The survival, replication, and virulence of mycoplasmas depend on their ability to capture and import host-derived nutrients using poorly characterized membrane proteins. Previous studies on the important bovine pathogen Mycoplasma bovis demonstrated that the amino-terminal end of an immunogenic 226-kDa (P226) protein, encoded by milA (the full-length product of which has a predicted molecular weight of 303 kDa), had lipase activity. The predicted sequence of MilA contains glycosaminoglycan binding motifs, as well as multiple copies of a domain of unknown function (DUF445) that is also found in apolipoproteins.
KEYWORDS: Mycoplasma bovis, membrane protein, immunogenicity, lipid binding, GAG, ATPase activity
ABSTRACT
The survival, replication, and virulence of mycoplasmas depend on their ability to capture and import host-derived nutrients using poorly characterized membrane proteins. Previous studies on the important bovine pathogen Mycoplasma bovis demonstrated that the amino-terminal end of an immunogenic 226-kDa (P226) protein, encoded by milA (the full-length product of which has a predicted molecular weight of 303 kDa), had lipase activity. The predicted sequence of MilA contains glycosaminoglycan binding motifs, as well as multiple copies of a domain of unknown function (DUF445) that is also found in apolipoproteins. We mutagenized the gene to facilitate expression of a series of regions spanning the gene in Escherichia coli. Using monospecific antibodies against these recombinant proteins, we showed that MilA was proteolytically processed into 226-kDa and 50-kDa fragments that were both partitioned into the detergent phase by Triton X-114 phase fractionation. Trypsin treatment of intact cells showed that P226 was surface exposed. In vitro, the recombinant regions of MilA bound to 1-anilinonaphthalene-8-sulfonic acid and to a variety of lipids. The MilA fragments were also shown to bind heparin. Antibody against the carboxyl-terminal fragment inhibited the growth of M. bovis in vitro. This carboxyl end also bound and hydrolyzed ATP, suggestive of a potential role as an autotransporter. Our studies have demonstrated that DUF445 has lipid binding activity and that MilA is a multifunctional protein that may play multiple roles in the pathogenesis of infection with M. bovis.
INTRODUCTION
Mycoplasma bovis contributes to the bovine respiratory disease complex and poses a significant threat to livestock production worldwide (1, 2), resulting in its inclusion in the EU-funded DISCONTOOLS project in their disease database (https://www.discontools.eu/database.html) (3). Clinical disease caused by M. bovis includes mastitis, arthritis, genital disorders, keratoconjunctivitis, and otitis media (4, 5). The survival, replication, and virulence of mycoplasmas depend to a large extent on their ability to colonize and, as a consequence of their limited biosynthetic capabilities, to capture and import host-derived nutrients. The acquisition of nutrients by this important group of pathogens depends on a number of poorly characterized membrane-associated proteins. In spite of the considerable increase in genome sequence data over the past decade, many mycoplasma proteins remain hypothetical. Many predicted mycoplasma gene products, other than those involved in housekeeping functions, have little or no detectable sequence similarity to those characterized in other bacteria (6), limiting our capacity to extrapolate gene function from studies in other bacteria. Improved understanding of the molecular pathogenesis of Mycoplasma bovis, and of mycoplasmas in general, will thus depend on characterization of the function of these proteins that appear to be specific to the mycoplasmas (7–11). Experimental validation of the functions of surface proteins of M. bovis has been identified as one of the critical gaps in understanding about this pathogen (12). These functional studies are likely to require consideration of the possibility that many of them will play a number of roles during infection, as the evolutionary forces that have driven the reduction in the genome of the mycoplasmas are likely to have limited functional redundancy and selected for acquisition of multifunctionality in proteins. Some progress has been made in predicting gene function in mycoplasmas by examining metabolomic differences between wild-type organisms and mutants in which specific genes have been disrupted by insertion of a transposon (13–15).
Functional prediction methods can complement primary sequence annotations. While computational three-dimensional protein structural modeling has limitations, it can suggest biochemical functions and/or biological roles of a protein about which relatively little is known (16). As many of the proteins of M. bovis have no known function and many have homology only with other mycoplasma proteins of unknown function, ab initio structural modeling and comparisons using the Protein Data Bank (PDB) structural database may provide hints about their functions, providing a starting point for in vitro functional studies on purified proteins.
The gene milA (MBOVPG45_0710) of M. bovis encodes a very large membrane protein that was shown in previous studies to be detected by antibodies from experimentally infected calves (17). The amino terminal end of the protein was shown to have lipase activity, but the functions of the carboxyl 75% of MilA have not been investigated. While it is likely that the role of the remainder of the protein may be linked to the lipase activity that was detected previously (17), given the prevalence of proteins with moonlighting functions in mycoplasmas it is appropriate to consider the possibility of other activities as well. Previous studies aimed at determining the dispensability of genes in M. bovis using transposon mutagenesis did not detect any mutants in which milA was disrupted (18, 19), suggesting that this gene may be essential in M. bovis (18).
The aim of this study was to further investigate the function of MilA, initially by using bioinformatic approaches to identify potential functional domains and predict likely activities. These predicted activities were explored by expressing different regions of the full-length protein in E. coli and assessing their biochemical function in vitro. In addition, monospecific antisera were raised against these regions to examine the effect of antibody responses against different parts of MilA on the metabolic activity of M. bovis.
RESULTS
Genomic location, features, and bioinformatic analyses of milA.
In order to identify potential functional domains within MilA, we analyzed the sequence of the milA and its homologues. The coding sequence of the milA gene is located between nucleotides 814575 and 822587 in the genome of M. bovis strain PG45 (GenBank accession number CP002188). The region upstream of milA contains a gene that has not been assigned a function (MBOVPG45_0709, a hypothetical protein), while the region downstream has a putative rpoC, coding for the DNA-directed RNA polymerase subunit beta (Fig. 1A), which is found in a similar position in most mycoplasma species in the Mycoplasma hominis cluster. The physicochemical and other features of MilA were predicted using ProtParam (ExPASy). M. bovis MilA had a predicted molecular weight of 303 kDa and an isoelectric point of 8.71, while its closest relative, the product of MAG_6100 of Mycoplasma agalactiae, had a predicted molecular weight of 302 kDa and an isoelectric point of 8.41. MilA homologues in other mycoplasma species are shown in Table 1. Most have molecular weights greater than 200 kDa, with the exception of those in Mycoplasma mobile (168 kDa) and Mycoplasma synoviae (174 kDa), and isoelectric points greater than 8.0, with the exception of those in Mycoplasma penetrans (4.66), Mycoplasma columbinum (5.07), M. mobile (5.12), M. synoviae (5.93) and Mycoplasma canis (5.97).
FIG 1.
Physical map of M. bovis PG45 milA. (A) The milA gene lies downstream of rpoB and rpoC and is followed by a pseudogene and an ISMbov6 transposase gene encoded on the opposite strand. The numbers below the genes indicate the number of amino acids encoded by the open reading frame (ORF). (B) Motifs and domains identified in milA, which include the GDSL lipase motif, a recurring domain of unknown function (DUF445) and an unassigned conserved protein domain, COG5283. (C) The recombinant regions expressed during this study. The numbers below the black rectangles indicate the numbers of amino acids in the recombinant MilA protein fragments.
TABLE 1.
