in the LumiCycle yielded stronger luciferase signals (data not shown). These results, taken together, were interesting because a larger number of cells per dish ought to have enhanced luciferase signal. This suggested that in Figure 1A, the clock suppressive effect of IFN-γ per cell was even greater than shown. We wondered whether suppression by IFN-γ meant PER2 expression was downregulated in cells or whether cells were simply desynchronizing. Desynchrony would also reduce amplitude because rhythms from separate cells would exhibit destructive interference; however, if cells were desynchronizing, in the presence of IFN-γ, then baseline luciferase signal should rise as time progressed, even as amplitude was reduced. We did not observe this effect in raw, non-detrended bioluminescence traces (Figure 1A), therefore our results exclude the possibility that PER2 suppression is mediated by cell desynchrony.
Because the circadian clock affects around 8% of the transcriptome (i.e., CCGs) (9) and inflammatory cytokines affected expression of the Bmal1 transcription factor (Supplemental Figure 2), we infer that overall rhythmicity of macrophages is likely affected. During attempts to characterize rhythmic phenotypes of macrophages such as phagocytosis or cytokine secretion in the presence of IFN-γ, we discovered that serum synchronization led to artifacts including quasi-rhythmicity in mPer1 Brdm1Per2Brdm1 macrophages similar to those found in previous studies (20, 64). Some effects may be a consequence of Per2 mRNA fluctuations in mPer1/2 BMDMs during 0–8 h after serum shock, seen in Figure 2, and dexamethasone- or forskolin-based synchronization did not resolve these issues. Thus, our in vitro-based model may have limitations for determining biological consequences, some of which might be resolved by utilizing in vivo studies. However, we note that whole mouse studies also present challenges; for instance, the SCN clock appears to respond to subcutaneous injections of IFN-α or IFN-γ only when these occur at lights-off (ZT12) but not at lights-on (ZT0) (43).
The mechanism of action by pro-inflammatory stimuli on the clock was not elucidated in this study. We approached the problem by first using genetics to ascertain whether the IFN receptor was involved (Figure 4A), then used pharmacological inhibitors to target components of the IFN-γ pathway (Figures 4B–D). We were surprised that, in our hands, none of the targeted components were involved. This suggested to us that an unknown, non-canonical pathway from IFN-γ to the clock was active. During the revision of this manuscript, a study provided evidence that imiquimod, through TLR7, triggers IFN regulatory factor 7 (Irf7) in mouse skin and also suppresses several core clock genes (65). Given that multiple pro-inflammatory stimuli were able to suppress PER2LUC, it is tantalizing to speculate that there may be a common player, such as Irf7, related to all of these stimuli.
Anti-inflammatory signals, especially IL-4 and dexamethasone, enhanced PER2LUC expression. In combination with the pro-inflammatory results, these results are reminiscent of the Th1/Th2 paradigm (66). From this, we suggest that the clock, upon sensing pro- or anti-inflammatory microenvironments, may participate in regulating gene expression profiles (i.e., metabolism, immune-related functions, transmigration, antigen presentation, or wound repair). Macrophages polarized to a pro-inflammatory subset require increased metabolic flux from glycolysis (67) whereas those polarized to an anti-inflammatory subset rely on the TCA cycle and fatty acid oxidation (48, 68). The possibility exists that either the circadian oscillator modulates the amplitude of its rhythms in response to glycolytic flux or that metabolic state of the cell is governed by the robustness of the clock. A recent review highlighted the emergence of interest in immunometabolism and circadian rhythms and the ability to therapeutically target related metabolic pathways (69). It is also tempting to speculate that the host gains flexibility and the ability to re-allocate resources by suppressing circadian clock-related transcription factors in the face of an emerging immunological threat. Our results revealed that the clock in macrophages is affected by diametrically opposing stimuli, and that these stimuli regulate the circadian clock architecture in complex ways. However, it remains to be determined how changes in PER2 amplitude in circadian rhythms affect macrophage biological behavior and circadian output.
