Abstract
Chronic respiratory diseases including chronic rhinosinusitis, otitis media, asthma, cystic fibrosis, non-CF bronchiectasis, and chronic obstructive pulmonary disease are a major public health burden. Patients suffering from chronic respiratory disease are prone to persistent, debilitating respiratory infections due to the decreased ability to clear pathogens from the respiratory tract. Such infections often develop into chronic, life-long complications that are difficult to treat with antibiotics due to the formation of recalcitrant biofilms. The microbial communities present in the upper and lower respiratory tracts change as these respiratory diseases progress, often becoming less diverse and dysbiotic, correlating with worsening patient morbidity. Those with chronic respiratory disease are commonly infected with a shared group of respiratory pathogens including Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, and Moraxella catarrhalis, among others. In order to understand the microbial landscape of the respiratory tract during chronic disease, we review the known inter-species interactions among these organisms and other common respiratory flora. We consider both the balance between cooperative and competitive interactions in relation to microbial community structure. By reviewing the major causes of chronic respiratory disease, we identify common features across disease states and signals that might contribute to community shifts. As microbiome shifts have been associated with respiratory disease progression, worsening morbidity, and increased mortality, these underlying community interactions likely have an impact on respiratory disease state.
Keywords: polymicrobial, biofilm, respiratory tract, pulmonary, antibiotic resistance
Introduction
Rates of chronic respiratory disease (CRD) have increased with time and represent a major global health burden, comprising the fourth greatest cause of death (Heron, 2017). Chronic diseases are constant, persistent health issues causing significant morbidity and mortality. Individuals with CRD often have underlying defects in airway clearance, resulting in chronic respiratory infections. Chronic infections also involve the formation of biofilms, recalcitrant microbial communities that are able to persist, resisting immune clearance, and antibiotic killing.
Throughout the upper and lower human respiratory tract, niche-specific microbial communities colonize the entire length of the airways. Many of these organisms are found in complex microbial communities known as biofilms, which are encapsulated in a polysaccharide matrix. The upper respiratory tract (URT) contains the highest density of bacteria in the respiratory tract, but many of the healthy normal flora are highly shared between anatomical sites. The superior regions of the respiratory tract, the sinonasal and nasal cavities, share a similar microbiome, including Corynebacterium spp. , Staphylococcus spp., and Cutibacterium acnes (Ramakrishnan et al., 2016; Man et al., 2017; Paramasivan et al., 2020). Beginning in the nasal cavity and extending to the oropharynx, other bacteria are found including Moraxella catarrhalis, Haemophilus spp., and Streptococcus spp. Most of the species found in the lower respiratory tract (LRT) are common oral flora, suggesting that microaspiration of mouth contents results in more colonization than anything inhaled from the air (Dickson and Huffnagle, 2015). Such organisms include Prevotella, Veillonella, and Rothia spp., in addition to Streptococcus spp. (Man et al., 2017). However, these studies inherently have potential limitations due to the nature of sample collection. In sampling the LRT, a bronchoscope must pass through the URT, providing a source of potential contamination. Many studies have concluded the community signatures of the LRT are likely not due to sample contamination through the collection of matched oral wash samples, repeated sampling of the same site, and through various routes of insertion (oral versus nasal) (Dickson et al., 2014; Bassis et al., 2015; Dickson and Huffnagle, 2015). Therefore, proper controls are essential when studying these distal sites.
The importance of the human microbiome to overall health is well-appreciated, and many studies have demonstrated that dysbiosis of the normal microbial communities in any particular anatomical site is strongly associated with disease progression. A healthy microbiome contains a diverse community of organisms. Such communities are markedly more stable and resistant to blooms of pathogenic species. Commensal bacteria maintain community homeostasis by secreting antimicrobial peptides and other molecules to suppress the growth of pathogens (Gallo, 2015). However, in dysbiotic communities associated with chronic disease, the diversity is markedly lower, often dominated by a few pathogenic species with diminished normal healthy flora. Notably, dysbiotic communities are more susceptible to inflammation and immune activation. The acquisition or presence of certain pathogenic species might drive spatial organization, community composition, and functional behaviors. Commensal flora usually limits the growth of pathogens, but when these communities are compromised, the proliferation of pathogenic species during dysbiosis may trigger inflammation and lead to chronic inflammation and disease progression (Lawley et al., 2009).
Early in life, a number of environmental factors likely shapes the developing microbiome and potentially dictates future respiratory health. Notably, by 1–2 months of age, infants' microbiomes diversify and develop a unique microbial signature in their upper airways, which seem to correlate with predisposition to respiratory infections (Bosch et al., 2016). Infants dominated by Corynebacterium spp., Staphylococcus spp., or Alloiococcus spp., had a lower predisposition to respiratory illnesses, whereas infants with Streptococcus, Moraxella, or Haemophilus dominated microbiomes had higher rates of respiratory illness and predisposition to asthma, likely due to the inflammatory potential of these organisms (Teo et al., 2015; Bosch et al., 2016). These outcomes depended largely on exposure to antibiotics as an infant, the presence of siblings, and season (Teo et al., 2015).
In patients with chronic respiratory disease, a number of factors contribute to an altered respiratory microbiome. The production of excess mucus in diseases such as cystic fibrosis and chronic rhinosinusitis provide additional nutrient sources as well as pockets of decreased oxygen availability, allowing for different species to colonize these new niches. Additionally, decreased mucociliary clearance resulting from the pathogenesis of diseases such as bronchiectasis and cystic fibrosis result in decreased microbial clearance and elimination, allowing environmental microbes increased access to these sites. Disease exacerbations and the acute worsening of respiratory symptoms result in many interventions, including antibiotic therapy and hospitalizations, that have the potential to disrupt the microbiome. Respiratory symptoms themselves also allow for microbial migration and elimination including cough, hyperventilation (microaspiration), and bronchoconstriction, resulting in altered oxygen availability, temperature, and pH (Dickson and Huffnagle, 2015). Approximately 75% of individuals experiencing chronic lower respiratory tract disease also develop esophageal reflux and dysfunction, allowing for microbial migration from the digestive tract into the respiratory tract (Dickson and Huffnagle, 2015).
Although many of these respiratory symptoms are shared among various chronic respiratory diseases, each is uniquely classified by the anatomical location of inflammation and infection. Different regions of the respiratory tract differ in their physiochemical properties, including temperature, pH, and nutrient availability, like oxygen (Dickson et al., 2016). These factors likely impact the microbial communities present and influence disease progression.
In the following review, we discuss the underlying pathophysiology of common chronic respiratory diseases, the microbial communities present in the diseased respiratory tract, and common pathogens and exacerbations that impact lung function. In relation to these communities, we discuss both cooperative and competitive species interactions that occur and how these likely impact disease state and the overall microbial communities. Although much more work is needed to truly understand daily microbiome changes in vivo, these interactions have the potential to underly many CRD manifestations.
The Microbiology of Chronic Respiratory Diseases
Chronic Rhinosinusitis
Chronic rhinosinusitis (CRS), the chronic inflammation of the nose and paranasal sinuses, affects ~31 million people in the US alone (Bose et al., 2016) (12.5% of US adults; 11% of European adults) with an economic burden of over 60 billion dollars (Caulley et al., 2015). CRS results in impaired mucociliary clearance and poor drainage of the sinuses, resulting in mucus accumulation, but this disease has been hypothesized to develop in response to microbial dysbiosis and associated inflammation (Lam et al., 2015; Jervis Bardy and Psaltis, 2016; Hoggard et al., 2017; Wagner Mackenzie et al., 2017; Copeland et al., 2018). The CRS sinuses are distinctly less diverse than healthy controls, however whether low diversity and dysbiosis is the cause of CRS or a consequence of the disease is still unclear (De Boeck et al., 2019). Regardless, the CRS microbiome is enriched in Cutibacterium acnes and Corynebacterium spp., with a known enrichment of Corynebacterium tuberculstearicum in CRS patients (Abreu et al., 2012). CRS patients also harbor increased number of pathogens including Staphylococcus aureus, coagulase-negative Staphylococcus spp., Streptococcus pneumoniae, and Haemophilus influenzae in their sinuses. Notably, the presence of S. aureus or H. influenzae correlates with the development of nasal polyps, inflammatory lesions which contribute to worsening disease morbidity (Bose et al., 2016; Chalermwatanachai et al., 2018). Patients with CRS often experience disease exacerbations, characterized by a sudden worsening of respiratory symptoms. These exacerbations have been associated with acquisition of other bacterial pathogens, including Pseudomonas aeruginosa, Proteus mirabilis, and Klebsiella pneumoniae (Bose et al., 2016).
The complex environment of the inflamed sinuses of CRS patients, consisting of an influx of inflammatory immune cells, effector molecules, and cationic antimicrobial peptides, likely have a direct impact on bacterial gene expression and resulting microbial community structure. The presence of certain species also likely has an impact on the resulting community structure and virulence of CRS pathogens. Specifically, the presence of large numbers of Corynebacterium spp. might drive S. aureus to be less virulent and more benign, whereas the presence of certain Proteobacteria, such as E. coli, might drive S. aureus to exhibit more pathogenic behavior (Ramakrishnan et al., 2015, 2016). Interspecies interactions might also contribute to worsening disease pathology. For example, as H. influenzae is associated with occurrence of nasal polyps in CRS patients, it is thought that H. influenzae might actually initiate and drive inflammation and impact the development of nasal polyposis (Chalermwatanachai et al., 2018). These associations might have important implications for treatment of CRS, including early elimination of H. influenzae or S. aureus to prevent the development of nasal polyps.
Otitis Media
Otitis media, inflammation of the middle ear, is the most frequently diagnosed illness in children under 15 and the primary cause for emergency room visits (Cassell et al., 1994). Approximately 80% of children will experience OM by age 3, affecting between 330 and 700 million people worldwide (Teele et al., 1989; Monasta et al., 2012; Mittal et al., 2015). Acute OM can progress to chronic suppurative OM (CSOM), which is associated with ear drum perforation and purulent discharge (Acuin, 2007). There are around 31 million new cases of CSOM per year, with 22.6% of cases in children under 5 years old (Monasta et al., 2012). Pathogens cause infection and subsequent disease by gaining access to the middle ear through the Eustachian tube. As early as six months of age, many children are already colonized by bacteria known to cause OM, including Moraxella catarrhalis, Streptococcus pneumoniae, and H. influenzae (Bakaletz, 2010; Qureishi et al., 2014). Of these species, S. pneumoniae is the most virulent and inflammatory, causing a highly symptomatic disease, while H. influenzae and M. catarrhalis are more likely silent, chronic infections (Bakaletz, 2010). However, P. aeruginosa and S. aureus are the most common pathogens found in CSOM cases (Mittal et al., 2015). An important aspect of OM pathophysiology is the presence of bacterial biofilms in the ear, allowing for chronic, persistent infection. The presence of biofilms in the middle ear exudate has been confirmed by microscopy (Kaya et al., 2013; Gu et al., 2014). Particularly, all three major pathogens (H. influenzae, S. pneumoniae, and M. catarrhalis) have been found together in polymicrobial biofilms (Kaya et al., 2013).
Continuing to understand how OM pathogens contribute to disease pathology is important to understand treatments. Colonization is likely an important step in dictating the course of disease progression. Natural competition likely occurs in the middle ear and Eustachian tube to impact pathogenesis and microbial communities. For example, the presence of Corynebacterium spp. is often inversely correlated with S. pneumoniae and S. aureus colonization (Kumpitsch et al., 2019). Additionally, children with recurrent OM who were vaccinated with the 7-valent pneumococcal vaccine had an increased incidence of S. aureus and H. influenzae disease and colonization, also suggesting a competitive relationship of S. pneumoniae with S. aureus and H. influenzae (Biesbroek et al., 2014).
Asthma
Over 300 million people suffer from asthma, and this number continues to climb (Nunes et al., 2017). This chronic inflammatory disease results in narrowing of the airways and reduction in airflow. Asthma is the most frequent chronic disease among pediatric patients (Boutin et al., 2017). During an asthma attack, the lining of the bronchial tubes become inflamed, narrowing the airways and resulting in widespread reduction in airflow. Many factors have been associated with the development of asthma including geographical location, viral infection, and antibiotic exposure (Ni et al., 2019). There is evidence suggesting that infection with Chlamydia pneumoniae or Mycoplasma pneumoniae may precede the development of asthma (Hahn et al., 1991). Other studies have suggested that exposure to S. aureus and its associated enterotoxins early in life might also be a risk factor for development of asthma (Redinbo, 2014). S. aureus enterotoxins function as superantigens, which might result in damage of the lower and upper respiratory tract and eosinophil accumulation. To support this hypothesis, antibodies specific to S. aureus enterotoxins are more commonly found in asthma patients as compared to healthy controls (Redinbo, 2014).
