The cryptic plasmid pCM is critical for chlamydial colonization in the gastrointestinal tract. Nevertheless, orally inoculated plasmid-free Chlamydia sp. was still able to colonize the gut. Surprisingly, orally inoculated Chlamydia sp. deficient in only plasmid-encoded pGP3 was no longer able to colonize the gut. A comparison of live organism recoveries from individual gastrointestinal tissues revealed that pGP3-deficient Chlamydia sp. survived significantly better than plasmid-free Chlamydia sp.
KEYWORDS: Chlamydia, pGP3, gastrointestinal spreading, plasmid function
ABSTRACT
The cryptic plasmid pCM is critical for chlamydial colonization in the gastrointestinal tract. Nevertheless, orally inoculated plasmid-free Chlamydia sp. was still able to colonize the gut. Surprisingly, orally inoculated Chlamydia sp. deficient in only plasmid-encoded pGP3 was no longer able to colonize the gut. A comparison of live organism recoveries from individual gastrointestinal tissues revealed that pGP3-deficient Chlamydia sp. survived significantly better than plasmid-free Chlamydia sp. in small intestinal tissues. However, the small intestinal pGP3-deficient Chlamydia sp. failed to reach the large intestine, explaining the lack of live pGP3-deficient Chlamydia sp. in rectal swabs following an oral inoculation. Interestingly, pGP3-deficient Chlamydia sp. was able to colonize the colon following an intracolon inoculation, suggesting that pGP3-deficient Chlamydia sp. might be prevented from spreading from the small intestine to the large intestine. This hypothesis is supported by the finding that following an intrajejunal inoculation that bypasses the gastric barrier, pGP3-deficient Chlamydia sp. still failed to reach the large intestine, although similarly inoculated plasmid-free Chlamydia sp. was able to do so. Interestingly, when both types of organisms were intrajejunally coinoculated into the same mouse small intestine, plasmid-free Chlamydia sp. was no longer able to spread to the large intestine, suggesting that pGP3-deficient Chlamydia sp. might be able to activate an intestinal resistance for regulating Chlamydia sp. spreading. Thus, the current study has not only provided evidence for reconciling a previously identified conflicting phenotype but also revealed a potential intestinal resistance to chlamydial spreading. Efforts are under way to further define the mechanism of the putative intestinal resistance.
INTRODUCTION
Chlamydia sp. is frequently detected in the gastrointestinal (GI) tracts (1–8). However, neither the significance nor the mechanism of chlamydial colonization in the GI tract is known. The species Chlamydia muridarum that is well adapted to mice has been used extensively for investigating the pathogenic mechanisms of chlamydial infection in the genital tract (9–14), which has led to the discovery of pathogenic factors from both Chlamydia sp. (15–19) and the host (9, 13, 20–26). The cryptic plasmid, termed pCM (27, 28), is a key pathogenic factor since Chlamydia sp. that lacks the entire plasmid (also called plasmid-free Chlamydia sp.) (15, 18, 19, 29, 30) or only the plasmid-encoded pGP3 (also called pGP3-deficient Chlamydia sp.) (15) is significantly attenuated in inducing genital tract pathology.
In vivo and ex vivo imaging of a luciferase-expressing Chlamydia sp. revealed that the mouse genital Chlamydia organisms selectively spread to and established long-lasting colonization in the GI tract (3). Although the chlamydial long-lasting colonization is nonpathological in the GI tract (31–34), it may contribute to chlamydial pathogenicity in the genital tract (35). Furthermore, many chlamydial virulence factors identified in the mouse genital tract were found to be critical for Chlamydia sp. to colonize the GI tract (35–40). Thus, investigating how chlamydial virulence factors, such as the plasmid-encoded pGP3, modulate chlamydial interactions with GI mucosal tissue may be a productive approach to understand chlamydial pathogenicity in the genital tract.