Methods
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee at Dartmouth College. Mice were housed in a specific-pathogen-free environment in groups of 4, given ad libitum access to food and water, and maintained on a 12:12 light:dark (LD) cycle. We repeatedly backcrossed mPer2Luc mice and mPer1 Brdm1Per2Brdm1 mice (49, 52) to a C57BL/6J background to >97% homozygosity by SNP mapping. Mice lacking the IFN-α/β and IFN-γ receptors (AG129 or IFNαβγR−/−) in a 129 background (70, 71) were generously provided by Dr. David Leib from Geisel School of Medicine at Dartmouth. IFNαβγR−/− mice were crossed with mPer2Luc mice to generate mice heterozygous for both genes, then those were crossed to generate receptor homozygous (mPer2LucIFNαβγR−/−) and heterozygous (mPer2LucIFNαβγR+/−) offspring in a mixed background.
Cell Cultures, Synchronization, and Treatments
Bone marrow-derived macrophages (BMDMs) and peritoneal macrophages were isolated using standard procedures (72). Briefly, bone marrow was flushed from femurs and tibias of 4- to 8-week-old male mice, cells filtered through a 100 μm cell strainer (Fisher), and red blood cells lysed with ACK Lysing Buffer (Gibco) for 2 min. Bone marrow cells were plated at a density of 6 × 106 cells per 35 mm dish, unless otherwise described in the text. Bone marrow cells were then differentiated in culture for 6 days in DMEM (Corning) supplemented with 10% FBS (Gibco), 20% L-929 conditioned medium (LCM), 100 U/mL penicillin/streptomycin (Corning), and 2 mM L-Glutamine (Corning) at 37°C and 5% CO2, with a media change on day 3. Peritoneal macrophages were elicited by injecting 3% Brewer Thioglycolate Medium in the peritoneal cavity 4 days before flushing the peritoneum. Resulting macrophages were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin/streptomycin, and 2 mM L-Glutamine for at least 4 h before aspirating non-adherent cells and refreshing the media.
To prepare cultures for circadian clock synchronization, cells were first serum-starved for 24 h in Leibovitz's L-15 Medium (Gibco) supplemented with 5% LCM, 100 U/mL penicillin/streptomycin, and 2 mM L-Glutamine. The cells were then synchronized by serum-shock in Leibovitz's L-15 Medium supplemented with 50% FBS for 2 h (73). Subsequently, the cells were cultured in Leibovitz's L-15 Medium supplemented with 5% LCM, 100 U/mL penicillin/streptomycin, 2 mM L-Glutamine, 10 mM HEPES (Gibco), and 100 μM D-Luciferin (GoldBio) for bioluminescence monitoring.
Cultured cells were treated with cytokines, pathogen associated molecular patterns (PAMPs), agonists, or inhibitors. Macrophages were treated with IFN-γ (Peprotech), TNF-α (Peprotech), LPS-EK (InvivoGen), Pam3CSK4 (InvivoGen), and IL-4 (Peprotech), in different combinations at a 50 ng/mL concentration unless otherwise stated. Dexamethasone (Sigma), a glucocorticoid hormone analog that resets the mammalian circadian clock (74), was used at a 1 μM concentration. Inhibitors to multiple biological processes were used: STAT1 activation inhibitor Fludarabine (Selleckchem) at 20 μM, JAK2 inhibitor AZD1480 (Selleckchem) at 5 μM, and MEK inhibitor Selumetinib (Selleckchem) at 10 μM.
For resetting experiments (Figures 3C,D), plates were briefly removed from the LumiCycle and cytokines or glucocorticoids dissolved in DMSO were added at the indicated concentrations at a volume of 2 μL per 2 mL dish. For mock controls, an equivalent amount of DMSO (2 μL) was added. In order to minimize manipulation of the macrophages, the cytokines or glucocorticoids were left on the plates for the entire duration of the experiment. Mock treatments indicated that these manipulations did not themselves reset the clock. In these experiments, because cells were initially synchronized at the outset, treatments whose times span the day but that do not reset the clock will result in phases that align vertically whereas treatments that reset the clock will align diagonally in parallel to the time of treatment across the day.