The respiratory microbiome also has important implications for treatment response. Previously, patients who failed to respond to the inhaled corticosteroid fluticasone had more dysbiotic respiratory microbiomes, whereas those who responded favorably had microbiomes more similar to healthy controls (Durack et al., 2017). As compared to healthy individuals, individuals with asthma have a respiratory microbiome enriched in Haemophilus spp. , Neisseria spp. , Fusobacterium spp., and Porphyromonas spp. (Durack et al., 2017). Specifically, infection with Streptococcus pneumoniae was associated with asthma exacerbations (Iikura et al., 2015). Long-term, chronic colonization of the airways may result in neutrophilic accumulation observed in a subset of asthma patients. In those with neutrophilic asthma, M. catarrhalis was most often associated with worsening disease, but H. influenzae and Streptococcus spp. often also dominated the airway microbiome (Green et al., 2014). Infection with these three species was associated with increased duration of disease, worse lung function (FEV1), and higher sputum neutrophil counts and IL-8 concentration (Green et al., 2014).
Cystic Fibrosis
The genetic disease cystic fibrosis (CF) results from mutation in the cystic fibrosis transmembrane conductance regulator (CFTR), an ion channel primarily responsible for regulating chloride and bicarbonate transport (Elborn, 2016). This defect alters the pH of airway surface liquid, decreases mucociliary clearance, and impairs innate immunity. Chronic respiratory infections are the leading cause of death in people with CF, and disease exacerbations are a major cause of pulmonary function decline in CF patients (Stenbit and Flume, 2011). Early in life, the LRT of CF patients are predominately colonized with oral bacteria including Streptococcus spp., Prevotella spp., and Veillonella spp. (Zemanick et al., 2017) The most common CF pathogens isolated from young CF patients include S. aureus and H. influenzae. However, acute infections with these pathogens transition into chronic respiratory infections with age. As patients age, their microbiome becomes less diverse and more dominated with one or a few CF pathogens including P. aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, or S. aureus (Coburn et al., 2015). This shift might be due to respiratory viral co-infections, as preceding viral infections are associated with P. aeruginosa acquisition, development of chronic P. aeruginosa infection, and increased P. aeruginosa antibodies (Petersen et al., 1981; Johansen and Høiby, 1992; Collinson et al., 1996).
Specific CF pathogens likely strongly shape the microbial communities present in the CF lung and contribute to the microbial shifts documented as patients age. Specifically, the presence of P. aeruginosa and B. cepacia complex, pathogens commonly isolated in adult CF patients, notably impact the composition and presence of other species (Stressmann et al., 2012; Zemanick et al., 2017). The presence of either one of these organisms is associated with lower odds of infection by methicillin-sensitive S. aureus, S. maltophilia, or A. xylosoxidans in the future (Granchelli et al., 2018). These two pathogens are thought to directly contribute to declining microbial diversity due to their ability to compete with other species found in the CF lung, both limiting the acquisition of other pathogens and dominating over pre-established microbial communities. P. aeruginosa and B. cepacia complex co-infections are also reported, and co-infection is associated with worsening disease severity and lung function in both mice and humans, as compared to mono-infection (Isles et al., 1984; Jacques et al., 1998; Bragonzi et al., 2012).
Non-CF Bronchiectasis
Non-CF bronchiectasis comprises a disease characterized by abnormal widening of one or more airways, with extra mucus pooling in these airways (McShane et al., 2013). Bronchiectasis pathophysiology may be due to ciliary dyskinesia, autoimmune disease, immune deficiencies, hypersensitivities, TB, or, other infections. Approximately 350,000–500,000 adults have bronchiectasis in the US, and its incidence increases with age, especially in adults over the age of 60 (Chalmers and Elborn, 2015). People with bronchiectasis experience chronic cough, viscous sputum, frequent hospitalizations, and often one or more disease exacerbations per year. Exacerbations not only contribute to worsening morbidity, but also mortality, as patients who experience three or more exacerbations per year have double the mortality rate as those who have fewer (Chalmers et al., 2013). During exacerbation, bacterial density in the respiratory tract does not change, but microbial community shifts occur, including the increased prevalence of anaerobes in the LRT (Tunney et al., 2013). The most predominant species colonizing the LRT of bronchiectasis patients include H. influenzae, S. pneumoniae, S. aureus, P. aeruginosa, M. catarrhalis, Prevotella, and Veillonella. Over time, the lung microbiome of bronchiectasis patients seem to be largely dominated by either P. aeruginosa or H. influenzae, although these two organisms are rarely isolated together, suggesting there is natural competition among these species (Rogers et al., 2013; Woo et al., 2019). However, many studies have shown that patients infected with P. aeruginosa experience the most rapid decline in lung function (FEV1%) and have the highest hospital admission rates (Finch et al., 2015; McDonnell et al., 2015). In both CF and non-CF bronchiectasis, P. aeruginosa undergoes clonal selection and diversification over time, reflecting its ability to adapt to the diseased lung (Hilliam et al., 2017). These adaptations and mutations demonstrate the need for limited nutrients, biofilm formation, and competition with other organisms.
Chronic Obstructive Pulmonary Disease (COPD)
One of the greatest contributors to chronic respiratory disease is chronic obstructive pulmonary disease (COPD). This disease alone is the third-leading cause of death worldwide, and over 65 million people suffer from moderate to severe COPD (Quaderi and Hurst, 2018). COPD results in the chronic obstruction of airflow (obstructive bronchitis), impairing normal breathing, and/or destruction or the lung parenchyma, often caused by exposure to noxious gases including cigarette smoke. Patients with COPD experience symptoms including chronic cough, mucociliary dysfunction, excess mucus production, and breathlessness. This environment favors colonization from a number of bacteria, including predominant species such as H. influenzae, S. pneumoniae, and M. catarrhalis (Beasley et al., 2012).
COPD patients are found to have significantly lower microbial community diversity in the LRT (Quaderi and Hurst, 2018). Many factors seem to influence this shift. Disease exacerbations and corticosteroid use have been associated with decreased diversity and enrichment of specific species (Wang et al., 2016). It has been observed that, as the disease progresses and airways become more obstructed, the prevalence of P. aeruginosa, H. influenze, and other Gram-negative pathogens increase (Soler et al., 1999; Murphy et al., 2008; Engler et al., 2012). Disease exacerbations are most commonly associated with the presence of H. influenzae in the airways, but are also associated with acquisition of new strains of P. aeruginosa, H. influenzae, or M. catarrhalis (Soler et al., 1999; Mammen and Sethi, 2016). Notably, in microbiome analyses of COPD patients, those who were colonized with H. influenzae had many negative correlations with species found in healthy individuals (Wang et al., 2016). Therefore, the presence of H. influenzae might directly impact the composition of the lung microbiome. Finally, patients with low community diversity also had higher levels of serum IL-8, suggesting that the microbiome might drive inflammation and directly impact disease severity (Wang et al., 2016). By understanding the microbial interactions which lead to microbial community changes and decreased diversity, we can better understand how the microbiome contributes to disease progression and how to prevent these shifts.
Microbial Community Interactions
Even the earliest reports of microorganisms have noted that species are rarely found in isolation. Very few body sites in humans are thought to be truly sterile. With sequencing advances, we continue to discover that healthy humans harbor microbial communities in areas previously assumed to be sterile, including the lower respiratory tract where many species are found to co-exist (Harrison, 2007; Dickson and Huffnagle, 2015). Unfortunately, our understanding of bacterial pathogenesis is often restricted to species in mono-culture. How community members interact in combination is often different and difficult to predict by studying individual species. When pathogenesis is studied with the addition of other species or in polymicrobial environments, bacterial interactions, and associated disease severity sometimes changes (Duan et al., 2003; Sibley et al., 2008; Korgaonkar et al., 2013).
In the airways, bacteria are localized in biofilm communities to persist and resist clearing from the host. Biofilms have been directly detected in CRD including CRS (Cryer et al., 2004; Sanclement et al., 2005), CF (Bjarnsholt et al., 2013; Hoiby et al., 2017), OM (Hall-Stoodley et al., 2006; Bakaletz, 2012; Gu et al., 2014), and bronchiectasis (Marsh et al., 2015) and have been implicated in other diseases including COPD (Murphy et al., 2005; Pang et al., 2008). The spatial organization of these communities favor the close proximity of related cells due to growth and clonal expansion. This arrangement limits the diffusion of extracellular products away from a population of related cells to restrict access from competitors and reduce cheating. However, this dense environment also allows for close contact with competing species and renders a population vulnerable to direct antagonistic attacks.
Bacteria have evolved multiple mechanisms to interact with neighboring cells to acquire the nutrients necessary to survive, resist clearing from the host during infection, and persist over time. Such interactions have differing effects on other species, broadly resulting in either cooperative behaviors or competitive interactions. While many of these interactions rely on the use and exploitation of the greater nutritional environment and production of extracellular products, some of these interactions are more targeted, with specific cross-talk mediated by direct contact.
There have been a wealth of studies describing the pathogenesis and coordination of these behaviors in vitro, as well as a number of studies detailing known clinical associations and microbial community shifts during CRD. However, the role of these interactions during human disease is only speculative. Here, we will review known microbial interactions in light of observed disease shifts in an effort to identify connections and enhance our understanding of chronic respiratory disease progression.
Cooperative Behaviors
Although bacteria often compete for space and resources to persist in the body, a number of cooperative interactions have been described between species. Such interactions often positively impact both species and favor the good of the group rather than an individual cell (Figure 1). Many of these cooperative interactions have been described in common respiratory commensals and CRD pathogens.
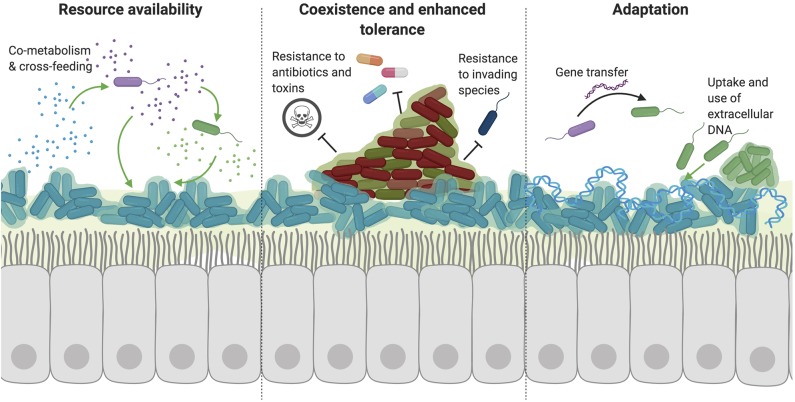
Figure 1.
Cooperative interactions favor the persistence of multiple species. A number of cooperative interactions have been described among species, which can broadly be broken down into three main categories. (1) Bacteria benefit from the resources released or metabolized by other species. Through mechanisms such as co-metabolism and cross-feeding, species can utilize complex nutrients that they may be unable to metabolize or access themselves. Additionally, the secretion of public goods from one species (such as siderophores, enzymes, or signaling molecules) also benefits neighboring bacteria by releasing resources into the extracellular environment. (2) The coexistence of multiple species of bacteria in a polymicrobial biofilm also allows the sharing of resources and cross-species signaling due to the close proximity of these species. Importantly, certain species can protect and shield other neighboring species in a biofilm through enhanced tolerance to antibiotics, immune radicals, and other toxic small molecules. (3) Interactions with other species also result in adaptation to improve survival in the host environment. While many organisms might evolve to evade toxic effects from competitive species, adaptation also occurs through horizontal gene transfer and uptake of exogenous bacterial DNA from the environment. The presence of this DNA alone can also aid in biofilm formation through incorporation into the extracellular biofilm matrix.
Cross-Feeding
Bacteria rely on specific nutrients to survive in the nutrient-restricted host environment of the respiratory tract (Armstrong, 2015). However, many nutrients are not readily available because they are sequestered by the host or require breakdown of larger molecules. Some species of bacteria are able to utilize and catabolize complex molecules to access needed nutrients. Other species can use the metabolites produced by these organisms as a source of energy or nutrient sources in a process called cross-feeding (Seth and Taga, 2014). Many carbohydrate sources are available in the respiratory tract and are implicated in polymicrobial community interactions, as recently reviewed by Armbruster et al. (2019), but one common example is mucus, predominately comprised of mucins. Mucin is one of the most abundant molecules in the respiratory tract and is produced in large quantities in CRD, including CF and COPD. These molecules are comprised of an amino acid backbone with oligosaccharide side chains. Terminal domains on the oligosaccharide chains form a highly cross-linked structure, making most of these nutrients inaccessible to bacteria (Flynn et al., 2016). Species including P. aeruginosa and S. aureus cannot efficiently use mucin as a sole carbon source (Flynn et al., 2016). However, the presence of other commensal species including Rothia mucilaginosa, Streptococcus spp., Prevotella spp., and Veillonella spp., cooperate to degrade mucin and liberate useable metabolites for other species (Flynn et al., 2016; Gao et al., 2018). These species, along with other anaerobes, posess glycoside hydrolase, sulfatase, sialidase, alpha-fucosidase, and endopeptidase activities (Bradshaw et al., 1994; Wright et al., 2000; Inui et al., 2015). The unique enzymatic activities of anaerobic species act synergistically to release fermentative metabolites, including amino acids, such as glutamate, and short-chain fatty acids, including proprionate, acetate, and butyrate (Bradshaw et al., 1994; Mirković et al., 2015).