MilA of M. bovis and its orthologues in other mycoplasmas
| Mycoplasma species | GenBank protein accession no. | ORFc | Mol wt (kDa)b | Isoelectric point (pI)a | No. of amino acids (% identity)b |
|---|---|---|---|---|---|
| M. bovis PG45 | ADR24994.1 | MBOVPG45_0710 | 303 | 8.71 | 2,670 (100) |
| M. bovis Hubei-1 | AEI90366.1 | MMB_0654 | 302 | 8.41 | 2,665 (84) |
| M. bovis H0801 | AIA34223.1 | Mbov_0693 | 302 | 8.41 | 2,665 (84) |
| M. bovis CQ-W70 | AIA34223.1 | K668_03265 | 302 | 8.45 | 2,665 (84) |
| M. agalactiae PG2 | WP_011949767.1 | MAG_6100 | 302 | 8.31 | 2,667 (75) |
| M. agalactiae 5632 | CBH40886.1 | MAGa6830 | 302 | 8.41 | 2,669 (76) |
| M. primatum ATCC 25948 | WP_029513523.1 | T386_RS0102100 | 314 | 8.98 | 2,743 (34) |
| M. columbinum | WP_029891842.1 | MSCF7_01871 | 348 | 5.07 | 3,050 (27) |
| M. meleagridis ATCC 25294 | WP_046096832.1 | MMELEA_03960 | 356 | 9.07 | 3,094 (26) |
| M. bovigenitalium 51080 | WP_004419557.1 | MBVG_2180 | 216 | 9.06 | 1,897 (25) |
| M. fermentans PG18 | BAH69610.1 | MBIO_0345 | 205 | 8.89 | 1,788 (25) |
| M. fermentans JER | ADN68869.1 | MFE_02570 | 205 | 8.89 | 1,788 (25) |
| M. californicum ST-6 | AIA29521.1 | MCFN_01900 | 204 | 9.00 | 1,787 (25) |
| M. pulmonis | WP_010925117.1 | MYPU_3130 | 324 | 8.86 | 2,819 (25) |
| M. hominis ATCC 23114 | CAX37166.1 | MHO_0320 | 307 | 8.61 | 2,671 (25) |
| M. crocodyli | ADE19662.1 | MCRO_0279 | 386 | 8.04 | 3,422 (24) |
| M. canis | AKF41005.1 | AAW50_00940 | 380 | 5.97 | 3,346 (24) |
| M. hyopneumoniae 232 | AAV27616.1 | mhp539 | 229 | 8.85 | 1,973 (23) |
| M. hyopneumoniae J | AAZ44609.2 | MHJ_0523 | 233 | 9.03 | 2,004 (23) |
| M. hyopneumoniae 7448 | AAZ53888.2 | MHP7448_0522 | 229 | 8.85 | 2,004 (23) |
| M. conjunctivae | CAT05082.1 | MCJ_003940 | 220 | 8.10 | 1,941 (23) |
| M. penetrans HF-2 | WP_011076982.1 | MYPE1550 | 386 | 4.66 | 3,317 (23) |
| M. hyorhinis DBS 1050 | AHA41059.1 | Q453_0318 | 212 | 9.04 | 1,838 (22) |
| M. hyorhinis GDL-1 | AEX14068.1 | MYM_0289 | 212 | 9.04 | 1,838 (22) |
| M. synoviae | AAZ43740.1 | MS53_0328 | 174 | 5.93 | 1,533 (21) |
| M. mobile | AAT27911.1 | MMOB4250 | 168 | 5.12 | 1,460 (18) |
| Ureaplasma urealyticum | ACI60264.1 | UUR10_0289 | 381 | 8.28 | 3,332 (24) |
Geneious version 7.1.5 was used for amino acid alignment and calculation of the percentage identity.
ProtParam (ExPaSy) was used to compute these parameters.
ORF, open reading frame.
Using TMpred, a total of three transmembrane helices were predicted, located between residues 8 and 28, 556 and 574, and 2529 and 2553. PSORTb was used to predict the cellular localization of the protein and MilA was predicted to be a membrane protein, with a score of 9.52 (out of 10). The sequence was analyzed with SignalP (Gram-positive setting), which identified a putative signal peptide cleavage site between residues 23 and 24.
The multifunctionality of MilA was analyzed using motif searches and the Kyoto Encyclopedia of Genes and Genomes (KEGG) and NCBI databases. The domains identified included: a recurring domain of unknown function, DUF445, along the whole length of the gene; one DUF2397; a lipase_GDSL_2 superfamily domain at the N-terminal end; and a phosphoenolpyruvate (PEP)/pyruvate binding domain between residues 200 and 264. DUF445 is found in apolipoproteins, which are involved in lipid binding and transport. GDSL lipases and esterases are hydrolytic enzymes with multifunctional properties (20). The ability of this region of MilA, and that of a related domain in Mycoplasma hyopneumoniae, to hydrolyze lipids has been shown previously (17, 21). Enzymes with a PEP/pyruvate binding domain are associated with ATP-binding and or hydrolytic activities (22).
Cloning and expression of the milA gene.
To study biochemical and other functional activities of MilA, we cloned and expressed a series of regions of milA (MBOVPG45_0710) in E. coli. This was achieved by changing the TGA tryptophan codons in the gene to TGG in order to achieve full-length expression of each of the regions of MilA. Eighteen tryptophan codons were identified in the sequence of the M. bovis strain PG45 milA gene. The MilA-AB fragment could not be expressed successfully, so the shorter length MilA-ab fragment was constructed to optimize expression and solubility (Fig. 1C). Optimal expression of MilA-ab was achieved by incubation at 30°C with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), that of MilA-CD at 37°C with 1 mM IPTG, and that of MilA-EF at 37°C with 0.5 mM IPTG. The recombinant glutathione S-transferase (GST)-tagged fusion proteins were purified by glutathione affinity chromatography and had molecular weights of approximately 93 kDa (GST-MilA-ab), 139 kDa (GST-MilA-CD), and 105 kDa (GST-MilA-EF), as determined by SDS-PAGE (see Fig. S1A and B in the supplemental material). Western blotting was used to confirm the expression of the fusion protein by probing with goat anti-GST antibody (results not shown).
M. bovis MilA is a proteolytically processed surface localized protein.
Previous studies identified an immunoreactive fragment of MilA of about 226 kDa (P226) using Western blotting and peptide fingerprinting (17), but not a protein of 303 kDa, the predicted size of the full-length product of milA. To assess whether MilA was posttranslationally cleaved, freshly cultured M. bovis cells were treated with different concentrations of trypsin, and then Western blots of the whole-cell proteins were probed with pooled antisera raised against MilA fragments spanning the whole of the milA coding sequence. An immunoreactive protein of 226 kDa (P226) was degraded by trypsin, while another protein of ∼50 kDa (P50) was not affected by trypsin treatment. An immunoreactive 226-kDa protein was also detectable in the culture supernatant but not in uninoculated culture medium, indicating that MilA was being secreted into the medium or posttranslationally cleaved from the cell surface (Fig. 2B). P226 was recognized by both anti-MilA-ab and anti-MilA-EF sera, while P50 was only recognized by anti-MilA-EF serum (Fig. 2C and D). To further confirm that MilA was an integral membrane protein, M. bovis whole-cell proteins were fractionated into hydrophilic and hydrophobic phases using Triton X-114, and the fractions were subjected to SDS-PAGE and Western blotting. Both P226 and P50 were detected in the detergent phase but not in the aqueous phase (see Fig. S2 in the supplemental material).
FIG 2.
Proteolytic processing of MilA. Proteins in trypsin-treated whole M. bovis PG45 cells, uninoculated M. bovis broth, and the supernatant from a M. bovis broth culture were resolved by SDS-4 to 20% gradient PAGE and either (A) stained with Coomassie blue or (B) transferred onto a polyvinylidene difluoride (PVDF) membrane and probed with a pool of antisera raised in rats against recombinant MilA fragments spanning the whole length of the gene. In addition, whole M. bovis cells were either not treated (NT), or were treated with increasing concentrations of trypsin (0.5, 1, 4, 8, 16, or 32 μg/ml), then their proteins were resolved by SDS-PAGE, transferred onto a PVDF membrane, and probed with rat antisera against MilA-ab (C) or MilA-EF (D). Antibody binding was detected using the Clarity Western ECL blotting substrate (Bio-Rad). (B) The 226- and 50-kDa cleavage fragments of MilA can be seen, with the 226-kDa fragment present in both the mycoplasma cells and the supernatant and susceptible to trypsin cleavage, while the 50-kDa fragment was only detected in the mycoplasma cells and was not cleaved by trypsin. The 226-kDa fragment was detected by antisera against recombinant fragments from the amino and carboxyl ends of the full-length protein (C and D), while the 50-kDa fragment was only detected by the antiserum against the carboxyl end (D). M, PageRuler prestained protein ladder (Thermo Scientific).
Immunogenicity of MilA fragments.
The immunogenicity of the amino terminal end of MilA was demonstrated in previous Western immunoblotting and enzyme-linked immunosorbent assay (ELISA) studies (17), but the immunogenicity of the remainder of the 226-kDa product and that of the carboxyl-terminal 50-kDa product was not assessed. The immunogenicity of the remaining fragments was tested by Western blotting using hyperimmune serum from rabbits inoculated with M. bovis strain PG45 and with sera from infected calves. The N and C termini were immunogenic, while the middle portion (MilA-CD) did not show significant immunoreactivity (Fig. S1C) in Western blots. Western blot analysis of 7 additional strains of M. bovis using pooled monospecific antisera against the three recombinant fragments of MilA detected 226-kDa and 50-kDa proteins in all the strains (see Fig. S3A in the supplemental material). In order to determine whether proteins crossreactive with MilA occurred in other mycoplasma species, we performed Western blot analysis on whole-cell proteins from M. bovis, M. agalactiae, Mycoplasma ovipneumoniae, Mycoplasma mycoides subsp. capri, Mycoplasma arginini, and Mycoplasma gallisepticum using the pooled monospecific antisera against the three recombinant fragments of MilA. Weak binding was detected to proteins of around 226 kDa in M. agalactiae and M. mycoides subsp. capri. A polypeptide of about 130 kDa was detected in M. gallisepticum. Binding to proteins of around 50 kDa in all species was also detected, although the sizes differed slightly in each species. A strongly reactive protein of approximately 100 kDa was detected in M. mycoides subsp. capri. In M. agalactiae, the species most closely related to M. bovis, another weakly immunoreactive fragment of about 60 kDa was detected (Fig. S3B). Apparent orthologues of milA were found in the genomes of M. gallisepticum R(low) (MGA_1203; cytadherence-associated protein Hlp2), M. arginini (MARG145_0372), M. mycoides subsp. capri (MLC_6100), and M. agalactiae (MAGa6830).