Cellular Real-Time Bioluminescence Monitoring and Analysis
Cells harboring Per2Luc (49) were grown to confluence in a 35 mm diameter tissue culture dish (Corning), and before bioluminescence monitoring, sealed with a 40 mm circular cover glass (Thermo) using silicone high-vacuum grease (Dow Corning) (75). Data were collected in a LumiCycle 32 (ActiMetrics) luminometer for 48–72 h. Raw circadian bioluminescence data were PMT-normalized by subtracting PMT-specific bioluminescence signal without cells to remove baseline signal. Quantification of amplitude was done by subtracting these bioluminescence first peak values by the first trough values in raw data; % change for amplitude was then calculated by normalizing to the max peak value within the mock treatment group. A second order Butterworth filter (baseline-subtraction method in LumiCycle analysis software) and detrending (subtracting luminescence values based on linear regression) was applied to raw luminescence values to minimize noise where indicated, allowing for better period estimates, and were used in several figures for clarity. Period estimates were done by subtracting the time of the second peak from the first peak in rhythms passed through the Butterworth filter.
RNA Purification, Quantitative RT-PCR, and Primers
Macrophages at different circadian times had supernatants aspirated, and were then frozen immediately at −80°C. Total RNA was then extracted from macrophages using the RNeasy Mini Kit (Qiagen). RNA quality and quantity were assessed by NanoDrop (Thermo), and RNA frozen at −80°C until used. RNA was reverse transcribed, and cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen) primed with oligo(dT). cDNA was amplified using iTaq SYBR Green (Bio-Rad) and the StepOnePlus System (Applied Biosystems) using the following primers: Per2-F: GCTGCAGTAGTGAGCAGTCT, Per2-R: CTCCGCAGGGCATACTTCAA, Nr1d1-F: AGGTGGTAGAGTTTGCCAAACAC, Nr1d1-R: CACCATCAGCACCTCAAAGGT, Eif2a-F: CAACGTGGCAGCCTTACA, Eif2a-R: TTTCATGTCATAAAGTTGTAGGTTAGG, Bmal1-F: GACCTACTCTCCGGTTCCCT, Bmal1-R: GCATATTCTAACTGGTAGTCAGTGG, Rora-F: CGCAGCGATGAAAGCTCAAAT, Rora-R: CAGGAGTAGGTGGCATTGCT, Cry2-F: GCAAGGACTCCTGAGACTGGA, Cry2-R: CGTCTGTTGGTGATTGGCTT. Eif2a was used as a non-circadian reference gene (51).
Flow Cytometry
To assess purity of cells, macrophages were dissociated from tissue culture dishes using a non-enzymatic CellStripper Dissociation Reagent (Corning), blocked with anti-mouse CD16/32 (BioLegend), stained with Brilliant Violet 421 anti-mouse F4/80 (BioLegend) and Alexa Fluor 488 anti-mouse/human CD11b (BioLegend) or Zombie Red Fixable Viability Dye (BioLegend), fixed with 4% paraformaldehyde (BioLegend), and analyzed using a MACSQuant VYB (Miltenyi Biotec). Analysis of data was performed using FlowJo software (Becton, Dickson & Company).
Statistics
Throughout the paper, groups were compared using Student's two-tailed t-tests or one-way ANOVA with post-hoc Tukey tests. Circadian rhythmicity was determined by the Cosinor method and program (Cosinor Periodogram v3.1) as previously described (76). Statistical analyses were performed using Prism 5 software (GraphPad) or Excel software (Microsoft).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at Dartmouth College.
Author Contributions
SC, KF, JD, and JL contributed conception and design of the study. SC performed the statistical analyses and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Brent L. Berwin, Ph.D. and Robert A. Cramer, Ph.D. for their scientific input and editing of the manuscript. We thank David A. Leib, Ph.D. for his generous gift of the IFNαβγR−/− mouse strain. We also thank Wei Wang for her help in crossing these mice to our mPer2Luc mice.