Notably, these metabolites were found to be increased in adult CF patient sputum (Mirković et al., 2015). Therefore, anaerobic species which are found in high numbers in sputum from people with CRD, can support the growth of common pathogens including P. aeruginosa, S. aureus, and Burkholderia cenocepacia through the cooperative utilization and metabolism of mucins (Tunney et al., 2008; Flynn et al., 2016). Such behavior increases available resources and results in cooperation (Figure 1).
Public Goods
In addition to nutrients, bacteria can also produce extracellular products that benefit neighboring cells. Although an individual cell experiences an energetic cost in metabolism, the production is beneficial for the surrounding population (Zhao et al., 2019). The production of these products occurs when bacteria are in a dense biofilm community where many of the surrounding cells are related organisms. These processes are often coordinated by quorum sensing, where bacteria release signal molecules (autoinducers) as a measure of cell density. Once this signal reaches a certain concentration, a change in gene expression occurs (Miller and Bassler, 2001). In this way, an organism regulates the production of goods until it is present at a sufficient cell density to benefit from production. A number of extracellular bacterial products can be exploited as public goods including extracellular enzymes, surfactants, exopolysaccharides, virulence factors, and siderophores. For example, the P. aeruginosa polysaccharide Psl functions as a public good, as it is an important factor for attachment to surfaces and biofilm formation (Irie et al., 2017). Production of the Psl polysaccharide was found to benefit bacterial populations and has social benefits for P. aeruginosa strains that do not produce Psl (Hall-Stoodley et al., 2006). However, production of public goods is highly prone to exploitation by social cheating. In this way, “cheaters” benefit from the production of public goods but do not expend energy for production. Species, such as P. aeruginosa, limit cheating by coregulating the production of public goods (such as protease) with the production of virulence factors to limit the growth of other organisms, including pyocyanin, hydrogen cyanide, and rhamnolipid production (Smalley et al., 2015).
Inter-Species Signaling
Quorum sensing is also involved in inter-species signaling. Although known autoinducer molecules are quite diverse, one signal is highly conserved across many species. This signal, autoinducer-2 (AI-2), is produced by the LuxS synthase found in bacteria including Haemophilus spp., Staphylococcus spp., and Streptococcus spp. (Federle and Bassler, 2003). In H. influenzae, AI-2 is necessary for biofilm maturation, persistence, and chronic infection in a chinchilla model (Armbruster et al., 2009). However, AI-2 also has important roles in inter-species gene regulation and the formation of mixed-species biofilms. Specifically, M. catarrhalis displays increased biofilm formation and enhanced antibiotic tolerance in co-culture with H. influenzae (Armbruster et al., 2010). Although M. catarrhalis does not encode LuxS, it is able to detect the production of AI-2 by H. influenzae in order to promote biofilm, allowing M. catarrhalis to “eavesdrop” on nearby species. The addition of exogenous AI-2 precursor alone to mono-cultures of M. catarrhalis was sufficient to increase biofilm (Armbruster et al., 2010). Similar responses to AI-2 also occur in P. aeruginosa, which also lacks LuxS synthase. In response to AI-2, P. aeruginosa produces phenazines, elastase, rhamnolipids, flagellin, and other genes, likely altering its interactions with nearby microbes (Duan et al., 2003). AI-2 has been detected from CF patient sputum, suggesting that this molecule is especially important for bacterial pathogenesis in vivo (Duan et al., 2003).
Formation of Polymicrobial Biofilms
In the respiratory tract, pathogens encounter many other organisms which may alter the organization of bacterial communities. For example, changes in P. aeruginosa and S. aureus biofilm formation have been shown to result from co-infection with other organisms, notably respiratory viruses such as respiratory syncytial virus and rhinovirus (Hendricks et al., 2016; Kiedrowski et al., 2018). Bacterial species can also impact neighboring bacteria to increase biofilm formation, facilitating enhanced tolerance, and persistence of both species. These changes are sometimes due to altered bacterial signaling and metabolite production that occurs during polymicrobial infections. As described above, AI-2 production by H. influenzae enhances the biofilm formation of M. catarrhalis (Armbruster et al., 2010). Many other species also display increased biofilm formation during co-culture. Although many of the specific signals preceding this change are still unknown, a number of observations have been made. First, clinical isolates of P. aeruginosa and Burkholderia cepacia from CF patients were observed to form mixed species biofilms during co-culture (Tomlin et al., 2001). Other studies found that P. aeruginosa conditioned media enhanced the attachment of B. cepacia to epithelial cells, suggesting that P. aeruginosa signals to B. cepacia to enhance biofilm formation through an unknown mechanism (Saiman et al., 1990). Additionally, the presence of P. aeruginosa also enhanced subsequent biofilm formation of Klebsiella pneumoniae (Stewart et al., 1997).
Altered gene expression during co-culture in polymicrobial biofilms likely underlies most of these observed changes. Specifically, the relationship between H. influenzae and S. pneumoniae is well-documented. These two pathogens both reach higher cell densities during co-culture as either do alone (Margolis et al., 2010; Cope et al., 2011). Additionally, H. influenzae reaches a higher cell density when invading established populations of S. aureus or S. pneumoniae (Margolis et al., 2010). Transcription of the H. influenzae type IV pili occurred only in the presence of S. pneumoniae, as compared to monoculture. Expression of type IV pili was also confirmed in diseased tissue from CRS patients. The type IV pili is an essential factor for H. influenzae biofilm formation and might also mediate direct contact with other species during co-culture, so induction of type IV pili during co-culture is likely an important factor mediating colonization and persistence of H. influenzae during respiratory infection (Jurcisek et al., 2007).
Coaggregation of bacterial species may also impact the development of polymicrobial biofilms, as the association of genetically distinct bacteria has previously been shown to enhance bacterial adhesion preceding biofilm formation (Rickard et al., 2003). A few examples of coaggregation have been reported in the respiratory tract, as M. catarrhalis can coaggregate with Streptococcus pyogenes, enhancing the colonization and adherence of S. pyogenes to epithelial cells (Lafontaine et al., 2004). However, the role of coaggregation in the respiratory tract is still poorly understood (Humphreys et al., 2017).
Enhanced Tolerance
The presence of other microbes can confer higher antimicrobial resistance and enhanced protection from immune radicals to the entire microbial population through a variety of mechanisms (Figure 1). First, P. aeruginosa production of extrapolymeric substance (EPS), an essential component of the biofilm matrix, provides protection to other neighboring species (Billings et al., 2013; Periasamy et al., 2015). P. aeruginosa produces three different polysaccharides with various roles in biofilm formation—Psl, Pel, and alginate (Hentzer et al., 2001; Colvin et al., 2012). All three polysaccharides have been documented to confer antibiotic resistance to other bacteria. Non-mucoid strains of P. aeruginosa primarily used Pel or Psl as a primary matrix polysaccharide. Psl has been found to confer protection to E. coli and S. aureus to colistin and tobramycin, respectively (Billings et al., 2013). This effect is thought to be due to binding and sequestration of antibiotics to the matrix, limiting its contact with the bacterial cell surface. Although no effect on other species has been described, Pel and extracellular DNA (eDNA) have both been shown to enhance resistance to aminoglycosides, suggesting they might also protect other species from aminoglycoside toxicity (Khan et al., 2010; Colvin et al., 2011; Chiang et al., 2013). Mucoid strains of P. aeruginosa are commonly isolated during chronic infection, especially in CF (Bjarnsholt et al., 2009). The conversion of P. aeruginosa to a mucoid phenotype involves mutations resulting in the overexpression of alginate (Hentzer et al., 2001). The expression of alginate is known to confer enhanced antibiotic tolerance of S. maltophilia to tobramycin (Pompilio et al., 2015) and ciprofloxacin (Magalhães et al., 2017) as well as S. aureus and Inquilinus limosus resistance to ciprofloxacin (Magalhães et al., 2017). Alginate is thought to impact antibiotic resistance by slowing or preventing antibiotic penetration into the biofilm matrix (Gordon et al., 1988; Nichols et al., 1988). The expression of alginate was also increased when P. aeruginosa and S. maltophilia are grown in a polymicrobial biofilm, suggesting this mechanism is important in polymicrobial environments (Pompilio et al., 2015).
Antibiotic tolerance can also be conferred to other species by enzymatic activity, delivery of these enzymes, and horizontal gene transfer resulting in the acquisition of antimicrobial resistance genes. The production of β-lactamase, which functions to cleave the β-lactam ring of β-lactam antibiotics, by non-typeable H. influenzae (NTHi) is shown to protect both planktonic and biofilm-associated S. pneumoniae from amoxicillin (Weimer et al., 2011). However, β-lactamases can also confer inter-species protection through the delivery in bacterial outer-membrane vesicles (OMVs). M. catarrhalis is able to protect S. pneumoniae and H. influenzae from amoxicillin treatment through the transfer of β-lactamases in OMVs (Schaar et al., 2011). Delivery of β-lactamase in OMVs is likely important to prevent enzymatic degradation during transfer to other cells, as shown for P. aeruginosa and S. aureus (Ciofu et al., 2000; Bomberger et al., 2009; Lee et al., 2013).
Polymicrobial communities also protect neighboring species from the host immune system. The presence of bacteria can modulate both immune cell function and any toxic substances they release. S. aureus alpha toxin (AT) is able to inhibit human mononuclear cell antibacterial activity (Cohen et al., 2016). Specifically, AT impaired phagocytosis of S. aureus, P. aeruginosa, and K. pneumoniae. Therefore, S. aureus AT allows proliferation of other microbes by inhibiting immune cell function. Immune cells, including macrophages, release toxic metabolites as signaling molecules which are capable of killing pathogens. Nitric oxide (NO), one such free radical, is capable of inducing DNA damage and degrading iron sulfur clusters, inhibiting bacterial growth (Wink and Mitchell, 1998). However, S. aureus is quite resistant to NO due to its detoxification abilities (Grosser et al., 2018). In co-culture with P. aeruginosa, S. aureus detoxifies NO to promote the survival of CF clinical strains of P. aeruginosa, consistent with findings showing S. aureus-P. aeruginosa co-infection in CF patients (Hoffman et al., 2010; Limoli et al., 2016). Additionally, bacterial relationships are also shaped by the presence of other host proteins involved in the immune response. Calprotectin, a protein released from neutrophils or other phagocytes, sequesters essential nutrients including iron, zinc, and manganese away from bacteria in order to inhibit their growth (Zygiel et al., 2019). Iron-, zinc-, and manganese- limited environments in turn repress the production of anti-staphylococcal factors in P. aeruginosa, promoting increased levels of P. aeruginosa and S. aureus co-colonization in vitro and in vivo (Wakeman et al., 2016).
DNA Availability
Extracellular DNA (eDNA) is an integral component of the biofilm matrix (Whitchurch et al., 2002), but also serves many other purposes in microbial communities (Figure 1). The presence of DNA in the host environment results from a variety of processes including lysis of cells due to lytic bacteriophage, autolysis, cell death, and active secretion of DNA (Steinmoen et al., 2002; Jurcisek et al., 2017). Extracellular DNA is produced by organisms including, P. aeruginosa, Streptococcus spp., Staphylococcus spp., and H. influenzae both on solid and liquid cultures (Allesen-Holm et al., 2006). Biofilm formation of many species relies on the incorporation of DNA into the biofilm matrix, as treatment with DNase is sufficient to inhibit biofilm formation (Moscoso et al., 2006; Rice et al., 2007). Additionally, the increased release of DNA during spontaneous phage lysis enhances biofilm formation of S. pneumoniae (Carrolo et al., 2010). Not only can eDNA serve a structural role for biofilm formation, but it also can be utilized as a nutrient source during nutrient-limiting conditions, also helping species persist. Specifically, P. aeruginosa encodes an extracellular deoxyribonuclease (DNase) that is capable of degrading DNA to utilize as a carbon, nitrogen, and phosphorous source (Mulcahy et al., 2010).
In addition to helping species survive in environments such as the respiratory tract, eDNA might also help organisms recover from damage caused by antibiotics or the immune system and also better adapt to an infection environment (Ibáñez de Aldecoa et al., 2017). Uptake of exogenous DNA is actually higher when bacteria live in a biofilm, rather than a planktonic state (Marks et al., 2012). The role of eDNA in DNA damage repair and gene transfer has been described for many respiratory pathogens (Ibáñez de Aldecoa et al., 2017). DNA damage resulting from insults including antibiotic treatment and the host immune system often induces the expression of many genes involved in competence. Competent organisms including H. influenzae and S. pneumoniae take up extracellular environmental DNA during this process, helping these organisms adapt by benefitting from genes that might be released from other bacterial species within a microbial community (Steinmoen et al., 2002).