The M. bovis MilA is a novel lipid-binding protein.
As analysis of the predicted sequence of MilA detected multiple DUF445 regions along the length of the protein, a domain also found in eukaryotic apolipoproteins, and the N-terminal region of MilA has lipase activity, so we hypothesized that other regions of MilA may be binding lipids and thus interacting with the lipase domain in the N-terminal region. We used protein-lipid overlay assays to assess the ability of the different recombinant fragments of MilA to bind lipids. All three regions of MilA were able to bind lipids. The N-terminal region (MilA-ab, containing the complete GDSL domain) appeared to have greater capacity to bind palmitic acid and cholesterol than the other MilA regions (Fig. 3). The peak excitation wavelength for 1-anilinonaphthalene-8-sulfonic acid (1,8-ANS) bound to MilA-CD was 385 nm, and the greatest emission was detected at 485 nm. The N-terminal (MilA-ab) and the C-terminal (MilA-EF) regions were able to bind significantly more 1,8-ANS than the middle region (MilA-CD) (P < 0.002) of MilA, with no significant difference in binding between MilA-ab and MilA-EF (Fig. 4). To further assess lipid binding to MilA, the capacity of oleic acid to compete with 1,8-ANS in binding to MilA-EF was examined. Increasing amounts of oleic acid competed with binding of 1,8-ANS to the protein (see Fig. S4 in the supplemental material). Fixed concentrations of the proteins were incubated with increasing concentrations of 1,8-ANS to determine the Michaelis-Menten kinetics of the binding reactions. MilA-CD had the highest Km (253.6 μM), with the Km values for binding to MilA-ab (54.61) and MilA-EF (53.11 μM) being similar (Table 2). We assessed whether the lipids oleic acid, palmitic acid, and butyric acid could displace bound 1,8-ANS from the different regions of MilA, but none of these lipids (even at concentrations as high as 100 μM) was able to displace 1,8-ANS bound to an equimolar concentration of protein (0.5 μM).
FIG 3.
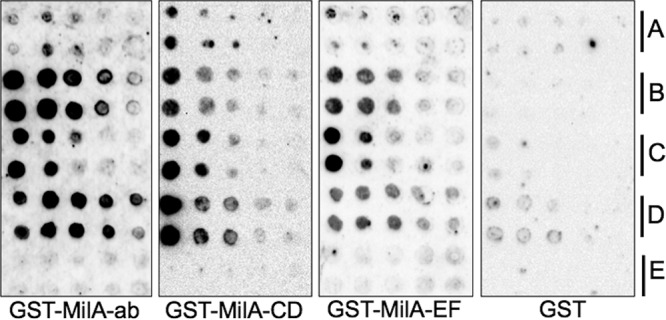
Lipid binding properties of M. bovis MilA. Duplicate rows of different lipids at doubling dilutions were dotted onto nitrocellulose membranes. The lipid blots were incubated with purified recombinant MilA-ab, MilA-CD, MilA-EF, or GST (as a negative control). Protein bound to the lipids was detected with goat anti-GST antibody, rabbit anti-goat horseradish peroxidase (HRP) conjugate, and the Clarity Western ECL blotting substrate (Bio-Rad). The lipids on the blots were (A) tributyrin, (B) palmitic acid, (C) oleic acid, (D) cholesterol, and (E) deoxycholic acid.
FIG 4.

The binding of M. bovis MilA fragments to the fluorescent probe 1,8-ANS. Recombinant protein fragments spanning MilA (1 μM each) were incubated with 5 μM 1,8-ANS for 3 min at room temperature, and fluorescence was detected by excitation at 385 nm with emission measured at 485 nm in a Hybrid multimode microplate reader (BioTek). The amount of fluorescence (relative fluorescence units [RFU]) corresponds to the amount of 1,8-ANS bound to protein. Data are presented as the mean ± standard deviation (SD); ****, significant differences at P < 0.0001; ***, P = 0.0002; **, P = 0.0026.
TABLE 2.
Kinetic analysis of the binding of M. bovis recombinant MilA to the fluorescent substrate 1,8-ANS
| Protein construct | Vmax (min−1)b | Km (μM) | R-squared value | Efficiency (min−1 · μM−1)a |
|---|---|---|---|---|
| MilA-ab | 41,121 | 54.61 | 0.9967 | 752.9 |
| MilA-CD | 130,802 | 253.6 | 0.9949 | 515.7 |
| MilA-EF | 61,909 | 53.11 | 0.9977 | 1,165.6 |
Efficiency = Vmax/Km.
Vmax, maximum rate of metabolism.
MilA binds heparin.
Bioinformatic analysis of the MilA sequence identified potential heparin-binding motifs. Glycosaminoglycan-binding proteins contain consensus sequences of two types, XBBXBX and XBBBXXBX, where B represents a basic residue and X is any other amino acid (23). MilA contained motifs that match the first of these consensus sequences (IKKAKE at positions 197 to 200, AKKLKN at positions 227 to 230 and EKKAKE at positions 2299 to 2302). Several heparin-binding proteins have been identified that do not contain the consensus sequences but that do have sequences that are relatively rich in basic residues (24, 25). MilA also contains relatively large numbers of basic amino acids across the length of its sequence.
Several mycoplasma proteins have been shown to interact with the extracellular matrix (ECM) molecule heparin (26–28). To determine the heparin-binding profile of M. bovis whole-cell lysates and to determine whether the different regions of MilA could bind heparin, Western blotting and ligand dot blot assays were performed. Heparin bound to many M. bovis proteins in a Western blot analysis (see Fig. S5 in the supplemental material), and heparin also bound to MilA-ab and MilA-EF, but not to MilA-CD, in a Western blot analysis (Fig. 5B). This was consistent with the detection of heparin binding motifs in the sequences of the N and C termini of MilA but not in the sequence of the middle region (MilA-CD). The specificity of the heparin-MilA binding was assessed by incubating MilA-ab with increasing concentrations of unlabeled heparin or with monospecific antisera against MilA-ab before addition of labeled heparin. The binding signals in a Western blot analysis diminished considerably as the concentration of unlabeled heparin or anti-MilA-ab antibody increased (Fig. 5C), confirming that MilA was specifically binding heparin. When proteins were progressively diluted in 2-fold series, the binding affinity for MilA-ab was lost after the third dilution, whereas MilA-EF showed binding up to the fifth dilution (Fig. 6A), and the heparin binding of MilA-EF was abridged when the protein was denatured (Fig. 6B).
FIG 5.

Heparin binding by different regions of MilA. (A) Coomassie blue-stained SDS-PAGE of MilA. (B) A duplicate blot of that shown in panel A after Western transfer and probing with biotinylated heparin (100 μg/ml). Binding was detected with goat anti-biotin HRP conjugate (Cell Signaling) and the Clarity Western ECL blotting substrate (Bio-Rad). GST and bovine serum albumin (BSA) served as a negative controls, while GST-0232 served as a positive control (unpublished data). (C) Specificity of heparin binding by MilA-ab. Protein was transferred onto PVDF membrane, blocked, cut into strips, and then treated with increasing amounts of unlabeled heparin or monospecific anti-MilA-ab before probing with biotinylated heparin (100 μg/ml). Binding was detected with goat anti-biotin HRP conjugate (Cell Signaling) and the Clarity Western ECL blotting substrate (Bio-Rad).
FIG 6.

Heparin affinity binding and effect of denaturation on binding of MilA to heparin. (A) Doubling dilutions of each of the proteins were blotted onto duplicate nitrocellulose membranes. Blots were stained with Coomassie blue (panel 1) or probed with biotinylated heparin (100 μg/ml) (panel 2). Binding was detected with goat anti-biotin HRP conjugate and ECL substrate (GE Healthcare). (B). Equimolar amounts of recombinant proteins in their native form and after denaturation by heating in cracking buffer were blotted onto nitrocellulose membrane, with GST-MilA-ab in column 1 and GST-MilA-EF in column 2. The first blot was stained with Coomassie blue, while the other blots were incubated in biotinylated heparin (100 μg/ml). Binding was detected with goat anti-biotin HRP conjugate and the ECL Chemiluminescence Substrate (GE Healthcare).
ATP binding and effect of ATP on the tryptic digestion pattern of MilA.
Bioinformatic analysis of MilA using ATPint identified some ATP-interacting residues across the length of the protein. We therefore assessed the ability of MilA to bind ATP using ATP-linked agarose. All three recombinant fragments of MilA were able to bind ATP-linked agarose, with the C-terminal fragment (MilA-EF) showing more binding capacity and the middle fragment (MilA-CD) the least (see Fig. S6 in the supplemental material).