Footnotes
Funding. This work was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health to JL (grant R35 GM118022) and JD (grant R35 GM118021).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00867/full#supplementary-material
References
- 1.Dunlap JC. Molecular bases for circadian clocks. Cell. (1999) 96:271–90. 10.1016/S0092-8674(00)80566-8 [DOI] [PubMed] [Google Scholar]
- 2.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. (2013) 13:190–8. 10.1038/nri3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW, et al. A matter of time: study of circadian clocks and their role in inflammation. J Leukoc Biol. (2016) 99:549–60. 10.1189/jlb.3RU1015-451R [DOI] [PubMed] [Google Scholar]
- 4.Man K, Loudon A, Chawla A. Immunity around the clock. Science. (2016) 354:999–1003. 10.1126/science.aah4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tognini P, Thaiss CA, Elinav E, Sassone-Corsi P. Circadian coordination of antimicrobial responses. Cell Host Microbe. (2017) 22:185–92. 10.1016/j.chom.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. (2001) 2:521–6. 10.1038/35081582 [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. (2004) 119:693–705. 10.1016/j.cell.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 8.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. (2012) 35:445–62. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. (2009) 106:21407–12. 10.1073/pnas.0906361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjona A, Silver AC, Walker WE, Fikrig E. Immunity's fourth dimension: approaching the circadian-immune connection. Trends Immunol. (2012) 33:607–12. 10.1016/j.it.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. (2012) 109:582–7. 10.1073/pnas.1106750109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. (2012) 36:251–61. 10.1016/j.immuni.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. (2013) 341:1483–8. 10.1126/science.1240636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis AM, Bellet MM, Sassone-Corsi P, O'neill LA. Circadian clock proteins and immunity. Immunity. (2014) 40:178–86. 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Curtis AM, Fagundes CT, Yang G, Palsson-Mcdermott EM, Wochal P, Mcgettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci USA. (2015) 112:7231–6. 10.1073/pnas.1501327112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrecque N, Cermakian N. Circadian clocks in the immune system. J Biol Rhythms. (2015) 30:277–90. 10.1177/0748730415577723 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Pati P, Xu Y, Chen F, Stepp DW, Huo Y, et al. Endotoxin disrupts circadian rhythms in macrophages via reactive oxygen species. PLoS ONE. (2016) 11:e0155075. 10.1371/journal.pone.0155075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci USA. (2018) 115:E8460–8. 10.1073/pnas.1800431115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobis CC, Labrecque N, Cermakian N. From immune homeostasis to inflammation, a question of rhythms. Curr Opin Physiol. (2018) 5:90–8. 10.1016/j.cophys.2018.09.001 [DOI] [Google Scholar]
- 20.Geiger SS, Curtis AM, O'neill LAJ, Siegel RM. Daily variation in macrophage phagocytosis is clock-independent and dispensable for cytokine production. Immunology. (2019) 157:122–36. 10.1111/imm.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ince LM, Zhang Z, Beesley S, Vonslow RM, Saer BR, Matthews LC, et al. Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J. (2019) 33:126–39. 10.1096/fj.201800026RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H, et al. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J Neurosci. (2000) 20:5867–73. 10.1523/JNEUROSCI.20-15-05867.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamia KA, Sachdeva UM, Ditacchio L, Williams EC, Alvarez JG, Egan DF, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. (2009) 326:437–40. 10.1126/science.1172156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. (1997) 89:641–53. 10.1016/S0092-8674(00)80245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. (1998) 280:1564–9. 10.1126/science.280.5369.1564 [DOI] [PubMed] [Google Scholar]