Competitive Behaviors
Although many cooperative effects have been described in vivo and in vitro, many pairwise combinations of species actually result in net negative effects due to competition (Foster and Bell, 2012). Adaptation in the presence of other bacterial species is also predicted to be almost exclusively competitive to ensure the survival of a given species (Figure 2). Competition is carefully and tightly regulated in response to signals from the host and other invading microbes in order to prevent unnecessary toxicity and conserve energy (Lobato-Márquez et al., 2016).
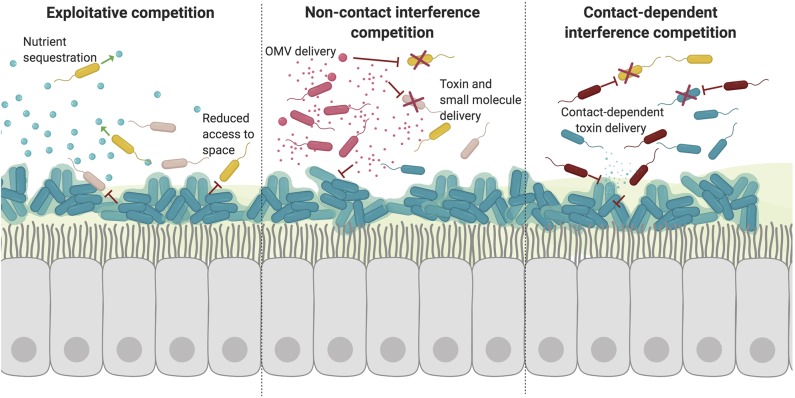
Figure 2.
Competitive interactions disrupt microbial communities. Competitive bacterial interactions can be exploitative or interfering, which is often energetically expensive and tightly regulated. (1) Exploitative competition takes advantage of extracellular nutrients and compounds released by other species. Such competition is often for trace metals which are actively sequestered but essential for bacterial growth. Other essential resources also include access to space. Formation of recalcitrant biofilms on epithelial surfaces restricts the colonization and growth of other species. (2) Bacteria can also interfere with other species growth and signaling through the active secretion and transport of toxic molecules. There are a variety of processes used to deliver toxic molecules over a distance including direct release into the extracellular environment and packaging into vesicles. (3) Finally, specific competition with neighboring bacteria can also occur via direct contact. In this way, bacteria can specifically deliver toxins directly to other cells in close proximity through mechanisms including contact-dependent inhibition and type VI secretion.
Exploitative Competition
Bacteria share the need for essential nutrients and substrates in order to survive in the respiratory tract. However, not all of these molecules are released as public goods. The host often serves as a reservoir for trace minerals and nutrients, yet actively sequesters these to prevent acquisition by pathogens in a process termed “nutritional immunity (Hood and Skaar, 2012).” In order to ensure access to these restricted resources, bacteria must confiscate these nutrients for themselves or circumvent the mechanisms other microbes use to access these nutrients. These competitive behaviors not only underlie a pathogen's success in the host environment, but also have important roles in the ecology and diversity of the respiratory microbiome, impacting chronic respiratory disease (Figure 2).
Nutrient Access
Trace metals including iron, zinc, manganese, and copper are essential nutrients for many different bacterial enzymes (Palmer and Skaar, 2016). The ability to acquire all of these nutrients certainly drives adaptation in the host and also likely influences inter-species competition to ensure survival. Of these nutrients, the best studied example is iron. Iron is an essential cofactor for the host and microbes alike, where it is used for enzymes involved in a variety of processes including DNA replication and metabolism. Iron is sequestered by the host by iron-binding proteins and intracellular iron stores (Hood and Skaar, 2012). Many bacterial species produce secreted iron-chelating molecules, termed siderophores, that allow bacteria to access and transport ferric iron for use (Miethke and Marahiel, 2007). Some species can also utilize xenosiderophores, siderophores produced by other species. The expression of siderophores and cognate receptors is greatly increased during iron-limiting conditions, as found in the host. Although the production of siderophores might benefit neighboring species who also express the cognate receptor (xenosiderophore receptors), their production is largely competitive, as bacteria must acquire and sequester iron in order to compete for finite resources in the host. For example, when P. aeruginosa overproduces siderophores it inhibits the growth of Streptococcus spp. by restricting the amount of iron available to Streptococcus spp. (Scott et al., 2019). Additionally, P. aeruginosa encodes many outer membrane receptors to benefit from xenosiderophores in order to sequester iron for itself. In co-culture with B. cepacia, P. aeruginosa can detect and respond to ornibactin produced by B. cepacia (Weaver and Kolter, 2004). By acquiring iron from other organisms, P. aeruginosa can easily acquire essential iron, while restricting iron acquisition from other microbes. Utilizing this iron source, rather than producing its own chelating molecules, prevents P. aeruginosa from expending the energy necessary to produce siderophores.
Iron can also be acquired through other mechanisms, including direct lysis of competing cells (Mashburn et al., 2005). In low iron conditions, P. aeruginosa can lyse S. aureus and S. pneumoniae to access their stored iron through activity of the LasA protease. The presence of S. aureus actually decreases P. aeruginosa's transcription of iron-regulated genes, as S. aureus alone can serve as an iron source for P. aeruginosa (Mashburn et al., 2005). Iron can also be acquired through heme breakdown, as 80% of host iron is present as ferriprotoporphyrin IX (heme) (Hammer and Skaar, 2011). Because heme is toxic at high concentrations, many species have developed mechanisms to process heme. S. aureus preferentially acquires iron from heme during iron-limiting conditions by utilizing hemolysins that function to lyse erythrocytes to gain access to hemoglobin and heme. In the presence of other Staphylococcus and Corynebacterium species, S. aureus has enhanced β-hemolytic activity, suggesting that S. aureus sequesters more iron away from other species in order to ensure its survival (Hebert and Hancock, 1985).
Communication Disruption
Cell to cell communication is an essential component of bacterial communities in order to modulate gene expression to best ensure survival. Although quorum sensing (QS) and inter-species communication can be used cooperatively in microbial communities, this pathway can also be exploited, allowing species to “eavesdrop” on neighboring species (Miller et al., 2018). QS can be blocked by inhibiting the production of signal, delivery of the signal, or detection of the signal. In bacteria this is often achieved by enzymatic activity, as many naturally occurring quorum quenching enzymes have been described. These enzymes are often either lactonases, which cleave the lactone ring of N-acyl homoserine lactones (AHLs) or acylases, which hydrolyze the amide bond of AHLs, both of which result in inactivation of the autoinducer. Lactonases have been described in species of Klebsiella and Acinetobacter, which are capable of attenuating the production of virulence factors, including P. aeruginosa elastase (Chan et al., 2011). The quorum quenching acylase PvdQ of P. aeruginosa is capable of inactivating the 3-oxo-C12-HSL signal in P. aeruginosa, and might also have some activity in decreasing the amount of C8-HSL signal in B. cenocepacia (Koch et al., 2014; Utari et al., 2018). Additionally, quorum quenching bacterial species capable of degrading AHLs were able to decrease P. aeruginosa biofilm formation and elastase production. These species also attenuated the virulence of P. aeruginosa and B. cenocepacia in a Caenorhabditis elegans model, supporting the role of QS in bacterial pathogenesis (Christiaen et al., 2014).
Growth interference
Bacteria produce an arsenal of enzymatic and non-enzymatic molecules which function to kill or interfere with microbial growth (Figure 2). Many metabolites with diverse functions from P. aeruginosa have antimicrobial properties, including pyocyanin and HQNO (Noto et al., 2017). P. aeruginosa produces four types of phenazines, redox-active pigments with broad-spectrum antibiotic properties and roles in virulence. One of these phenazines, pyocyanin, has been specifically demonstrated to reduce microbial community diversity (Norman et al., 2004). Pyocyanin inhibits the growth of many other species, including B. cepacia, allowing P. aeruginosa to outcompete B. cepacia in polymicrobial biofilms (Tomlin et al., 2001). However, the effects of pyocyanin also inhibit oxidative respiration in S. aureus (Filkins et al., 2015; Noto et al., 2017). As such, pyocyanin drives S. aureus into a respiration-deficient small colony variant (SCV) state and also leads to the formation of persister cells in A. baumannii (Bhargava et al., 2014; Noto et al., 2017). Another secondary metabolite of P. aeruginosa, 4-hydroxy-2-heptylquinoline N-oxide (HQNO), also functions by inhibiting respiratory electron transfer. Like pyocyanin, HQNO also inhibits the growth of S. aureus, while promoting the growth of SCVs, but increasing tolerance to antibiotics (Orazi and O'Toole, 2017). Additionally, HQNO also inhibits many S. aureus virulence factors including agr signaling, alpha hemolysin production, and cytochrome oxidase production (Mitchell et al., 2010).
Other species also produce a variety of secreted factors capable of restricting microbial growth. Previously, S. pneumoniae culture supernatants were found to inhibit the growth of H. influenzae, although H. influenzae culture supernatant had no effect on S. pneumoniae viability (Pericone et al., 2000). This growth inhibition is due to the production of hydrogen peroxide by S. pneumoniae, which is upregulated during co-culture with H. influenzae. Hydrogen peroxide by S. pneumoniae also was able to inhibit growth of M. catarrhalis and Neisseria meningitidis. P. aeruginosa also restricts Streptococcus constellatus growth and biofilm formation by the production of rhamnolipids, a type of bacterial surfactant (Price et al., 2015). Notably, tobramycin treatment of P. aeruginosa biofilms resulted in decreased rhamnolipid production, allowing for enhanced S. constellatus growth.
Many bacterial enzymes also function to inactivate toxic molecules produced by competing species. In fact, S. aureus produces catalase in order to decompose the hydrogen peroxide produced by S. pneumoniae, as previously described (Park et al., 2008). The production of catalase helps S. aureus survive S. pneumoniae in vitro and in vivo. However, many bacterial enzymes directly inhibit the growth of other microbes. Many of these enzymes function to degrade bacterial cell walls. The proteolytic activity of P. aeruginosa against S. aureus has been well-established (Kessler et al., 1993). The P. aeruginosa LasA endopeptidase cleaves peptide bonds resulting in the degradation of the S. aureus cell wall. Rather than being directly secreted, enzymes are also often packaged into extracellular vesicles for delivery. These outer-membrane vesicles (OMVs) are normally produced during cell growth and fuse with the Gram-negative cell membrane or adhere to Gram-positive cell walls in order to deliver cargo directly into a cell. P. aeruginosa OMVs seem to be particularly effective at killing other species. OMVs from P. aeruginosa contained peptidoglycan hydrolase, phospholipase C, protease, hemolysin, and alkaline protease (Kadurugamuwa and Beveridge, 1996; Li et al., 1998). These enzymes effectively lysed E. coli, other Pseudomonas spp., and S. aureus. Notably, delivery of peptidoglycan hydrolase is likely an important mechanism for cargo delivery and killing of Gram-positive species, suggesting that OMVs can target a diversity of bacterial species.
Finally, other secreted enzymes also function to inhibit bacterial virulence and persistence, rather than directly killing other species. One example is the Esp serine protease produced by Staphylococcus epidermidis. This protease degrades S. aureus biofilms and prevents further S. aureus biofilm formation (Vandecandelaere et al., 2014). Esp also inactivates S. aureus autolysins, preventing the release of DNA from S. aureus following lysis. As DNA is an essential component of the biofilm matrix, Esp prevents biofilm formation by restricting DNA availability. These findings complement associations documented in the nasal cavity of CRS patients, where negative correlations exist between S. epidermidis and S. aureus (Lina et al., 2003). Since S. epidermidis is often non-pathogenic, this represents one way that healthy microbial flora might restrict the growth and success of invading pathogens such as S. aureus.
Antibiotic Production
Many species of bacteria produce molecules specifically to kill neighboring cells and competitors. One class of antibacterial molecules that act to kill bacterial growth are bacteriocins. It is estimated that almost all species of bacteria produce at least one bacteriocin (Klaenhammer, 1988). Although various bacteriocins possess diverse antimicrobial activities, they share an extremely narrow specificity and high activity at very low concentrations. These antibacterial peptides bind to specific outer membrane receptors to be translocated through the membrane to access their specific target. Bacteriocins produced by P. aeruginosa, pyocins, have been well described. Over 90% of P. aeruginosa strains produce at least one pyocin, but often synthesize several different pyocins with DNase, tRNase, or pore-forming activities (Michel-Briand and Baysse, 2002). Although pyocins have intra-species activities, other species including H. influenzae are also sensitive to pyocins, likely due to similar outer membrane receptors (Filiatrault et al., 2001). Pyocin production is often in response to stress, including antibiotic treatment or immune radicals as often encountered in chronic respiratory disease. Specifically, P. aeruginosa pyocin production is induced following exposure to hydrogen peroxide, ciprofloxacin treatment, and anaerobic environments (Brazas and Hancock, 2005; Chang et al., 2005; Waite and Curtis, 2009). In an analysis of P. aeruginosa and B. cenocepacia CF clinical isolates, ~97% of P. aeruginosa strains displayed bacteriocin-like activity, while only ~16% of B. cenocepacia strains possessed bacteriocin-like activity. Many of the P. aeruginosa CF clinical isolates possessed more than one pyocin. Notably, 81% of P. aeruginosa isolates were able to inhibit B. cenocepacia, often due to RF-type pyocins (Bakkal et al., 2010).