The binding of ATP often results in conformational changes in the protein, and this can affect the tryptic digestion pattern of the protein (29). To test for this, the tryptic digestion products of MilA-EF bound to ATP were analyzed by Western blotting. In the absence of ATP, MilA-EF was digested into fragments of 45 kDa or less, while in the presence of ATP, proteolysis was markedly reduced and the pattern of degradation was altered (Fig. 7B). Many ATP-protein interactions require divalent cations. Addition of magnesium ions resulted in enhanced protection from the proteolytic effect of trypsin, and an altered pattern of proteolysis (Fig. 7C). EDTA was used to chelate the Mg2+ ions from the ATP binding assay. This led to a loss of the protective effect of ATP against proteolysis.
FIG 7.

Effect of ATP on tryptic digestion of MilA. Following tryptic digestion of 1 μg of recombinant MilA-EF in the presence or absence of 5 mM ATP and in the presence or absence of 5 mM MgCl2, the fragments were resolved by SDS-12% PAGE and detected by Western immunoblotting, using antisera raised against MilA-EF to probe the blot. (A) MilA-EF stained with Coomassie blue. (B) Western blot of tryptic fragments generated in the absence of MgCl2. (C) Western blot of tryptic digestion products generated in the presence of MgCl2. Lane 1, protein treated with trypsin only; lane 2, protein treated with trypsin in the presence of ATP; lane 3, protein treated with EDTA as a chelator prior to trypsin treatment. M, PageRuler prestained protein ladder (Thermo Scientific).
We further assessed the ability of MilA to hydrolyze ATP using the ATPase/GTPase Activity assay kit. The C-terminal region of MilA (MilA-EF) had ATPase activity, but the middle region (MilA-CD) did not (Fig. 8).
FIG 8.

ATP hydrolysis by MilA. Recombinant protein (60 pmol) was incubated with ATP (1 mM) for 50 min at room temperature and then the malachite green reagent was added and the reaction mixtures were incubated for a further 50 min at room temperature to detect free phosphate. Absorbance was read at 620 nm. BSA and GST were used as negative-control proteins, while inorganic phosphate was used as a positive control. Data are presented as the mean ± SD; ***, P = 0.0002.
Antibody directed against the C-terminal part of MilA inhibits growth of M. bovis 3683 in vitro.
Monospecific antisera were raised against recombinant MilA fragments spanning the length of the protein. These antisera were assessed for their growth inhibitory effect against M. bovis using the filter disc diffusion method on M. bovis agar. The antiserum against MilA-EF, which spanned the C-terminal part of MilA, inhibited the growth of M. bovis strain 3683 in vitro (see Fig. S7 in the supplemental material). Antisera against the other regions of MilA did not affect growth. Sera from unimmunized rats and anti-GST were used as negative controls, and they also had no effect on the growth of M. bovis.
DISCUSSION
M. bovis MilA is expressed during infection, as infected calves produce antibodies against it that are detectable by Western immunoblotting, and the amino terminal region has been shown to have lipase activity (17). Beyond these findings, the role and functions of MilA have not been elucidated. The predicted molecular mass of the mature MilA was calculated to be 303 kDa, but Western blot analysis with sera from experimentally infected calves, combined with mass spectrometric analysis, detected a protein of 226 kDa (P226) (17). We predicted that the remainder of the full-length product of milA may be cleaved from the cell surface posttranslationally or secreted, or may not be surface exposed. Analysis of recombinant constructs spanning the whole length of MilA showed that both the amino and carboxyl termini (MilA-ab and MilA-EF, respectively) were immunoreactive when probed with sera from infected calves, while the central region was not detected by antibodies in the sera of the same calves. The amino-terminal region of MilA, which contains the lipase domain, was the immunodominant region and has been used previously to develop a novel ELISA for detection of cattle that have been infected with M. bovis (17, 30). Western blot analysis of Triton-X-114-phase-separated M. bovis proteins showed that both P226 and a 50-kDa cleavage product of MilA, P50, segregated into the detergent fraction and were not detectable in the aqueous fraction, demonstrating that both these cleavage products of full-length MilA were integral membrane proteins. Surface exposure of P226 was demonstrated using mild trypsin treatment of intact M. bovis cells, but P50 was not affected by trypsin treatment. P50 was detected with antibody against the carboxyl-terminal region of MilA. Thus, this cleavage product of MilA appears to remain associated with the cell membrane and is immunogenic, but it is not exposed sufficiently to be degraded by trypsin. A putative transmembrane domain identified between amino acids 2532 and 2556 may anchor it in the membrane. P226 was also detectable in culture supernatant, possibly as a result of proteolytic cleavage from the cell surface, secretion, or cleavage during translocation across the membrane. A number of mycoplasma proteins have been identified as secretory proteins, including a fragment of the lipoprotein MALP-404 of Mycoplasma fermentans (31), P80 of M. hominis (32), P102 of M. hyopneumoniae (33), and, recently, a lipoprotein nuclease (MBOV_RS02825) of M. bovis (11). Homologues of milA were found in the genomes of a number of mycoplasma species, including M. agalactiae, M. fermentans, M. hyopneumoniae, M. arginini, and M. gallisepticum (Table 1). A common feature among these milA homologues is the presence of the Lipase_GDSL_2 motif, while regions homologous to the carboxyl 75% of MilA are generally restricted to the species most closely related to M. bovis.
The presence of a lipase domain and a recurring DUF445 along the entire length of MilA led to the hypothesis that MilA could be a lipid-binding protein, and protein-lipid overlay assays showed that all regions of the protein had some lipid binding activity, with the amino terminus (MilA-ab) showing the strongest activity, especially for palmitic acid. It is possible that triglycerides and other lipids are bound by MilA for degradation by the lipase in the amino terminal region and that the fatty acids that are released are captured by other domains on MilA and imported into the cell. Bioinformatic analysis using the TmPred algorithm detected three putative transmembrane domains, which could serve as channels for communication between the extracellular and cytosolic compartment. The role played by MilA in lipid binding and lipolysis concords with the persistence of M. bovis in the mammary gland (34, 35), where there are ample supplies of triglycerides to utilize for metabolic and biosynthetic activities.
The binding of cholesterol by MilA is worthy of note. Many mycoplasma species require an exogenous supply of fatty acids and sterols for growth (36). Mycoplasmas incorporate sterols into their cytoplasmic membrane, where they function as a regulator of membrane fluidity (36). The N-terminal (MilA-ab) and C-terminal (MilA-EF) regions of MilA bound the fluorescent substrate 1,8-ANS significantly more strongly than did the middle region (MilA-CD) (P = 0.0002 and P = 0.0024, respectively). However, there was no significant difference between the binding of 1,8-ANS to MilA-ab and MilA-EF. The middle region of MilA (MilA-CD) had a Km for binding 1,8-ANS that was 5 times that of the other two regions, indicating a low affinity for lipid substrates. While oleic acid could compete with 1,8-ANS for binding to MilA-EF, lipids were unable to displace bound 1,8-ANS. Although import of lipids is clearly an important function in the survival of mycoplasmas, as yet no protein has been shown to perform this function. Our study is the first to describe a cell surface lipid binding protein in a mycoplasma. Such binding is likely to be a prelude to import.
Lipoproteins within ABC transporter operons are predicted to function as substrate-binding proteins for the import of macromolecules (37). In the absence of other genes around milA suggestive of a transporter operon, we hypothesized that MilA may also function as a transporter and may require energy to carry out this putative autotransporter function. Structural prediction using I-TASSER indicated a similarity to a serine/threonine kinase (38), while binding site prediction using RaptorX identified putative nucleotide ligands such as AMP, GMP, and GTP. Hypothetically, MilA could be associated with ATP binding and or hydrolytic activities, catalyzing the reversible conversion of ATP to AMP, or of pyrophosphate to phosphoenolpyruvate (22). We therefore examined the ability of MilA to bind ATP by incubating the recombinant proteins with ATP-agarose and analyzing the bound proteins by SDS-PAGE and Western blotting. Multiple domains of MilA were able to bind ATP. Proteins with surface-localized sites for nucleotide binding have been described in eukaryotic and prokaryotic cells (39–41). Structural analyses and ligand binding site predictions using I-TASSER and Raptor-X identified some residues in MilA that could putatively bind AMP and GTP. Trypsin treatment of the recombinant proteins in the presence or absence of ATP demonstrated the likelihood that a conformational change in MilA occurs in response to the binding of ATP. This is often seen in nucleotide-binding proteins (29, 32). The data presented here also show that the ATP binding was Mg2+ dependent. The C-terminal region of MilA was shown to hydrolyze ATP to ADP, generating free inorganic phosphate. This indicates that this region of the protein may be able to generate energy to facilitate importation of lipids into the cell that have been generated by the N-terminal lipase of MilA (17).