- 26.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. (1998) 95:5474–9. 10.1073/pnas.95.10.5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. (2000) 103:1009–17. 10.1016/S0092-8674(00)00205-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin EAJr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. (1999) 286:768–71. 10.1126/science.286.5440.768 [DOI] [PubMed] [Google Scholar]
- 29.Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. (1999) 96:57–68. 10.1016/S0092-8674(00)80959-9 [DOI] [PubMed] [Google Scholar]
- 30.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. (1999) 98:193–205. 10.1016/S0092-8674(00)81014-4 [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. (2001) 107:855–67. 10.1016/S0092-8674(01)00610-9 [DOI] [PubMed] [Google Scholar]
- 32.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. (2002) 110:251–60. 10.1016/S0092-8674(02)00825-5 [DOI] [PubMed] [Google Scholar]
- 33.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, Mcnamara P, et al. A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron. (2004) 43:527–37. 10.1016/j.neuron.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, et al. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbalpha couple metabolism to the clock. Science. (2015) 348:1488–92. 10.1126/science.aab3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollinger T, Leutz A, Leliavski A, Skrum L, Kovac J, Bonacina L, et al. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE. (2011) 6:e29801. 10.1371/journal.pone.0029801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. J Immunol. (2011) 187:6291–300. 10.4049/jimmunol.1004030 [DOI] [PubMed] [Google Scholar]
- 37.Cao Q, Zhao X, Gery S, Chen ZS, Deng RS, Sun HB, et al. Circadian clock protein CRY controls B-cell intrinsic tolerance. Blood. (2015) 126:1029 10.1182/blood.V126.23.1029.1029 [DOI] [Google Scholar]
- 38.Nobis CC, Dubeau Laramee G, Kervezee L, Maurice De Sousa D, Labrecque N, Cermakian N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci USA. (2019) 116:20077–86. 10.1073/pnas.1905080116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, et al. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci USA. (2013) 110:9897–902. 10.1073/pnas.1120636110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O'neill JS, et al. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci USA. (2016) 113:10085–90. 10.1073/pnas.1601895113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiessling S, Dubeau-Laramee G, Ohm H, Labrecque N, Olivier M, Cermakian N. The circadian clock in immune cells controls the magnitude of leishmania parasite infection. Sci Rep. (2017) 7:10892. 10.1038/s41598-017-11297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Fuller KK, Dunlap JC, Loros JJ. Circadian clearance of a fungal pathogen from the lung is not based on cell-intrinsic macrophage rhythms. J Biol Rhythms. (2018) 33:99–105. 10.1177/0748730417745178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nat Med. (2001) 7:356–60. 10.1038/85507 [DOI] [PubMed] [Google Scholar]
- 44.Kwak Y, Lundkvist GB, Brask J, Davidson A, Menaker M, Kristensson K, et al. Interferon-gamma alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J Biol Rhythms. (2008) 23:150–9. 10.1177/0748730407313355 [DOI] [PubMed] [Google Scholar]
- 45.Mosser EA, Chiu CN, Tamai TK, Hirota T, Li S, Hui M, et al. Identification of pathways that regulate circadian rhythms using a larval zebrafish small molecule screen. Sci Rep. (2019) 9:12405. 10.1038/s41598-019-48914-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prior KF, O'donnell AJ, Rund SSC, Savill NJ, Van Der Veen DR, Reece SE. Host circadian rhythms are disrupted during malaria infection in parasite genotype-specific manners. Sci. Rep. (2019) 9:10905. 10.1038/s41598-019-47191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray PJ. Macrophage polarization. Annu Rev Physiol. (2017) 79:541–66. 10.1146/annurev-physiol-022516-034339 [DOI] [PubMed] [Google Scholar]
- 49.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. (2004) 101:5339–46. 10.1073/pnas.0308709101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosir R, Acimovic J, Golicnik M, Perse M, Majdic G, Fink M, et al. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol Biol. (2010) 11:60. 10.1186/1471-2199-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. (2001) 105:683–94. 10.1016/S0092-8674(01)00380-4 [DOI] [PubMed] [Google Scholar]