Contact-Dependent Toxin Delivery
Although there are many mechanisms bacteria use to compete with surrounding organisms in the local environment, a number of more intimate interactions also exist in order to specifically compete with cells in close proximity (Figure 2). These mechanisms are contact-dependent, as they rely on physical contact to transfer toxins and other molecules from cell-to-cell. Contact dependent inhibition (CDI) is one such mechanism used to compete with nearby cells. Bacteria that possess CDI systems form a rigid extracellular filament topped with a receptor binding domain which binds to its cognate outer membrane receptors on target bacteria, secreted by a type Vb secretion system (Anderson et al., 2014). Although this mechanism has largely been shown to mediate contact with closely related cells, more evidence has been found suggesting this activity might have broader specificity than previously suggested (Virtanen et al., 2019). Interaction with other bacteria targets secretion of CdiA effector proteins with C-terminal toxin activity which often function to disrupt membranes or degrade nucleic acid in order to inhibit bacterial growth. CDI systems are characteristically involved in discriminating neighbors in a polymicrobial community as a means of self-recognition and inhibition of CDI− bacteria (Anderson et al., 2014). Competition occurs on a single-cell level, as CDI+ cells create a single-cell-wide boundary between neighboring cells (Bottery et al., 2019). CDI systems of Burkholderia spp. are also responsible for excluding CDI− invading cells from pre-established biofilms (Anderson et al., 2014). However spatial organization and exclusion are not the only roles of CDI systems, delivery of CDI toxins to kin cells results in increased community behaviors including increased biofilm formation (Garcia et al., 2016). In P. aeruginosa, CDI systems were found to have decreased virulence in acute infection models, including in Galleria mellonella (Melvin et al., 2017). These downstream effects likely result from post-transcriptional regulation of virulence genes.
Other contact-dependent growth inhibition systems exist, including the recently-described type VI secretion system (T6SS). The T6SS is evolutionarily related to bacteriophage proteins (Leiman et al., 2009). These contact-dependent “molecular syringes” are contractile structures that puncture cell membranes to inject toxic effectors directly into nearby cells. Although many other bacterial secretion systems function solely in the delivery of effectors to eukaryotic host cells, the T6SS is unique in that it also delivers antibacterial effectors to neighboring bacteria (Hood et al., 2010). Toxic effectors have a variety of activities resulting in cell death or growth inhibition, including nucleic acid degradation, cell wall degradation, membrane permeabilization, and transcriptional and translational inhibition (Suarez et al., 2010; Russell et al., 2011, 2013; Sana et al., 2012; Jiang et al., 2014; Tang et al., 2018). T6SS-containing species also encode cognate immunity proteins to protect themselves from intoxication by sister cells. T6SSs have been described in ~25% of Gram-negative organisms (Bingle et al., 2008). Many respiratory pathogens, including P. aeruginosa, B. cepacia complex, K. pneumoniae, and Acinetobacter baumannii, encode multiple non-redundant T6SSs that function to deliver an arsenal of toxins to neighboring cells (Schwarz et al., 2010; Hachani et al., 2011). T6SS toxins from P. aeruginosa, B. cepacia complex, K. pneumoniae, A. baumannii, and the opportunistic pathogen Serratia marcescens have been shown to be toxic to competing species including E. coli, however the spectrum of toxic effects on diverse classes of bacteria is still poorly described (Jiang et al., 2014; Alcoforado Diniz and Coulthurst, 2015; Hsieh et al., 2019; Spiewak et al., 2019). Gram-positive species also possess specialized secretion systems, known as the type VII secretion system (Ates et al., 2016). This system likely impacts other microbes, however, less is known about this system and how it contributes to microbial community interactions.
Discussion/Conclusion
Microbial dysbiosis is a common marker of CRD, as those with CRD have less diversity and greater abundance of pathogenic organisms in their respiratory tract (Coburn et al., 2015; Durack et al., 2017; Copeland et al., 2018; Quaderi and Hurst, 2018). The altered community structure in the respiratory tract of CRD patients is likely shaped by respiratory symptoms and antibiotic treatment, but also is likely due to interactions within the microbial communities (Figure 3). Although many microbial interactions have been elegantly described, understanding how organisms interact in the host during CRD is still largely being elucidated. The cooperative and competitive interactions described herein may impact the resulting community structure and composition, yet there are likely many more interactions that occur that are still unknown or poorly understood. Specifically, how these interactions change in response to the environment of the host respiratory tract is still unknown. However, there are many common trends that occur across chronic respiratory diseases: (1) diseased respiratory microbiomes are dysbiotic and often result in the dominance of a single pathogenic organism over time, (2) low community diversity adversely affects lung function, and (3) disease symptoms and the respiratory environment impacts the microbiome. Although much about microbial interactions in the respiratory tract is unknown, many of the known in vitro microbial interactions do correlate with clinical observations. For example, Staphylococcus spp. and Streptococcus spp. have a negative correlation with one another in CRS and OM, consistent with the competitive interactions described previously. In addition to interactions with other bacteria, interactions with other organisms including viruses and fungi also impact bacterial communities. Although outside the scope of this review, viral infections are important in the pathogenesis of asthma, CF, COPD, and OM and likely impact the bacterial communities in the respiratory tract (Wedzicha, 2004; Bakaletz, 2010; Wark et al., 2012; Iikura et al., 2015). Therefore, understanding these bacterial-bacterial interactions and the polymicrobial community in the respiratory tract could be informative in understanding how to preserve microbial diversity and maintain lung function.
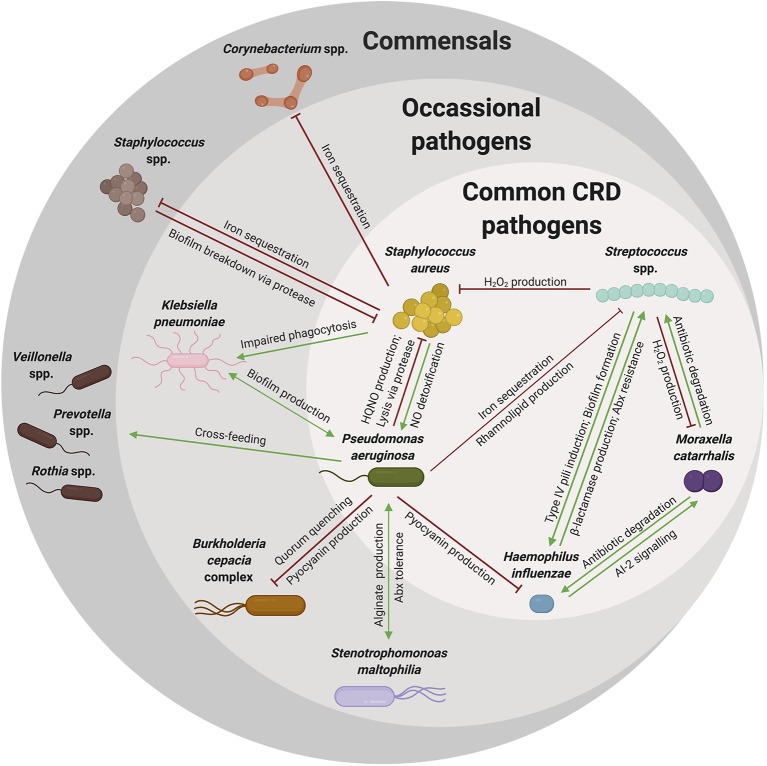
Figure 3.
A complex web of microbial interactions shape microbial communities. Many organisms reside in the respiratory tract of young patients with respiratory disease. However, as patients age, these communities become increasingly dysbiotic, likely due to interactions among colonizing organisms and newly acquired pathogens. Many interactions have been described between commensal organisms and pathogenic species that might explain the microbial community shifts noted over a patient's lifetime. As illustrated here, many of these pathogens are shared among the various types of chronic respiratory diseases (common CRD pathogens), while other pathogens are not shared among all CRD, but rather indicated in one or few CRD (occasional pathogens), but still contribute to notable community shifts. Commensal species are also an important component of the respiratory microbiome, directly involved in competition with pathogenic species. Some of the cooperative (green arrow) and competitive (red) interactions described in this review are summarized in this web, with the arrow indicating directionality of effects. These interactions are diverse and likely complicated by respiratory symptoms and therapeutic strategies. Although complex, an understanding of these interactions can explain observed microbial shifts and may give us insight into overarching microbial community dynamics.
Author Contributions
AW and JB wrote and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported in part by the National Institutes of Health (NIH R01HL142587, R01HL123771, R33HL137077 to JB) and the Cystic Fibrosis Foundation (BOMBER18G0 to JB). No persons employed by the funders played any role in the study, in preparation of the manuscript, or in the decision to publish.
References
- Abreu N. A., Nagalingam N. A., Song Y., Roediger F. C., Pletcher S. D., Goldberg A. N., et al. (2012). Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 4:151ra124. 10.1126/scitranslmed.3003783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuin J. (2007). Chronic suppurative otitis media. BMJ. Clin. Evid. 2007:0507. [PMC free article] [PubMed] [Google Scholar]
- Alcoforado Diniz J., Coulthurst S. J. (2015). Intraspecies competition in Serratia marcescens is mediated by type VI-secreted rhs effectors and a conserved effector-associated accessory protein. J. Bacteriol. 197, 2350–2360. 10.1128/JB.00199-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allesen-Holm M., Barken K. B., Yang L., Klausen M., Webb J. S., Kjelleberg S., et al. (2006). A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59, 1114–1128. 10.1111/j.1365-2958.2005.05008.x [DOI] [PubMed] [Google Scholar]
- Anderson M. S., Garcia E. C., Cotter P. A. (2014). Kind discrimination and competitive exclusion mediated by contact-dependent growth inhibition systems shape biofilm community structure. PLoS Pathog. 10:e1004076. 10.1371/journal.ppat.1004076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster C. E., Hong W., Pang B., Dew K. E., Juneau R. A., Byrd M. S., et al. (2009). LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface. Infect. Immun. 77:4081. 10.1128/IAI.00320-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster C. E., Hong W., Pang B., Weimer K. E. D., Juneau R. A., Turner J., et al. (2010). Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1, e00102–00110. 10.1128/mBio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster C. R., Coenye T., Touqui L., Bomberger J. M. (2019). Interplay between host-microbe and microbe-microbe interactions in cystic fibrosis. J. Cystic Fibrosis 19, S47–S53. 10.1016/j.jcf.2019.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K. (2015). Bacterial metabolism in the host environment: pathogen growth and nutrient assimilation in the mammalian upper respiratory tract. Microbiol. Spectrum 3. 10.1128/microbiolspec.MBP-0007-2014 [DOI] [PubMed] [Google Scholar]
- Ates L. S., Houben E. N. G., Bitter W. (2016). Type VII secretion: a highly versatile secretion system. Microbiol. Spectr. 4. 10.1128/microbiolspec.VMBF-0011-2015 [DOI] [PubMed] [Google Scholar]
- Bakaletz L. O. (2010). Immunopathogenesis of polymicrobial otitis media. J. Leukoc. Biol. 87, 213–222. 10.1189/jlb.0709518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L. O. (2012). Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatr. Respir. Rev. 13, 154–159. 10.1016/j.prrv.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkal S., Robinson S. M., Ordonez C. L., Waltz D. A., Riley M. A. (2010). Role of bacteriocins in mediating interactions of bacterial isolates taken from cystic fibrosis patients. Microbiology 156, 2058–2067. 10.1099/mic.0.036848-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassis C. M., Erb-Downward J. R., Dickson R. P., Freeman C. M., Schmidt T. M., Young V. B., et al. (2015). Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 6, e00037–e00015. 10.1128/mBio.00037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley V., Joshi P. V., Singanayagam A., Molyneaux P. L., Johnston S. L., Mallia P. (2012). Lung microbiology and exacerbations in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 7, 555–569. 10.2147/COPD.S28286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava N., Sharma P., Capalash N. (2014). Pyocyanin stimulates quorum sensing-mediated tolerance to oxidative stress and increases persister cell populations in Acinetobacter baumannii. Infect. Immun. 82, 3417–3425. 10.1128/IAI.01600-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesbroek G., Wang X., Keijser B. J. F., Eijkemans R. M. J., Trzcinski K., Rots N. Y., et al. (2014). Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg. Infect. Dis. 20, 201–210. 10.3201/eid2002.131220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings N., Ramirez Millan M., Caldara M., Rusconi R., Tarasova Y., Stocker R., et al. (2013). The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 9:e1003526. 10.1371/journal.ppat.1003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L. E. H., Bailey C. M., Pallen M. J. (2008). Type VI secretion: a beginner's guide. Curr. Opin. Microbiol. 11, 3–8. 10.1016/j.mib.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Alhede M., Alhede M., Eickhardt-Sorensen S. R., Moser C., Kuhl M., et al. (2013). The in vivo biofilm. Trends Microbiol. 21, 466–474. 10.1016/j.tim.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Jensen P. O., Fiandaca M. J., Pedersen J., Hansen C. R., Andersen C. B., et al. (2009). Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 44, 547–558. 10.1002/ppul.21011 [DOI] [PubMed] [Google Scholar]