Surface-exposed proteins can interact with host extracellular matrix components, potentially playing a role in the initial steps in pathogenesis. We identified some sequences in MilA (AKKLKN and EKKAKE; residues 227 to 230 and 2299 to 2302, respectively) that could interact with heparin. Multiple putative heparin binding proteins were detected when Western blots of whole-cell proteins were probed with biotin-labeled heparin (Fig. S5). Ligand blotting studies also showed that MilA-ab and MilA-EF bound heparin, but that MilA-CD did not. This concurs with our hypothesis that the middle region of MilA (MilA-CD) may not be exposed on the surface for possible interactions with host extracellular matrix components (42, 43). To the best of our knowledge, MilA is the first M. bovis protein shown to interact with heparin.
The presence of MilA homologues in multiple mycoplasma species implies that this large protein may play an important role in these organisms, and transposon mutagenesis studies on M. bovis have not detected insertions in the gene that encodes it, indicating that it may be essential (18, 44). Antibody against the carboxyl-terminal region of MilA inhibited the growth of M. bovis 3683 in vitro, demonstrating that this region was exposed on the cell surface and that it has an essential role in growth of M. bovis in vitro. As there is evidence that vaccination with some attenuated mycoplasmas, with whole-cell antigens, or with some specific subunit antigens can exacerbate some mycoplasmoses, the identification of specific immunogens that induce protective immunity, but not immunopathological responses, is necessary for the development of effective subunit vaccines. Immunogens that induce growth-inhibitory antibody might be expected to induce a protective humoral immune response. The carboxyl-terminal region of MilA could, therefore, be a target of protective immunity and a promising candidate for inclusion in subunit vaccines against M. bovis (45–49).
Conclusions.
The findings of this study have shown that MilA binds both triglycerides and fatty acids. These activities, in concert with the previously characterized lipase activity in the amino-terminal region of this protein, suggest that this large protein is likely to have a role in the capture and processing of triglycerides in preparation for import of fatty acids. This would fit with the mammary gland habitat of some of the mycoplasmas with genes encoding homologues of this large protein, as the mammary gland is an environment rich in triglycerides. This study is the first to identify a lipid binding protein in mycoplasmas. The presence of putative transmembrane domains and the ability of MilA to bind and hydrolyze ATP is an indication that this membrane protein may also act as an autotransporter. Adherence of MilA to heparin is indicative of a further role as an adhesin, and thus that this protein may make a complex contribution to the pathogenesis of infection with M. bovis, while the capacity of the carboxyl terminus to induce growth-inhibitory antibodies implies a role in the induction of protective immunity. Our findings also demonstrate that DUF445, which is widely distributed across proteins of prokaryotes and eukaryotes, may be a lipid-binding domain. This study furthers our understanding of the biology of M. bovis and has the potential to contribute to the development of novel preventive methodologies to control this economically important bovine pathogen.
MATERIALS AND METHODS
Ethical statement.
Animal experiments were performed according to the international guiding principles for biomedical research involving animals with approval of The University of Melbourne Animal Ethics Committee (approvals 1011894.1 and 1413074).
Trypsin treatment of intact M. bovis cells.
An initial culture containing 1.58 × 107 color-changing units (CCU) per ml (13) of M. bovis PG45 was passaged at 1:10 dilution in M. bovis broth (18) and grown for 18 h at 37°C to reach the logarithmic phase (1.2 × 106 CCU/ml). Culture (20 ml) was pelleted by centrifugation at 13,000 × g for 5 min. The cell pellet was washed in phosphate-buffered saline (PBS) and resuspended in 400 μl PBS, and protein was quantitated using the BCA protein assay kit (Thermo Scientific) according to the manufacturer’s instructions. For each concentration of trypsin, 20 μg of this cell suspension was diluted with PBS to a final volume of 200 μl. To partially digest the cell surface proteins of the intact M. bovis PG45, trypsin (Sigma-Aldrich) was added to separate aliquots of the cell suspension to a final concentration of 32, 16, 8, 4, 1, 0.5, or 0 μg/ml. Samples were incubated at 37°C for 30 min and centrifuged at 13,000 × g for 5 min, and the supernatant was discarded. The cell pellets were resuspended in SDS-PAGE lysis buffer and heated at 95°C for 5 min. The cell lysate was then analyzed by SDS-12% PAGE and stained with Coomassie blue or transferred onto a polyvinylidene difluoride (PVDF) membrane for Western immunoblotting.
Triton X-114 phase partitioning.
Triton X-114 phase partitioning of M. bovis proteins was performed as described previously (50).
SDS-PAGE and Western immunoblotting.
Whole-cell proteins of M. bovis and purified GST-tagged recombinant proteins were resolved by SDS-PAGE through 7, 10 or 12% polyacrylamide gels and transferred onto PVDF membranes (Amersham Biosciences). The PageRuler prestained protein ladder (Thermo Scientific) was used as a molecular weight standard. After transfer, membranes were incubated overnight at 4°C in 5% (wt/vol) skim milk in PBS. The membranes were washed thrice for 5 min each with washing buffer (PBS containing 0.05% Tween 20 [vol/vol]) and then incubated with gentle rocking for 1 h at room temperature with a 1:100 dilution of pooled sera from calves experimentally infected with M. bovis 3686 (17) or with a 1:500 dilution of monospecific antisera raised against MilA in washing buffer containing 0.05% skim milk (wt/vol). Membranes were washed thrice for 5 min each in washing buffer and incubated at room temperature for 1 h with gentle rocking in a 1:2,000 dilution of horseradish peroxidase (HRP)-conjugated sheep anti-bovine antibody (Dako) or HRP-conjugated rabbit anti-rat antibody (Dako) in washing buffer containing 0.05% skim milk (wt/vol). The immunoreactive protein bands were detected using the ECL substrate (GE Healthcare) or Clarity Western ECL blotting substrate (Bio-Rad). Images were acquired using a ChemiDoc XRS+ system (Bio-Rad Laboratories).
Analysis of secreted or posttranslationally cleaved M. bovis proteins.
M. bovis PG45 was cultured in M. bovis broth for 18 h to reach the logarithmic phase of growth as stated previously. Cells were pelleted by centrifugation at 13,000 × g for 5 min at 4°C, and the supernatant (containing the secreted proteins or posttranslationally cleaved proteins) was carefully aspirated into a clean 1.5 ml Eppendorf tube and stored at −20°C. The cell pellet was resuspended in 1 ml cold PBS and centrifuged again before being resuspended in 200 μl PBS and stored at −20°C. SDS-PAGE lysis buffer was added to samples containing 10 μg of proteins from the supernatant and pellet, then they were heated at 95°C for 5 min and the proteins within them separated by SDS-12% PAGE. The gel was then stained with Coomassie blue or subjected to Western immunoblotting.
Polyclonal antibody production.
Healthy Sprague Dawley rats were used to raise antisera against the recombinant fusion proteins. Each rat was inoculated with 50 μg of recombinant protein in solution emulsified in Freund’s complete adjuvant (Sigma-Aldrich) by subcutaneous injection. The rats were given booster inoculations with 50 μg of the proteins in Freund’s incomplete adjuvant (Sigma-Aldrich) at day 21. A second booster in Freund’s incomplete adjuvant was given 2 weeks after the first booster. Blood was collected by cardiac puncture under anesthesia with isoflurane at day 45. The sera were stored at −20°C until further use. Production of antibodies against whole-cell proteins of M. bovis strain PG45 in rabbits has been described previously (51).
Immunogenicity of M. bovis MilA.
Seven M. bovis strains isolated between 1993 and 2007 (see Table S2 in the supplemental material) were used to assess the immunogenicity of MilA by Western immunoblotting. Cross-reactivity with other mycoplasmas, including M. agalactiae strain PG2, M. ovipneumoniae strain M12579, M. mycoides subspecies capri strain 1699, M. arginini strain GE3, and M. gallisepticum strain Ap3AS, was also assessed by Western immunoblotting. Whole mycoplasma cells (10 μg from each species) were lysed in SDS-PAGE lysis buffer for 5 min at 95°C and the lysate subjected to SDS-12% PAGE. The proteins were then electroblotted onto a PVDF membrane, which was then blocked in 5% skim milk and probed with monospecific antisera raised against different recombinant fragments of MilA. The identity of each of the mycoplasma species was confirmed using a universal Mycoplasma genus PCR assay targeting the 16S rRNA gene (52) and species-specific PCR assays using the primers listed in Table S4 in the supplemental material.
Growth inhibition assays.
Complement in monospecific antisera was inactivated by incubation at 56°C for 30 min. The growth-inhibitory effect of each of the monospecific antisera against recombinant fragments of MilA was examined by using 25-μl samples of 1:200 dilutions of a logarithmic-phase culture of M. bovis strain 3683 (1.2 × 106 CCU/ml) to make running drop cultures on an M. bovis agar plate (18). After the running drop inocula had been allowed to dry for 5 min, a 25-μl sample of heat-inactivated serum was inoculated onto a 5-mm-diameter sterile blank susceptibility disk (Oxoid), which was then carefully placed on the running drop culture. The agar plate was incubated at 37°C for 2 to 4 days. Heat-inactivated sera from GST-immunized and from unimmunized rats were used as negative controls, while gentamicin (3.125 μg/disk) was used as a positive control. Assays were carried out in duplicate in at least two independent experiments. The cultures were examined for any zones of growth inhibition using an SZX-ILLB200 stereo microscope (Olympus), and images were captured using a Dino-Eye eyepiece camera (AnMo Electronics Corporation). Fiji (53) was used for all image analysis and manipulations.