- 53.Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. (1993) 150:5080–5. [PubMed] [Google Scholar]
- 54.Winfree AT. Corkscrews and singularities in fruit flies: resetting behaviour of the circadian eclosion rhythm. In: Michael M, editor. Biochronometry. Washington, DC: National Academy of Sciences; (1971). p. 81–106. [Google Scholar]
- 55.Johnson CH, Elliott J, Foster R, Honma KI, Kronauer R. Fundamental properties of circadian rhythms. In: Dunlap JC, Loros JJ, DeCoursey P, editors. Chronobiology Biological Timekeeping. Sinauer Associates, Inc. Sunderland, MA. (2004) 67–105. [Google Scholar]
- 56.Sadir R, Forest E, Lortat-Jacob H. The heparan sulfate binding sequence of interferon-gamma increased the on rate of the interferon-gamma-interferon-gamma receptor complex formation. J Biol Chem. (1998) 273:10919–25. 10.1074/jbc.273.18.10919 [DOI] [PubMed] [Google Scholar]
- 57.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity. (2009) 31:539–50. 10.1016/j.immuni.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. (1998) 94:557–72. 10.1042/cs0940557 [DOI] [PubMed] [Google Scholar]
- 59.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. (1998) 160:5936–44. [PubMed] [Google Scholar]
- 60.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. (1993) 362:248–50. 10.1038/362248a0 [DOI] [PubMed] [Google Scholar]
- 61.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, et al. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA. (2007) 104:12843–8. 10.1073/pnas.0701466104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hurley JM, Jankowski MS, De Los Santos H, Crowell AM, Fordyce SB, Zucker JD, et al. Circadian proteomic analysis uncovers mechanisms of post-transcriptional regulation in metabolic pathways. Cell Syst. (2018) 7:613–26 e615. 10.1016/j.cels.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. (2014) 24:90–9. 10.1016/j.tcb.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heipertz EL, Harper J, Lopez CA, Fikrig E, Hughes ME, Walker WE. Circadian rhythms influence the severity of sepsis in mice via a TLR2-dependent, leukocyte-intrinsic mechanism. J Immunol. (2018) 201:193–201. 10.4049/jimmunol.1701677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenberg EN, Marshall ME, Jin S, Venkatesh S, Dragan M, Tsoi LC, et al. Circadian control of interferon-sensitive gene expression in murine skin. Proc Natl Acad Sci USA. (2020) 117:5761–71. 10.1073/pnas.1915773117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. (2000) 164:6166–73. 10.4049/jimmunol.164.12.6166 [DOI] [PubMed] [Google Scholar]
- 67.Ardawi MS. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by lungs of the rat. Biochimie. (1991) 73:557–62. 10.1016/0300-9084(91)90023-T [DOI] [PubMed] [Google Scholar]
- 68.Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. (1963) 17:487–501. 10.1083/jcb.17.3.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carroll RG, Timmons GA, Cervantes-Silva MP, Kennedy OD, Curtis AM. Immunometabolism around the clock. Trends Mol Med. (2019) 25:612–25. 10.1016/j.molmed.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 70.Van Den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. (1995) 69:4792–6. 10.1128/JVI.69.8.4792-4796.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasieka TJ, Collins L, O'connor MA, Chen Y, Parker ZM, Berwin BL, et al. Bioluminescent imaging reveals divergent viral pathogenesis in two strains of Stat1-deficient mice, and in alphassgamma interferon receptor-deficient mice. PLoS ONE. (2011) 6:e24018. 10.1371/journal.pone.0024018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. (2008) Chapter 14:Unit 14.1. 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. (1998) 93:929–37. 10.1016/S0092-8674(00)81199-X [DOI] [PubMed] [Google Scholar]
- 74.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. (2000) 289:2344–7. 10.1126/science.289.5488.2344 [DOI] [PubMed] [Google Scholar]
- 75.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Meth Enzymol. (2005) 393:288–301. 10.1016/S0076-6879(05)93012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. (2007) 38:275–325. 10.1080/09291010600903692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.