- Bomberger J. M., Maceachran D. P., Coutermarsh B. A., Ye S., O'Toole G. A., Stanton B. A. (2009). Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. 10.1371/journal.ppat.1000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A. A. T. M., Levin E., van Houten M. A., Hasrat R., Kalkman G., Biesbroek G., et al. (2016). Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 9, 336–345. 10.1016/j.ebiom.2016.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Grammer L. C., Peters A. T. (2016). Infectious chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 4, 584–589. 10.1016/j.jaip.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottery M. J., Passaris I., Dytham C., Wood A. J., van der Woude M. W. (2019). Spatial organization of expanding bacterial colonies is affected by contact-dependent growth inhibition. Curr. Biol. 29, 3622–3634.e3625. 10.1016/j.cub.2019.08.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin R. C. T., Petersen C., Finlay B. B. (2017). Microbial insights into asthmatic immunopathology. A forward-looking synthesis and commentary. Ann. Am. Thoracic Soc. 14, S316–S325. 10.1513/AnnalsATS.201707-534AW [DOI] [PubMed] [Google Scholar]
- Bradshaw D. J., Homer K. A., Marsh P. D., Beighton D. (1994). Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140, 3407–3412. 10.1099/13500872-140-12-3407 [DOI] [PubMed] [Google Scholar]
- Bragonzi A., Farulla I., Paroni M., Twomey K. B., Pirone L., Lore N. I., et al. (2012). Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS ONE 7:e52330. 10.1371/journal.pone.0052330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazas M. D., Hancock R. E. W. (2005). Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrobial. Agents Chemother. 49, 3222–3227. 10.1128/AAC.49.8.3222-3227.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrolo M., Frias M. J., Pinto F. R., Melo-Cristino J., Ramirez M. (2010). Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS ONE 5:e15678. 10.1371/journal.pone.0015678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G., Archer G., Beam T., Gilchrist M., Goldmann D., Hooper D., et al. (1994). Report of the ASM Task Force on Antibiotic Resistance. Washington, DC: American Society for Microbiology. [Google Scholar]
- Caulley L., Thavorn K., Rudmik L., Cameron C., Kilty S. J. (2015). Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: results of the US medical expenditure panel survey. J. Allergy Clin. Immunol. 136, 1517–1522. 10.1016/j.jaci.2015.08.037 [DOI] [PubMed] [Google Scholar]
- Chalermwatanachai T., Vilchez-Vargas R., Holtappels G., Lacoere T., Jáuregui R., Kerckhof F.-M., et al. (2018). Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci. Rep. 8:7926. 10.1038/s41598-018-26327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J. D., Elborn J. S. (2015). Reclaiming the name 'bronchiectasis'. Thorax 70:399. 10.1136/thoraxjnl-2015-206956 [DOI] [PubMed] [Google Scholar]
- Chalmers J. D., Goeminne P., Aliberti S., McDonnell M. J., Lonni S., Davidson J., et al. (2013). The Bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 189, 576–585. 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.-G., Atkinson S., Mathee K., Sam C.-K., Chhabra S. R., Cámara M., et al. (2011). Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 11:51. 10.1186/1471-2180-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Small D. A., Toghrol F., Bentley W. E. (2005). Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics. 6:115. 10.1186/1471-2164-6-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang W.-C., Nilsson M., Jensen P. Ø., Høiby N., Nielsen T. E., Givskov M., et al. (2013). Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrobial. Agents Chemother. 57:2352. 10.1128/AAC.00001-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaen S. E. A., Matthijs N., Zhang X.-H., Nelis H. J., Bossier P., Coenye T. (2014). Bacteria that inhibit quorum sensing decrease biofilm formation and virulence in Pseudomonas aeruginosa PAO1. Pathog. Dis. 70, 271–279. 10.1111/2049-632X.12124 [DOI] [PubMed] [Google Scholar]
- Ciofu O., Beveridge T. J., Kadurugamuwa J., Walther-Rasmussen J., Hoiby N. (2000). Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45, 9–13. 10.1093/jac/45.1.9 [DOI] [PubMed] [Google Scholar]
- Coburn B., Wang P. W., Diaz Caballero J., Clark S. T., Brahma V., Donaldson S., et al. (2015). Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 5:10241. 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T. S., Hilliard J. J., Jones-Nelson O., Keller A. E., O'Day T., Tkaczyk C., et al. (2016). Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci. Transl. Med. 8:329ra331. 10.1126/scitranslmed.aad9922 [DOI] [PubMed] [Google Scholar]
- Collinson J., Nicholson K. G., Cancio E., Ashman J., Ireland D. C., Hammersley V., et al. (1996). Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51, 1115–1122. 10.1136/thx.51.11.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin K. M., Gordon V. D., Murakami K., Borlee B. R., Wozniak D. J., Wong G. C. L., et al. (2011). The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 7:e1001264. 10.1371/journal.ppat.1001264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin K. M., Irie Y., Tart C. S., Urbano R., Whitney J. C., Ryder C., et al. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 14, 1913–1928. 10.1111/j.1462-2920.2011.02657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope E. K., Goldstein-Daruech N., Kofonow J. M., Christensen L., McDermott B., Monroy F., et al. (2011). Regulation of virulence gene expression resulting from Streptococcus pneumoniae and nontypeable Haemophilus influenzae interactions in chronic disease. PLoS ONE 6:e28523. 10.1371/journal.pone.0028523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland E., Leonard K., Carney R., Kong J., Forer M., Naidoo Y., et al. (2018). Chronic rhinosinusitis: potential role of microbial dysbiosis and recommendations for sampling sites. Front. Cell. Infect. Microbiol. 8:57. 10.3389/fcimb.2018.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer J., Schipor I., Perloff J. R., Palmer J. N. (2004). Evidence of bacterial biofilms in human chronic sinusitis. ORL. J. Otorhinolaryngol. Relat. Spec. 66, 155–158. 10.1159/000079994 [DOI] [PubMed] [Google Scholar]
- De Boeck I., Wittouck S., Martens K., Claes J., Jorissen M., Steelant B., et al. (2019). Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere 4, e00532–e00519. 10.1128/mSphere.00532-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. P., Erb-Downward J. R., Freeman C. M., Walker N., Scales B. S., Beck J. M., et al. (2014). Changes in the lung microbiome following lung transplantation include the emergence of two distinct pseudomonas species with distinct clinical associations. PLoS ONE 9:e97214. 10.1371/journal.pone.0097214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. P., Erb-Downward J. R., Martinez F. J., Huffnagle G. B. (2016). The microbiome and the respiratory tract. Annu. Rev. Physiol. 78, 481–504. 10.1146/annurev-physiol-021115-105238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. P., Huffnagle G. B. (2015). The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog. 11:e1004923. 10.1371/journal.ppat.1004923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Dammel C., Stein J., Rabin H., Surette M. G. (2003). Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50, 1477–1491. 10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- Durack J., Lynch S. V., Nariya S., Bhakta N. R., Beigelman A., Castro M., et al. (2017). Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 140, 63–75. 10.1016/j.jaci.2016.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn J. S. (2016). Cystic fibrosis. Lancet 388, 2519–2531. 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- Engler K., Muhlemann K., Garzoni C., Pfahler H., Geiser T., von Garnier C. (2012). Colonisation with Pseudomonas aeruginosa and antibiotic resistance patterns in COPD patients. Swiss. Med. Wkly. 142:w13509. 10.4414/smw.2012.13509 [DOI] [PubMed] [Google Scholar]
- Federle M. J., Bassler B. L. (2003). Interspecies communication in bacteria. J. Clin. Invest. 112, 1291–1299. 10.1172/JCI20195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiatrault M. J., Munson R. S., Jr., Campagnari A. A. (2001). Genetic analysis of a pyocin-resistant lipooligosaccharide (LOS) mutant of Haemophilus ducreyi: restoration of full-length LOS restores pyocin sensitivity. J. Bacteriol. 183, 5756–5761. 10.1128/JB.183.19.5756-5761.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins L. M., Graber J. A., Olson D. G., Dolben E. L., Lynd L. R., Bhuju S., et al. (2015). Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a Cystic Fibrosis Model. J. Bacteriol. 197:2252. 10.1128/JB.00059-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch S., McDonnell M. J., Abo-Leyah H., Aliberti S., Chalmers J. D. (2015). A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann. Am. Thorac. Soc. 12, 1602–1611. 10.1513/AnnalsATS.201506-333OC [DOI] [PubMed] [Google Scholar]
- Flynn J. M., Niccum D., Dunitz J. M., Hunter R. C. (2016). Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathog. 12:e1005846. 10.1371/journal.ppat.1005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. R., Bell T. (2012). Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 22, 1845–1850. 10.1016/j.cub.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Gallo R. L. (2015). S. epidermidis influence on host immunity: more than skin deep. Cell Host Microbe 17, 143–144. 10.1016/j.chom.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Gallagher T., Zhang Y., Elbadawi-Sidhu M., Lai Z., Fiehn O., et al. (2018). Tracking polymicrobial metabolism in cystic fibrosis airways: Pseudomonas aeruginosa metabolism and physiology are influenced by rothia mucilaginosa-derived metabolites. mSphere 3, e00151–e00118. 10.1128/mSphere.00151-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E. C., Perault A. I., Marlatt S. A., Cotter P. A. (2016). Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc. Natl. Acad. Sci. U.S.A. 113, 8296–8301. 10.1073/pnas.1606323113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. A., Hodges N. A., Marriott C. (1988). Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J. Antimicrob. Chemother. 22, 667–674. 10.1093/jac/22.5.667 [DOI] [PubMed] [Google Scholar]
- Granchelli A. M., Adler F. R., Keogh R. H., Kartsonaki C., Cox D. R., Liou T. G. (2018). Microbial interactions in the cystic fibrosis airway. J. Clin. Microbiol. 56:e00354-18. 10.1128/JCM.00354-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. J., Wiriyachaiporn S., Grainge C., Rogers G. B., Kehagia V., Lau L., et al. (2014). Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 9:e100645. 10.1371/journal.pone.0100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser M. R., Paluscio E., Thurlow L. R., Dillon M. M., Cooper V. S., Kawula T. H., et al. (2018). Genetic requirements for Staphylococcus aureus nitric oxide resistance and virulence. PLoS Pathog. 14:1006907. 10.1371/journal.ppat.1006907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Keyoumu Y., Long L., Zhang H. (2014). Detection of bacterial biofilms in different types of chronic otitis media. Eur. Arch. Otorhinolaryngol. 271, 2877–2883. 10.1007/s00405-013-2766-8 [DOI] [PubMed] [Google Scholar]
- Hachani A., Lossi N. S., Hamilton A., Jones C., Bleves S., Albesa-Jové D., et al. (2011). Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J. Biol. Chem. 286, 12317–12327. 10.1074/jbc.M110.193045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn D. L., Dodge R. W., Golubjatnikov R. (1991). Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA 266, 225–230. 10.1001/jama.1991.03470020051031 [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L., Hu F. Z., Gieseke A., Nistico L., Nguyen D., Hayes J., et al. (2006). Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296, 202–211. 10.1001/jama.296.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer N. D., Skaar E. P. (2011). Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu. Rev. Microbiol. 65, 129–147. 10.1146/annurev-micro-090110-102851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F. (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153, 917–923. 10.1099/mic.0.2006/004077-0 [DOI] [PubMed] [Google Scholar]
- Hebert G. A., Hancock G. A. (1985). Synergistic hemolysis exhibited by species of staphylococci. J. Clin. Microbiol. 22, 409–415. 10.1128/JCM.22.3.409-415.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M. R., Lashua L. P., Fischer D. K., Flitter B. A., Eichinger K. M., Durbin J. E., et al. (2016). Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc. Natl. Acad. Sci. U.S.A. 113:1642. 10.1073/pnas.1516979113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M., Teitzel G. M., Balzer G. J., Heydorn A., Molin S., Givskov M., et al. (2001). Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395. 10.1128/JB.183.18.5395-5401.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. (2017). Deaths: Leading Causes for 2017. National Vital Statistics Reports. Centers for Disease Control and Prevention. Hyattsville, MD. [Google Scholar]
- Hilliam Y., Moore M. P., Lamont I. L., Bilton D., Haworth C. S., Foweraker J., et al. (2017). Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur. Respir. J. 49:1602108. 10.1183/13993003.02108-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. R., Richardson A. R., Houston L. S., Kulasekara H. D., Martens-Habbena W., Klausen M., et al. (2010). Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog. 6:e1000712. 10.1371/journal.ppat.1000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard M., Wagner Mackenzie B., Jain R., Taylor M. W., Biswas K., Douglas R. G. (2017). Chronic rhinosinusitis and the evolving understanding of microbial ecology in chronic inflammatory mucosal disease. Clin. Microbiol. Rev. 30:321. 10.1128/CMR.00060-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N., Bjarnsholt T., Moser C., Jensen P. O., Kolpen M., Qvist T., et al. (2017). Diagnosis of biofilm infections in cystic fibrosis patients. Apmis 125, 339–343. 10.1111/apm.12689 [DOI] [PubMed] [Google Scholar]