Protein lipid overlay assay.
Stock 500 mM solutions of lipids (tributyrin, oleic acid, deoxycholic acid, cholesterol, and palmitic acid; Sigma-Aldrich) were prepared in chloroform-methanol (2:1) before further dilution with lipid diluent (methanol:chloroform:water in a ratio of 2:1:0.8) to create a doubling dilution series. A 1.5-μl sample of each dilution of the lipid was dot blotted onto Hybond-C Extra nitrocellulose membrane (Amersham), and the blots were allowed to dry at room temperature for 1 h. The membrane was blocked in 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% Tween 20, and 0.002% fatty acid-free bovine serum albumin (Sigma-Aldrich) (blocking buffer) with gentle rocking for 1 h at room temperature. The membrane was then incubated in fresh buffer containing 30 nM recombinant GST-MilA fragment overnight at 4°C. Over a period of 50 min, the membrane was washed 10 times in Tris-buffered saline (TBS) containing Tween 20 (TBS-T; 50 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.1% [vol/vol] Tween 20), and incubated for 1 h at room temperature in a 1:2,000 dilution of goat anti-GST polyclonal antibody (GE Healthcare) in TBS-T. The membrane was washed 10 times over 50 min in TBS-T and then incubated for 1 h in a 1:2,000 dilution of HRP-conjugated rabbit anti-goat polyclonal antibody (Dako) in TBS-T. The membrane was washed 12 times over 1 h in TBS-T, and binding was detected using the Clarity Western ECL blotting substrate (Bio-Rad) according to the manufacturer’s instructions, with images captured using the ChemiDoc XRS+ system (Bio-Rad). GST protein was used as a negative control.
1-Anilinonaphthalene-8-sulfonic acid binding studies.
Ligand binding to MilA was assessed using the fluorescent probe 1,8-ANS (Sigma-Aldrich). Recombinant fragments of MilA (1 μM each) were dispensed into the wells of a black 96-well plate (Thermo Scientific). The 1,8-ANS probe was added to a final concentration of 5 μM in a final volume of 100 μl. The samples were mixed for 30 s on a plate shaker (Titertek; Flow Laboratories) and incubated at room temperature for 5 min under dim light. After initially shaking the plate at 567 cpm for 4 s, the fluorescence was measured at 25°C in a Hybrid multimode microplate reader (BioTek) using slit widths of 5 nm for both excitation and emission. To obtain normalized fluorescence spectra, 1 μM recombinant GST-MilA-CD was incubated with 5 μM 1,8-ANS and the emission spectrum obtained by taking fluorescence readings at a constant excitation of 385 nm, while the excitation spectrum was obtained by measuring emission at 485 nm over a range of excitation wavelengths. The peak wavelengths for excitation and emission were then used for the remaining assays. A fixed amount of protein (0.5 μM) was incubated with increasing amounts of the substrate (0 to 20 μM), and the fluorescence intensity was measured. The Michaelis-Menten equation in GraphPad Prism v. 8 was then used to calculate the binding parameters. The fluorescence reading for a control reaction that did not contain any of the proteins was subtracted from all other fluorescence readings. Triplicate assays were performed in at least two independent experiments.
Competition/displacement assay.
To assess whether fatty acids could compete with 1,8-ANS for binding to MilA, fixed amounts of recombinant MilA-EF (60 pmol) and 1,8-ANS (30 pmol) and increasing amounts of oleic acid (0 to 4 mM) were incubated at room temperature for 10 min and the fluorescence was measured. A displacement assay was employed to assess the ability of various lipids to displace 1,8-ANS bound to MilA fragments. Each of the recombinant MilA fragments (0.5 μM final concentration) in PBS at pH 7.4 were mixed with an equimolar amount of 1,8-ANS and incubated at room temperature for 5 min under dim light. Increasing concentrations of competitor lipid (diluted from a 10 mM stock in absolute ethanol) were added to the MilA/1,8-ANS complex, the solution mixed for 30 s, incubated again for 5 min, and fluorescence then measured. Assays were performed in triplicate in two independent experiments.
Heparin binding studies.
(i) Western blot binding assay. Sixty pmol each of recombinant protein fragments of MilA, GST (negative control), and GST-0232 (positive control) were separated by SDS-12% PAGE and transferred onto PVDF membranes. In a separate assay, 10 μg of lysed whole-cell proteins of M. bovis strains 3683, PG45, and a derivative of PG45 (mutant Δ416) were separated by SDS-12% PAGE and transferred onto PVDF membranes. The membranes were incubated in blocking buffer for 1 h at room temperature and then washed thrice for 5 min each in TBS-T. The membranes were then incubated at room temperature for 1 h in 100 μg biotinylated heparin/ml of TBS-T. The membrane was again washed with TBS-T and incubated in goat anti-biotin antibody conjugated to HRP as described above. The antigen-antibody complex was visualized by chemiluminescence using the Clarity Western ECL blotting substrate (Bio-Rad) according to the manufacturer’s instructions. Images were captured using the ChemiDoc XRS+ system (Bio-Rad). The specificity of heparin binding by MilA was assessed by incubating PVDF membranes to which MilA-ab had been applied in solutions containing unlabeled heparin (0, 10, 50, 100, or 500 IU/ml) or monospecific anti-MilA-ab antiserum (1:6,400, 1:3,200, 1:1,600, 1:400, 1:100 dilutions, or with no antisera) for 20 min before addition of biotinylated heparin.
(ii) Ligand dot blot assay. Purified proteins were diluted to 250 μg/ml in PBS. To denature samples, proteins were diluted in cracking buffer (60 mM Tris [pH 6.8], 1% SDS, 1% β-mercaptoethanol, and 10% glycerol) and heated for 5 min at 95°C. A 5-μl volume of diluted protein was spotted onto Hybond-C Extra nitrocellulose membrane (Amersham), and the membrane allowed to dry for 10 min at room temperature. The membrane was blocked in 2% bovine serum albumin (BSA) in TBS for 1 h at room temperature and then washed thrice for 5 min each with TBS-T. The membrane was then incubated for 1 h in 100 μg biotinylated heparin (Sigma-Aldrich) per ml of 1% BSA–TBS-T with gentle rocking to allow binding of the heparin to the immobilized protein. Following binding, the membrane was then washed thrice, each time for 5 min, in TBS-T. In order to detect protein-heparin interaction, HRP-conjugated goat anti-biotin antibody (Cell Signaling), diluted 1:2,000 in 1% BSA-TBS-T, was added to the membrane. After 1 h of incubation, the blot was washed thrice for 5 min each with TBS-T. The antigen-antibody complex was visualized by chemiluminescence using the Clarity Western ECL substrate (Bio-Rad) according to the manufacturer’s instructions, and images were captured using the ChemiDoc XRS+ system (Bio-Rad). To ensure that equivalent amounts of protein had been blotted at each position, a duplicate blot was stained with Coomassie brilliant blue immediately after the proteins had been spotted onto nitrocellulose membrane and the membrane had been dried.
ATP binding studies.
(i) Binding of MilA fragments to ATP using ATP-agarose. A PEP/pyruvate binding domain was identified in the predicted sequence of MilA, implying a role in binding and/or hydrolysis of ATP. The ability of MilA to bind ATP was also predicted by I-Tasser, RaptorX, and ATPint (54). ATP binding was assessed using the ATP-agarose method (55). MilA-ab, MilA-CD, MilA-EF, GST, and BSA (60 pmol each) were separately incubated with 50 μl 4% beaded agarose, to which ATP had been covalently linked via N-6 with an 11-atom spacer (A6888; Sigma-Aldrich), diluted in a buffer containing 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2 to a final volume of 300 μl, with mixing every 30 min for 2 h at 4°C. The agarose was subjected to five cycles of washing in cold TBS-T and centrifugation at 12,000 × g for 5 min. The agarose beads were then resuspended in SDS-PAGE sample buffer, heated for 2 min at 95°C, and bound proteins were separated in SDS-4 to 20% polyacrylamide gradient gels. Proteins were transferred onto PVDF membrane and analyzed by Western immunoblotting. The membrane was probed with goat anti-GST polyclonal antibody (1:2,000; GE Healthcare) and rabbit anti-goat HRP-conjugated polyclonal antibody (Dako) (1:2000). The antigen-antibody complex was visualized by chemiluminescence using ECL substrate (GE Healthcare), and the image was captured using the ChemiDoc XRS+ system (Bio-Rad).
(ii) Effect of ATP on tryptic digestion of MilA. The effect of binding to ATP on the susceptibility of purified recombinant MilA-EF (0.1 g/ml) to the proteolytic effect of trypsin was assessed using a method described previously (29). Samples of the protein were incubated at 4°C for 10 min with or without 5 mM ATP and with or without 5 mM MgCl2. Trypsin was then added to a final concentration of 0.18 mg/ml and proteolysis was allowed to proceed for 20 min at 4°C. The reaction was terminated by the addition of SDS-PAGE sample buffer and immediate heating at 95°C for 3 min. The digested protein fragments were separated by SDS-12% PAGE and the digestion patterns were analyzed by Western immunoblotting using a monospecific antiserum against MilA-EF at a dilution of 1:500. HRP-conjugated goat anti-rat polyclonal antibody at a dilution of 1:2,000 was used as the secondary antibody (Dako). The role of metal ions in ATP binding was determined by the addition of EDTA (10 mM) as a chelator before the trypsin treatment. The antigen-antibody complex was visualized by chemiluminescence using ECL substrate (GE Healthcare), and the image was captured using the ChemiDoc XRS+ system (Bio-Rad).
(iii) ATPase assay. Recombinant regions of MilA were purified as described above but were dialyzed in Tris-buffered saline to avoid any contamination with phosphate, which would affect the subsequent assays. ATPase activity of the recombinant regions of MilA was assessed using the ATPase/GTPase activity assay kit (MAK113; Sigma-Aldrich) according to the manufacturer’s instructions, with minor modifications. Recombinant proteins (60 pmol) were mixed with ATP (1 mM; Sigma-Aldrich), and the reaction mixtures were incubated for 50 min at room temperature in a 96-well microtiter plate. Malachite green reagent was then added and the reactions incubated for a further 50 min at room temperature to detect the release of phosphate from the ATP. The absorbance of each well at 620 nm was measured with a Hybrid multimode microplate reader (BioTek). The absorbance from a blank reaction performed without any protein was subtracted from the absorbance readings for all reactions. All reactions were performed in triplicates of at least two independent experiments. BSA and GST (60 pmol each) were used as negative-control proteins, while inorganic phosphate (12.5 μM) was used as a positive control.
Data analysis.
Statistical analyses were performed using Prism version 8.0e for Mac OS X (GraphPad Software, Inc., San Diego, CA). When the means of two groups were compared, a two-tailed unpaired t test was used. A one-way analysis of variance, followed by Tukey’s multiple-comparison test, was used to compare three or more means in the 1,8-ANS binding, growth inhibition, and ATPase activity assays. A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bob Geyer and Cynthia Brown for their excellent technical assistance. We also thank James R. Gilkerson for his help during the production of antisera in rats.
James Yazah Adamu was supported by MIFRS and MIRS scholarships from The University of Melbourne and by a Staff Study Fellowship from the University of Maiduguri.
We declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nicholas R. 2011. Bovine mycoplasmosis: silent and deadly. Vet Rec 168:459–462. doi: 10.1136/vr.d2468. [DOI] [PubMed] [Google Scholar]
- 2.Arcangioli MA, Duet A, Meyer G, Dernburg A, Bezille P, Poumarat F, Le Grand D. 2008. The role of Mycoplasma bovis in bovine respiratory disease outbreaks in veal calf feedlots. Vet J 177:89–93. doi: 10.1016/j.tvjl.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien D, Scudamore J, Charlier J, Delavergne M. 2016. DISCONTOOLS: a database to identify research gaps on vaccines, pharmaceuticals and diagnostics for the control of infectious diseases of animals. BMC Vet Res 13:1. doi: 10.1186/s12917-016-0931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchenau I, Poumarat F, Le Grand D, Linkner H, Rosengarten R, Hewicker-Trautwein M. 2010. Expression of Mycoplasma bovis variable surface membrane proteins in the respiratory tract of calves after experimental infection with a clonal variant of Mycoplasma bovis type strain PG45. Res Vet Sci 89:223–229. doi: 10.1016/j.rvsc.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Caswell JL, Archambault M. 2007. Mycoplasma bovis pneumonia in cattle. Anim Health Res Rev 8:161–186. doi: 10.1017/S1466252307001351. [DOI] [PubMed] [Google Scholar]
- 6.Sirand-Pugnet P, Lartigue C, Marenda M, Jacob D, Barre A, Barbe V, Schenowitz C, Mangenot S, Couloux A, Segurens B, de Daruvar A, Blanchard A, Citti C. 2007. Being pathogenic, plastic, and sexual while living with a nearly minimal bacterial genome. PLoS Genet 3:e75. doi: 10.1371/journal.pgen.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczepanek SM, Frasca S, Schumacher VL, Liao X, Padula M, Djordjevic SP, Geary SJ. 2010. Identification of lipoprotein MslA as a neoteric virulence factor of Mycoplasma gallisepticum. Infect Immun 78:3475–3483. doi: 10.1128/IAI.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masukagami Y, Tivendale KA, Mardani K, Ben-Barak I, Markham PF, Browning GF. 2013. The Mycoplasma gallisepticum virulence factor lipoprotein MslA is a novel polynucleotide binding protein. Infect Immun 81:3220–3226. doi: 10.1128/IAI.00365-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitiku F, Hartley CA, Sansom FM, Coombe JE, Mansell PD, Beggs DS, Browning GF. 2018. The major membrane nuclease MnuA degrades neutrophil extracellular traps induced by Mycoplasma bovis. Vet Microbiol 218:13–19. doi: 10.1016/j.vetmic.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Tivendale KA, Markham PF, Browning GF. 2015. Disruption of the membrane nuclease gene (MBOVPG45_0215) of Mycoplasma bovis greatly reduces cellular nuclease activity. J Bacteriol 197:1549–1558. doi: 10.1128/JB.00034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Zhao G, Guo YS, Menghwar H, Chen YY, Chen HC, Guo AZ. 2016. Mycoplasma bovis MBOV_RS02825 encodes a secretory nuclease associated with cytotoxicity. Int J Mol Sci 17:628. doi: 10.3390/ijms17050628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calcutt MJ, Lysnyansky I, Sachse K, Fox LK, Nicholas RAJ, Ayling RD. 2018. Gap analysis of Mycoplasma bovis disease, diagnosis and control: an aid to identify future development requirements. Transbound Emerg Dis 65(Suppl 1):91–109. doi: 10.1111/tbed.12860. [DOI] [PubMed] [Google Scholar]
- 13.Masukagami Y, De Souza DP, Dayalan S, Bowen C, O’Callaghan S, Kouremenos K, Nijagal B, Tull D, Tivendale KA, Markham PF, McConville MJ, Browning GF, Sansom FM. 2017. Comparative metabolomics of Mycoplasma bovis and Mycoplasma gallisepticum reveals fundamental differences in active metabolic pathways and suggests novel gene annotations. mSystems 2:e00055-17. doi: 10.1128/mSystems.00055-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masukagami Y, Nijagal B, Tseng CW, Dayalan S, Tivendale KA, Markham PF, Browning GF, Sansom FM. 2018. Metabolite profiling of Mycoplasma gallisepticum mutants, combined with bioinformatic analysis, can reveal the likely functions of virulence-associated genes. Vet Microbiol 223:160–167. doi: 10.1016/j.vetmic.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Masukagami Y, Nijagal B, Mahdizadeh S, Tseng C-W, Dayalan S, Tivendale KA, Markham PF, Browning GF, Sansom FM. 2019. A combined metabolomic and bioinformatic approach to investigate the function of transport proteins of the important pathogen Mycoplasma bovis. Vet Microbiol 234:8–16. doi: 10.1016/j.vetmic.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Kemege KE, Hickey JM, Lovell S, Battaile KP, Zhang Y, Hefty PS. 2011. Ab initio structural modeling of and experimental validation for Chlamydia trachomatis protein CT296 reveal structural similarity to Fe(II) 2-oxoglutarate-dependent enzymes. J Bacteriol 193:6517–6528. doi: 10.1128/JB.05488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wawegama NK, Browning GF, Kanci A, Marenda MS, Markham PF. 2014. Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clin Vaccine Immunol 21:196–202. doi: 10.1128/CVI.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Markham PF, Browning GF. 2014. Genes found essential in other mycoplasmas are dispensable in Mycoplasma bovis. PLoS One 9:e97100. doi: 10.1371/journal.pone.0097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee N. 2009. Characterization of an ATP-binding cassette (ABC) transport system involved in nucleoside uptake in Mycoplasma bovis strain M23, and discovery of its pathogenicity genes. PhD dissertation. Iowa State University, Ames, IA. [Google Scholar]