- Hood M. I., Skaar E. P. (2012). Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood R. D., Singh P., Hsu F., Güvener T., Carl M. A., Trinidad R. R. S., et al. (2010). A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7, 25–37. 10.1016/j.chom.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P. F., Lu Y. R., Lin T. L., Lai L. Y., Wang J. T. (2019). Klebsiella pneumoniae type VI secretion system contributes to bacterial competition, cell invasion, type-1 fimbriae expression, and in vivo colonization. J. Infect. Dis. 219, 637–647. 10.1093/infdis/jiy534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. J., Kumar N., McBain A. J. (2017). Low incidence of coaggregation amongst bacteria isolated from the upper respiratory tract in health and disease. J. Med. Microbiol. 66, 1338–1341. 10.1099/jmm.0.000567 [DOI] [PubMed] [Google Scholar]
- Ibáñez de Aldecoa A. L., Zafra O., González-Pastor J. E. (2017). Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front. Microbiol. 8:1390. 10.3389/fmicb.2017.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iikura M., Hojo M., Koketsu R., Watanabe S., Sato A., Chino H., et al. (2015). The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS ONE 10:e0123584. 10.1371/journal.pone.0123584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui T., Walker L. C., Dodds M. W. J., Hanley A. B. (2015). Extracellular glycoside hydrolase activities in the human oral cavity. Appl. Environ. Microbiol. 81:5471. 10.1128/AEM.01180-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y., Roberts A. E. L., Kragh K. N., Gordon V. D., Hutchison J., Allen R. J., et al. (2017). The Pseudomonas aeruginosa PSL polysaccharide is a social but noncheatable trait in biofilms. mBio 8, e00374–e00317. 10.1128/mBio.00374-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., et al. (1984). Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104, 206–210. 10.1016/S0022-3476(84)80993-2 [DOI] [PubMed] [Google Scholar]
- Jacques I., Derelle J., Weber M., Vidailhet M. (1998). Pulmonary evolution of cystic fibrosis patients colonized by Pseudomonas aeruginosa and/or Burkholderia cepacia. Eur. J. Pediatr. 157, 427–431. 10.1007/s004310050844 [DOI] [PubMed] [Google Scholar]
- Jervis Bardy J., Psaltis A. J. (2016). Next generation sequencing and the microbiome of chronic rhinosinusitis: a primer for clinicians and review of current research, its limitations, and future directions. Ann. Otol. Rhinol. Laryngol. 125, 613–621. 10.1177/0003489416641429 [DOI] [PubMed] [Google Scholar]
- Jiang F., Waterfield N. R., Yang J., Yang G., Jin Q. (2014). A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15, 600–610. 10.1016/j.chom.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Johansen H. K., Høiby N. (1992). Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 47, 109–111. 10.1136/thx.47.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek J. A., Bookwalter J. E., Baker B. D., Fernandez S., Novotny L. A., Munson R. S., Jr., et al. (2007). The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol. Microbiol. 65, 1288–1299. 10.1111/j.1365-2958.2007.05864.x [DOI] [PubMed] [Google Scholar]
- Jurcisek J. A., Brockman K. L., Novotny L. A., Goodman S. D., Bakaletz L. O. (2017). Nontypeable Haemophilus influenzae releases DNA and DNABII proteins via a T4SS-like complex and ComE of the type IV pilus machinery. Proc. Natl. Acad. Sci. U.S.A. 114, E6632–E6641. 10.1073/pnas.1705508114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Beveridge T. J. (1996). Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767. 10.1128/JB.178.10.2767-2774.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya E., Dag I., Incesulu A., Gurbuz M. K., Acar M., Birdane L. (2013). Investigation of the presence of biofilms in chronic suppurative otitis media, nonsuppurative otitis media, and chronic otitis media with cholesteatoma by scanning electron microscopy. Sci. World J. 2013:638715. 10.1155/2013/638715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E., Safrin M., Olson J. C., Ohman D. E. (1993). Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 268, 7503–7508. [PubMed] [Google Scholar]
- Khan W., Bernier S. P., Kuchma S. L., Hammond J. H., Hasan F., O'Toole G. A. (2010). Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. Int. Microbiol. 13, 207–212. 10.2436/20.1501.01.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski M. R., Gaston J. R., Kocak B. R., Coburn S. L., Lee S., Pilewski J. M., et al. (2018). Staphylococcus aureus biofilm growth on cystic fibrosis airway epithelial cells is enhanced during respiratory syncytial virus coinfection. mSphere 3, e00341–e00318. 10.1128/mSphere.00341-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. (1988). Bacteriocins of lactic acid bacteria. Biochimie 70, 337–349. 10.1016/0300-9084(88)90206-4 [DOI] [PubMed] [Google Scholar]
- Koch G., Nadal-Jimenez P., Reis C. R., Muntendam R., Bokhove M., Melillo E., et al. (2014). Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc. Natl. Acad. Sci. U.S.A. 111:1568. 10.1073/pnas.1311263111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar A., Trivedi U., Rumbaugh K. P., Whiteley M. (2013). Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. U.S.A. 110:1059. 10.1073/pnas.1214550110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpitsch C., Koskinen K., Schöpf V., Moissl-Eichinger C. (2019). The microbiome of the upper respiratory tract in health and disease. BMC Biol. 17:87. 10.1186/s12915-019-0703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine E. R., Wall D., Vanlerberg S. L., Donabedian H., Sledjeski D. D. (2004). Moraxella catarrhalis coaggregates with Streptococcus pyogenes and modulates interactions of S. pyogenes with human epithelial. cells. Infect. Immun 72:6689 10.1128/IAI.72.11.6689-6693.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K., Schleimer R., Kern R. C. (2015). The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr. Allergy Asthma Rep. 15:41. 10.1007/s11882-015-0540-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. D., Clare S., Walker A. W., Goulding D., Stabler R. A., Croucher N., et al. (2009). Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 77, 3661–3669. 10.1128/IAI.00558-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Lee E.-Y., Kim S.-H., Kim D.-K., Park K.-S., Kim K. P., et al. (2013). Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrobial. Agents Chemother. 57, 2589–2595. 10.1128/AAC.00522-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman P. G., Basler M., Ramagopal U. A., Bonanno J. B., Sauder J. M., Pukatzki S., et al. (2009). Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U.S.A. 106:4154. 10.1073/pnas.0813360106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Clarke A. J., Beveridge T. J. (1998). Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180, 5478–5483. 10.1128/JB.180.20.5478-5483.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli D. H., Yang J., Khansaheb M. K., Helfman B., Peng L., Stecenko A. A., et al. (2016). Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur. J. Clin. Microbiol. Infect. Dis. 35, 947–953. 10.1007/s10096-016-2621-0 [DOI] [PubMed] [Google Scholar]
- Lina G., Boutite F., Tristan A., Bes M., Etienne J., Vandenesch F. (2003). Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl. Environ. Microbiol. 69, 18–23. 10.1128/AEM.69.1.18-23.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato-Márquez D., Díaz-Orejas R., García-del Portillo F. (2016). Toxin-antitoxins and bacterial virulence. FEMS Microbiol. Rev. 40, 592–609. 10.1093/femsre/fuw022 [DOI] [PubMed] [Google Scholar]
- Magalhães A. P., Lopes S. P., Pereira M. O. (2017). Insights into cystic fibrosis polymicrobial consortia: the role of species interactions in biofilm development, phenotype, and response to in-use antibiotics. Front. Microbiol. 7:146. 10.3389/fmicb.2016.02146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen M. J., Sethi S. (2016). COPD and the microbiome. Respirology 21, 590–599. 10.1111/resp.12732 [DOI] [PubMed] [Google Scholar]
- Man W. H., de Steenhuijsen Piters W. A. A., Bogaert D. (2017). The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat. Rev. Microbiol. 15:259. 10.1038/nrmicro.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E., Yates A., Levin B. R. (2010). The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol. 10:59. 10.1186/1471-2180-10-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks L. R., Reddinger R. M., Hakansson A. P. (2012). High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio 3, e00200–00212. 10.1128/mBio.00200-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. L., Thornton R. B., Smith-Vaughan H. C., Richmond P., Pizzutto S. J., Chang A. B. (2015). Detection of biofilm in bronchoalveolar lavage from children with non-cystic fibrosis bronchiectasis. Pediatr. Pulmonol. 50, 284–292. 10.1002/ppul.23031 [DOI] [PubMed] [Google Scholar]
- Mashburn L. M., Jett A. M., Akins D. R., Whiteley M. (2005). Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 187:554. 10.1128/JB.187.2.554-566.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell M. J., Jary H. R., Perry A., MacFarlane J. G., Hester K. L., Small T., et al. (2015). Non cystic fibrosis bronchiectasis: a longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir. Med. 109, 716–726. 10.1016/j.rmed.2014.07.021 [DOI] [PubMed] [Google Scholar]
- McShane P. J., Naureckas E. T., Tino G., Strek M. E. (2013). Non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 188, 647–656. 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- Melvin J. A., Gaston J. R., Phillips S. N., Springer M. J., Marshall C. W., Shanks R. M. Q., et al. (2017). Pseudomonas aeruginosa contact-dependent growth inhibition plays dual role in host-pathogen interactions. mSphere 2, e00336–e00317. 10.1128/mSphere.00336-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Briand Y., Baysse C. (2002). The pyocins of Pseudomonas aeruginosa. Biochimie 84, 499–510. 10.1016/S0300-9084(02)01422-0 [DOI] [PubMed] [Google Scholar]
- Miethke M., Marahiel M. A. (2007). Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71, 413–451. 10.1128/MMBR.00012-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. L., Kjos M., Abrudan M. I., Roberts I. S., Veening J. W., Rozen D. E. (2018). Eavesdropping and crosstalk between secreted quorum sensing peptide signals that regulate bacteriocin production in Streptococcus pneumoniae. ISME. J. 12, 2363–2375. 10.1038/s41396-018-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. B., Bassler B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- Mirković B., Murray M. A., Lavelle G. M., Molloy K., Azim A. A., Gunaratnam C., et al. (2015). The role of short-chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway. Am. J. Respir. Crit. Care Med. 192, 1314–1324. 10.1164/rccm.201505-0943OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G., Séguin D. L., Asselin A.-E., Déziel E., Cantin A. M., Frost E. H., et al. (2010). Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N- oxide. BMC Microbiol. 10:33. 10.1186/1471-2180-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Lisi C. V., Gerring R., Mittal J., Mathee K., Narasimhan G., et al. (2015). Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J. Med. Microbiol. 64, 1103–1116. 10.1099/jmm.0.000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasta L., Ronfani L., Marchetti F., Montico M., Vecchi Brumatti L., Bavcar A., et al. (2012). Burden of disease caused by otitis media: systematic review and global estimates. PLoS ONE 7:e36226. 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso M., García E., López R. (2006). Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 188, 7785–7795. 10.1128/JB.00673-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H., Charron-Mazenod L., Lewenza S. (2010). Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ. Microbiol. 12, 1621–1629. 10.1111/j.1462-2920.2010.02208.x [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Brauer A. L., Eschberger K., Lobbins P., Grove L., Cai X., et al. (2008). Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177, 853–860. 10.1164/rccm.200709-1413OC [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Kirkham C., Sethi S., Lesse A. J. (2005). Expression of a peroxiredoxin-glutaredoxin by Haemophilus influenzae in biofilms and during human respiratory tract infection. FEMS Immunol. Med. Microbiol. 44, 81–89. 10.1016/j.femsim.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Ni J., Friedman H., Boyd B. C., McGurn A., Babinski P., Markossian T., et al. (2019). Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatrics 19:225. 10.1186/s12887-019-1594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Dorrington S. M., Slack M. P., Walmsley H. L. (1988). Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 32, 518–523. 10.1128/AAC.32.4.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman R. S., Moeller P., McDonald T. J., Morris P. J. (2004). Effect of pyocyanin on a crude-oil-degrading microbial community. Appl. Environ. Microbiol. 70, 4004–4011. 10.1128/AEM.70.7.4004-4011.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto M. J., Burns W. J., Beavers W. N., Skaar E. P. (2017). Mechanisms of pyocyanin toxicity and genetic determinants of resistance in Staphylococcus aureus. J. Bacteriol. 199, e00221–e00217. 10.1128/JB.00221-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes C., Pereira A. M., Morais-Almeida M. (2017). Asthma costs and social impact. Asthma Res. Pract. 3:1. 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazi G., O'Toole G. A. (2017). Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio 8, e00873–e00817. 10.1128/mBio.00873-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. D., Skaar E. P. (2016). Transition metals and virulence in bacteria. Annu. Rev. Genet. 50, 67–91. 10.1146/annurev-genet-120215-035146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang B., Hong W., West-Barnette S. L., Kock N. D., Swords W. E. (2008). Diminished ICAM-1 expression and impaired pulmonary clearance of nontypeable Haemophilus influenzae in a mouse model of chronic obstructive pulmonary disease/emphysema. Infect. Immun. 76, 4959–4967. 10.1128/IAI.00664-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivan S., Bassiouni A., Shiffer A., Dillon M. R., Cope E. K., Cooksley C., et al. (2020). The international sinonasal microbiome study: A multicentre, multinational characterization of sinonasal bacterial ecology. Allergy. 10.1111/all.14276 [DOI] [PubMed] [Google Scholar]