- 20.Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF. 2004. GDSL family of serine esterases/lipases. Prog Lipid Res 43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt JA, Browning GF, Markham PF. 2007. Mycoplasma hyopneumoniae mhp379 is a Ca2+-dependent, sugar-nonspecific exonuclease exposed on the cell surface. J Bacteriol 189:3414–3424. doi: 10.1128/JB.01835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzberg O, Chen CC, Kapadia G, McGuire M, Carroll LJ, Noh SJ, Dunaway-Mariano D. 1996. Swiveling-domain mechanism for enzymatic phosphotransfer between remote reaction sites. Proc Natl Acad Sci U S A 93:2652–2657. doi: 10.1073/pnas.93.7.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardin AD, Weintraub H. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21–32. doi: 10.1161/01.ATV.9.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Margalit H, Fischer N, Bensasson SA. 1993. Comparative analysis of structurally defined heparin-binding sequences reveals a distinct spatial distribution of basic residues. J Biol Chem 268:19228–19231. [PubMed] [Google Scholar]
- 25.Pethe K, Aumercier M, Fort E, Gatot C, Locht C, Menozzi FD. 2000. Characterization of the heparin-binding site of the mycobacterial heparin-binding hemagglutinin adhesin. J Biol Chem 275:14273–14280. doi: 10.1074/jbc.275.19.14273. [DOI] [PubMed] [Google Scholar]
- 26.Yavlovich A, Rottem S. 2007. Binding of host extracellular matrix proteins to Mycoplasma fermentans and its effect on adherence to, and invasion of HeLa cells. FEMS Microbiol Lett 266:158–162. doi: 10.1111/j.1574-6968.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins C, Geary SJ, Gladd M, Djordjevic SP. 2007. The Mycoplasma gallisepticum OsmC-like protein MG1142 resides on the cell surface and binds heparin. Microbiol-Sgm 153:1455–1463. doi: 10.1099/mic.0.2006/004937-0. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins C, Wilton JL, Minion FC, Falconer L, Walker MJ, Djordjevic SP. 2006. Two domains within the Mycoplasma hyopneumoniae cilium adhesin bind heparin. Infect Immun 74:481–487. doi: 10.1128/IAI.74.1.481-487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider E, Wilken S, Schmid R. 1994. Nucleotide-induced conformational changes of MalK, a bacterial ATP binding cassette transporter protein. J Biol Chem 269:20456–20461. [PubMed] [Google Scholar]
- 30.Wawegama NK, Markham PF, Kanci A, Schibrowski M, Oswin S, Barnes TS, Firestone SM, Mahony TJ, Browning GF. 2016. Evaluation of an IgG ELISA as a serological assay for detection of Mycoplasma bovis infection in feedlot cattle. J Clin Microbiol 54:1269–1275. doi: 10.1128/JCM.02492-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis KL, Wise KS. 2002. Site-specific proteolysis of the MALP-404 lipoprotein determines the release of a soluble selective lipoprotein-associated motif-containing fragment and alteration of the surface phenotype of Mycoplasma fermentans. Infect Immun 70:1129–1135. doi: 10.1128/IAI.70.3.1129-1135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopfe M, Hoffmann R, Henrich B. 2004. P80, the HinT interacting membrane protein, is a secreted antigen of Mycoplasma hominis. BMC Microbiol 4:46. doi: 10.1186/1471-2180-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djordjevic SP, Cordwell SJ, Djordjevic MA, Wilton J, Minion FC. 2004. Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesin. Infect Immun 72:2791–2802. doi: 10.1128/IAI.72.5.2791-2802.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox LK, Muller FJ, Wedam ML, Schneider CS, Biddle MK. 2008. Clinical Mycoplasma bovis mastitis in prepubertal heifers on 2 dairy herds. Can Vet J 49:1110–1112. [PMC free article] [PubMed] [Google Scholar]
- 35.Pothmann H, Spergser J, Elmer J, Prunner I, Iwersen M, Klein-Jöbstl D, Drillich M. 2015. Severe Mycoplasma bovis outbreak in an Austrian dairy herd. J Vet Diagn Invest 27:777–783. doi: 10.1177/1040638715603088. [DOI] [PubMed] [Google Scholar]
- 36.Kornspan JD, Rottem S. 2012. The phospholipid profile of mycoplasmas. J Lipids 2012:1–8. doi: 10.1155/2012/640762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browning GF, Marenda MS, Noormohammadi AH, Markham PF. 2011. The central role of lipoproteins in the pathogenesis of mycoplasmoses. Vet Microbiol 153:44–50. doi: 10.1016/j.vetmic.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 38.Wang XJ, Ran TT, Zhang X, Xin JY, Zhang ZH, Wu TW, Wang WW, Cai G. 2017. 3.9 angstrom structure of the yeast Mec1-Ddc2 complex, a homolog of human ATR-ATRIP. Science 358:1206–1209. doi: 10.1126/science.aan8414. [DOI] [PubMed] [Google Scholar]
- 39.Juliani MH, Klein C. 1981. A protein kinase of the plasma membrane of Dictyostelium discoideum. Biochim Biophys Acta 662:256–264. doi: 10.1016/0005-2744(81)90037-1. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Fernandes JR, Dutra PML, Rodrigues CO, Saad-Nehme J, Lopes A. 1997. Mg-dependent ecto-ATPase activity in Leishmania tropica. Arch Biochem Biophys 341:40–46. doi: 10.1006/abbi.1997.9933. [DOI] [PubMed] [Google Scholar]
- 41.Nickel V, Prehm S, Lansing M, Mausolf A, Podbielski A, Deutscher J, Prehm P. 1998. An ectoprotein kinase of group C streptococci binds hyaluronan and regulates capsule formation. J Biol Chem 273:23668–23673. doi: 10.1074/jbc.273.37.23668. [DOI] [PubMed] [Google Scholar]
- 42.Robinson MW, Buchtmann KA, Jenkins C, Tacchi JL, Raymond BBA, To J, Chowdhury PR, Woolley LK, Labbate M, Turnbull L, Whitchurch CB, Padula MP, Djordjevic SP. 2013. MHJ_0125 is an M42 glutamyl aminopeptidase that moonlights as a multifunctional adhesin on the surface of Mycoplasma hyopneumoniae. Open Biol 3:130017. doi: 10.1098/rsob.130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balasubramanian S, Kannan TR, Baseman JB. 2008. The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infect Immun 76:3116–3123. doi: 10.1128/IAI.00173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josi C, Burki S, Vidal S, Dordet-Frisoni E, Citti C, Falquet L, Pilo P. 2019. Large-scale analysis of the Mycoplasma bovis genome identified non-essential, adhesion- and virulence-related genes. Front Microbiol 10:2085. doi: 10.3389/fmicb.2019.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulongo M, Prysliak T, Perez-Casal J. 2013. Vaccination of feedlot cattle with extracts and membrane fractions from two Mycoplasma bovis isolates results in strong humoral immune responses but does not protect against an experimental challenge. Vaccine 31:1406–1412. doi: 10.1016/j.vaccine.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 46.Mulongo M, Frey J, Smith K, Schnier C, Wesonga H, Naessens J, McKeever D. 2015. Vaccination of cattle with the N terminus of LppQ of Mycoplasma mycoides subsp. mycoides results in type III immune complex disease upon experimental infection. Infect Immun 83:1992–2000. doi: 10.1128/IAI.00003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczepanek SM, Majumder S, Sheppard ES, Liao X, Rood D, Tulman ER, Wyand S, Krause DC, Silbart LK, Geary SJ. 2012. Vaccination of BALB/c mice with an avirulent Mycoplasma pneumoniae P30 mutant results in disease exacerbation upon challenge with a virulent strain. Infect Immun 80:1007–1014. doi: 10.1128/IAI.06078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cimolai N, Cheong ACH, Morrison BJ, Taylor GP. 1996. Mycoplasma pneumoniae reinfection and vaccination: protective oral vaccination and harmful immunoreactivity after re-infection and parenteral immunization. Vaccine 14:1479–1483. doi: 10.1016/S0264-410X(96)00068-0. [DOI] [PubMed] [Google Scholar]
- 49.Boothby JT, Jasper DE, Thomas CB. 1986. Experimental intramammary inoculation with Mycoplasma bovis in vaccinated and unvaccinated cows: effect on the mycoplasmal infection and cellular inflammatory response. Cornell Vet 76:188–197. [PubMed] [Google Scholar]
- 50.Duffy MF, Walker ID, Browning GF. 1997. The immunoreactive 116 kDa surface protein of Mycoplasma pneumoniae is encoded in an operon. Microbiol-Uk 143:3391–3402. doi: 10.1099/00221287-143-10-3391. [DOI] [PubMed] [Google Scholar]
- 51.Cottew GS. 1970. Mycoplasmas isolated from cattle in Australia. Australian Vet J 46:378–381. doi: 10.1111/j.1751-0813.1970.tb15577.x. [DOI] [PubMed] [Google Scholar]
- 52.van Kuppeveld FJ, van der Logt JT, Angulo AF, van Zoest MJ, Quint WG, Niesters HG, Galama JM, Melchers WJ. 1992. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol 58:2606–2615. doi: 10.1128/AEM.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chauhan JS, Mishra NK, Raghava G. 2009. Identification of ATP binding residues of a protein from its primary sequence. BMC Bioinformatics 10:434. doi: 10.1186/1471-2105-10-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grenert JP, Johnson BD, Toft DO. 1999. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem 274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