- Park B., Nizet V., Liu G. Y. (2008). Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J. Bacteriol. 190, 2275–2278. 10.1128/JB.00006-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S., Nair H. A. S., Lee K. W. K., Ong J., Goh J. Q. J., Kjelleberg S., et al. (2015). Pseudomonas aeruginosa PAO1 exopolysaccharides are important for mixed species biofilm community development and stress tolerance. Front. Microbiol. 6:851. 10.3389/fmicb.2015.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericone C. D., Overweg K., Hermans P. W., Weiser J. N. (2000). Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68, 3990–3997. 10.1128/IAI.68.7.3990-3997.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. T., Hoiby N., Mordhorst C. H., Lind K., Flensborg E. W., Bruun B. (1981). Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma–possible synergism with Pseudomonas aeruginosa. Acta Paediatr. Scand. 70, 623–628. 10.1111/j.1651-2227.1981.tb05757.x [DOI] [PubMed] [Google Scholar]
- Pompilio A., Crocetta V., De Nicola S., Verginelli F., Fiscarelli E., Di Bonaventura G. (2015). Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 6:951. 10.3389/fmicb.2015.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Naimie A. A., Griffin E. F., Bay C., O'Toole G. A. (2015). Tobramycin-treated Pseudomonas aeruginosa PA14 enhances Streptococcus constellatus 7155 biofilm formation in a cystic fibrosis model system. J. Bacteriol. 198, 237–247. 10.1128/JB.00705-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaderi S. A., Hurst J. R. (2018). The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 3:e4. 10.1017/gheg.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureishi A., Lee Y., Belfield K., Birchall J. P., Daniel M. (2014). Update on otitis media - prevention and treatment. Infect. Drug. Resist. 7, 15–24. 10.2147/IDR.S39637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V. R., Hauser L. J., Feazel L. M., Ir D., Robertson C. E., Frank D. N. (2015). Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J. Allergy Clin. Immunol. 136, 334–342.e331. 10.1016/j.jaci.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V. R., Hauser L. J., Frank D. N. (2016). The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 24, 20–25. 10.1097/MOO.0000000000000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbo M. R. (2014). The microbiota, chemical symbiosis, and human disease. J. Mol. Biol. 426, 3877–3891. 10.1016/j.jmb.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K. C., Mann E. E., Endres J. L., Weiss E. C., Cassat J. E., Smeltzer M. S., et al. (2007). The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 104, 8113–8118. 10.1073/pnas.0610226104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard A. H., Gilbert P., High N. J., Kolenbrander P. E., Handley P. S. (2003). Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11, 94–100. 10.1016/S0966-842X(02)00034-3 [DOI] [PubMed] [Google Scholar]
- Rogers G. B., van der Gast C. J., Cuthbertson L., Thomson S. K., Bruce K. D., Martin M. L., et al. (2013). Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 68:731. 10.1136/thoraxjnl-2012-203105 [DOI] [PubMed] [Google Scholar]
- Russell A. B., Hood R. D., Bui N. K., LeRoux M., Vollmer W., Mougous J. D. (2011). Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343. 10.1038/nature10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., LeRoux M., Hathazi K., Agnello D. M., Ishikawa T., Wiggins P. A., et al. (2013). Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496, 508–512. 10.1038/nature12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Cacalano G., Prince A. (1990). Pseudomonas cepacia adherence to respiratory epithelial cells is enhanced by Pseudomonas aeruginosa. Infect. Immun. 58, 2578–2584. 10.1128/IAI.58.8.2578-2584.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana T. G., Hachani A., Bucior I., Soscia C., Garvis S., Termine E., et al. (2012). The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 287, 27095–27105. 10.1074/jbc.M112.376368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanclement J. A., Webster P., Thomas J., Ramadan H. H. (2005). Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 115, 578–582. 10.1097/01.mlg.0000161346.30752.18 [DOI] [PubMed] [Google Scholar]
- Schaar V., Nordström T., Mörgelin M., Riesbeck K. (2011). Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrobial. Agents Chemother. 55:3845. 10.1128/AAC.01772-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., West T. E., Boyer F., Chiang W.-C., Carl M. A., Hood R. D., et al. (2010). Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068. 10.1371/journal.ppat.1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Li K., Filkins L. M., Zhu B., Kuchma S. L., Schwartzman J. D., et al. (2019). Pseudomonas aeruginosa can inhibit growth of streptococcal species via siderophore production. J. Bacteriol. 201, e00014–00019. 10.1128/JB.00014-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth E. C., Taga M. E. (2014). Nutrient cross-feeding in the microbial world. Front. Microbiol. 5:350. 10.3389/fmicb.2014.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. D., Duan K., Fischer C., Parkins M. D., Storey D. G., Rabin H. R., et al. (2008). Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 4:e1000184. 10.1371/journal.ppat.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley N. E., An D., Parsek M. R., Chandler J. R., Dandekar A. A. (2015). Quorum sensing protects Pseudomonas aeruginosa against cheating by other species in a laboratory coculture model. J. Bacteriol. 197:3154. 10.1128/JB.00482-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler N., Ewig S., Torres A., Filella X., Gonzalez J., Zaubet A. (1999). Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur. Respir. J. 14, 1015–1022. 10.1183/09031936.99.14510159 [DOI] [PubMed] [Google Scholar]
- Spiewak H. L., Shastri S., Zhang L., Schwager S., Eberl L., Vergunst A. C., et al. (2019). Burkholderia cenocepacia utilizes a type VI secretion system for bacterial competition. Microbiol. Open 8:e00774 10.1002/mbo3.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmoen H., Knutsen E., Håvarstein L. S. (2002). Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc. Natl. Acad. Sci. U.S.A. 99, 7681–7686. 10.1073/pnas.112464599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbit A. E., Flume P. A. (2011). Pulmonary exacerbations in cystic fibrosis. Curr. Opin. Pulm. Med. 17, 442–447. 10.1097/MCP.0b013e32834b8c04 [DOI] [PubMed] [Google Scholar]
- Stewart P. S., Camper A. K., Handran S. D., Huang C., Warnecke M. (1997). Spatial distribution and coexistence of Klebsiella pneumoniae and Pseudomonas aeruginosa in biofilms. Microb. Ecol. 33, 2–10. 10.1007/s002489900002 [DOI] [PubMed] [Google Scholar]
- Stressmann F. A., Rogers G. B., van der Gast C. J., Marsh P., Vermeer L. S., Carroll M. P., et al. (2012). Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax 67, 867–873. 10.1136/thoraxjnl-2011-200932 [DOI] [PubMed] [Google Scholar]
- Suarez G., Sierra J. C., Erova T. E., Sha J., Horneman A. J., Chopra A. K. (2010). A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP Ribosylation of actin. J. Bacteriol. 192:155. 10.1128/JB.01260-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. Y., Bullen N. P., Ahmad S., Whitney J. C. (2018). Diverse NADase effector families mediate interbacterial antagonism via the type VI secretion system. J. Biol. Chem. 293, 1504–1514. 10.1074/jbc.RA117.000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teele D. W., Klein J. O., Rosner B., Group G. B. O. M. S. (1989). Epidemiology of otitis media during the first seven years of life in children in greater boston: a prospective, Cohort Study. J. Infect. Dis. 160, 83–94. 10.1093/infdis/160.1.83 [DOI] [PubMed] [Google Scholar]
- Teo S. M., Mok D., Pham K., Kusel M., Serralha M., Troy N., et al. (2015). The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17, 704–715. 10.1016/j.chom.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin K. L., Coll O. P., Ceri H. (2001). Interspecies biofilms of Pseudomonas aeruginosa and Burkholderia cepacia. Can. J. Microbiol. 47, 949–954. 10.1139/w01-095 [DOI] [PubMed] [Google Scholar]
- Tunney M. M., Einarsson G. G., Wei L., Drain M., Klem E. R., Cardwell C., et al. (2013). Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am. J. Respir. Crit. Care Med. 187, 1118–1126. 10.1164/rccm.201210-1937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunney M. M., Field T. R., Moriarty T. F., Patrick S., Doering G., Muhlebach M. S., et al. (2008). Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 177, 995–1001. 10.1164/rccm.200708-1151OC [DOI] [PubMed] [Google Scholar]
- Utari P. D., Setroikromo R., Melgert B. N., Quax W. J. (2018). PvdQ quorum quenching acylase attenuates Pseudomonas aeruginosa virulence in a mouse model of pulmonary infection. Front. Cell Infect. Microbiol. 8:119. 10.3389/fcimb.2018.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecandelaere I., Depuydt P., Nelis H. J., Coenye T. (2014). Protease production by Staphylococcus epidermidis and its effect on Staphylococcus aureus biofilms. Pathog. Dis. 70, 321–331. 10.1111/2049-632X.12133 [DOI] [PubMed] [Google Scholar]
- Virtanen P., Waneskog M., Koskiniemi S. (2019). Class II contact-dependent growth inhibition (CDI) systems allow for broad-range cross-species toxin delivery within the Enterobacteriaceae family. Mol. Microbiol. 111, 1109–1125. 10.1111/mmi.14214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner Mackenzie B., Waite D. W., Hoggard M., Douglas R. G., Taylor M. W., Biswas K. (2017). Bacterial community collapse: a meta-analysis of the sinonasal microbiota in chronic rhinosinusitis. Environ. Microbiol. 19, 381–392. 10.1111/1462-2920.13632 [DOI] [PubMed] [Google Scholar]
- Waite R. D., Curtis M. A. (2009). Pseudomonas aeruginosa PAO1 pyocin production affects population dynamics within mixed-culture biofilms. J. Bacteriol. 191, 1349–1354. 10.1128/JB.01458-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman C. A., Moore J. L., Noto M. J., Zhang Y., Singleton M. D., Prentice B. M., et al. (2016). The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 7:11951. 10.1038/ncomms11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Bafadhel M., Haldar K., Spivak A., Mayhew D., Miller B. E., et al. (2016). Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 47:1082. 10.1183/13993003.01406-2015 [DOI] [PubMed] [Google Scholar]
- Wark P. A. B., Tooze M., Cheese L., Whitehead B., Gibson P. G., Wark K. F., et al. (2012). Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur. Respir. J. 40:510. 10.1183/09031936.00202311 [DOI] [PubMed] [Google Scholar]
- Weaver V. B., Kolter R. (2004). Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J. Bacteriol. 186, 2376–2384. 10.1128/JB.186.8.2376-2384.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzicha J. A. (2004). Role of viruses in exacerbations of chronic obstructive pulmonary disease. Pro. Am. Thoracic Soc. 1, 115–120. 10.1513/pats.2306030 [DOI] [PubMed] [Google Scholar]
- Weimer K. E., Juneau R. A., Murrah K. A., Pang B., Armbruster C. E., Richardson S. H., et al. (2011). Divergent mechanisms for passive pneumococcal resistance to beta-lactam antibiotics in the presence of Haemophilus influenzae. J. Infect. Dis. 203, 549–555. 10.1093/infdis/jiq087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch C. B., Tolker-Nielsen T., Ragas P. C., Mattick J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science 295:1487. 10.1126/science.295.5559.1487 [DOI] [PubMed] [Google Scholar]
- Wink D. A., Mitchell J. B. (1998). Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 25, 434–456. 10.1016/S0891-5849(98)00092-6 [DOI] [PubMed] [Google Scholar]
- Woo T. E., Lim R., Heirali A. A., Acosta N., Rabin H. R., Mody C. H., et al. (2019). A longitudinal characterization of the non-cystic fibrosis Bronchiectasis airway microbiome. Sci. Rep. 9:6871. 10.1038/s41598-019-42862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. P., Rosendale D. I., Robertson A. M. (2000). Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 190, 73–79. 10.1111/j.1574-6968.2000.tb09265.x [DOI] [PubMed] [Google Scholar]
- Zemanick E. T., Wagner B. D., Robertson C. E., Ahrens R. C., Chmiel J. F., Clancy J. P., et al. (2017). Airway microbiota across age and disease spectrum in cystic fibrosis. Eur. Respir. J. 50:1700832. 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Liu L., Chen X., Huang T., Du L., Lin J., et al. (2019). Behavioral heterogeneity in quorum sensing can stabilize social cooperation in microbial populations. BMC Biol. 17:20. 10.1186/s12915-019-0639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygiel E. M., Nelson C. E., Brewer L. K., Oglesby-Sherrouse A. G., Nolan E. M. (2019). The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J. Biol. Chem. 294, 3549–3562. 10.1074/jbc.RA118.006819 [DOI] [PMC free article] [PubMed] [Google Scholar]