In current study, we compared plasmid-free Chlamydia sp. and pGP3-deficient Chlamydia sp. with wild-type Chlamydia sp. for their colonization in mouse GI tract. Although we have recently shown that the plasmid is required for improving Chlamydia fitness in different regions of the GI tract (41), it remains unclear whether plasmid-encoded pGP3 can improve chlamydial colonization in intestinal tissues in addition to its role in promoting chlamydial evasion of acidic killings (40). Despite the critical role of the plasmid in chlamydial colonization in the GI tract (36, 38, 40, 41), plasmid-free Chlamydia sp. was still able to reach the large intestine following an oral inoculation (41). However, orally inoculated pGP3-deficient Chlamydia sp. was no longer able to do so (38, 40). After reproducing the above-mentioning conflicting observations under the same experimental condition in the current study, we carefully examined the live chlamydial organism distributions along the mouse GI tract tissues. We found that pGP3-deficient Chlamydia sp. survived significantly better than plasmid-free Chlamydia sp. in the small intestine but failed to reach the large intestine, explaining the lack of live pGP3-deficient Chlamydia sp. in rectal swabs following an oral inoculation. This phenotype was further confirmed using an intrajejunal inoculation model. Interestingly, pGP3-deficient Chlamydia sp. successfully colonized the colon following a direct intracolon inoculation, indicating that a lack of live pGP3-deficient Chlamydia sp. in the large intestine following oral or intrajejunal inoculation is not due to its inability to colonize the colon tissues but, instead, its failure to spread from the small intestine to large intestine. Furthermore, when both plasmid-free and pGP3-deficient Chlamydia organisms were intrajejunally coinoculated into the small intestine of the same mouse, the plasmid-free Chlamydia sp. was no longer able to spread to the large intestine. This observation suggests that pGP3-deficient Chlamydia sp. might activate an intestinal resistance to prevent both types of organisms from spreading to the large intestine. Thus, the current study has not only provided an explanation for the conflicting observations on the relative roles of intact plasmid versus pGP3 in chlamydial colonization in the GI tract but also revealed a potential intestinal resistance for regulating Chlamydia sp. spreading in the gut.
RESULTS
Chlamydia sp. that lacks the entire plasmid is still able to colonize mouse gastrointestinal tract while Chlamydia sp. missing the plasmid-encoded pGP3 fails to do so regardless of the inoculation dose.
Both the cryptic plasmid and the plasmid-encoded pGP3 are important for promoting chlamydial colonization in the gastrointestinal tract (36, 38, 41). However, these previous studies also revealed conflicting observations that plasmid-free Chlamydia sp. (lacking the entire plasmid) colonized the GI tract better than the pGP3-deficient Chlamydia sp. (missing only the plasmid-encoded pGP3). Here, we carefully compared the colonization of plasmid-free Chlamydia sp. versus pGP3-deficient Chlamydia sp. following an oral inoculation at different doses (Fig. 1). As shown previously (31), the plasmid-positive (wild type) Chlamydia sp. was able to productively colonize the GI tract at an inoculation dose of as low as 1,000 inclusion-forming units (IFUs) per mouse, maintaining a steady colonization starting on day 7. Plasmid-free Chlamydia sp. was able to establish a stable colonization only at an inoculation dose of 1 × 105 IFUs per mouse or higher, and the colonization was also significantly delayed. When mice were inoculated with 1 × 104 IFUs, only minimal numbers of live organisms were recovered from some mice on days 21 and 28. These observations demonstrate a critical role of the cryptic plasmid in improving chlamydial colonization in the GI tract, which is consistent with our previous work (41). However, pGP3-deficient Chlamydia sp. failed to colonize the GI tract regardless of the inoculation dose. No live organisms were recovered from the rectal swabs, even with an oral inoculation dose of 1 × 107 IFUs per mouse. Thus, we not only confirmed the previous conflicting observations but also revealed a quantitative difference that pGP3-deficient Chlamydia sp. is at least 10,000-fold less efficient than plasmid-free Chlamydia sp. in colonizing the GI tract following an oral inoculation. The next question is what causes the conflicting observations.
FIG 1.

Comparison of live organism shedding from mice orally infected with different Chlamydia organisms. Groups of C57 mice (n = 3 to 5) were inoculated orally or intragastrically with C. muridarum with intact plasmid (wild-type Chlamydia sp., clone CMpGFP; a to c) or a plasmid that carries a premature stop codon in pgp3 gene (pGP3-deficient Chlamydia sp., clone CMpGP3S; g to i) or without any plasmid (plasmid-free Chlamydia sp., clone CMUT3.G5; d to f) at different inoculation doses ranging from 104 to 107 inclusion forming units (IFUs) per mouse, as indicated on the right of the figure. All mice were monitored for live chlamydial organism shedding in rectal swabs on days 3 and 7 and weekly thereafter, as indicated along the x axis. The live organisms recovered from rectal swabs were expressed as log10 IFUs, as shown along the left y axis. No live pGP3-deficient Chlamydia organism was detected in any rectal swabs regardless of the inoculation dose (AUC, Wilcoxon signed-rank test, P < 0.05 for h versus e, P < 0.01 for i versus f).
pGP3-deficient Chlamydia sp. is capable of colonizing the small intestine but unable to reach the large intestine following an oral inoculation.
To address the above conflicting observations, we compared the live organism recoveries from different GI tract tissues of mice orally inoculated with plasmid-free Chlamydia sp. versus pGP3-deficient Chlamydia sp. (Fig. 2). When the oral inoculation dose was at 1 × 105 IFUs per mouse, live organisms were recovered only from the large intestinal tissues of the plasmid-free Chlamydia-infected mice on days 14 and 21 after inoculation. This observation is consistent with the result from Fig. 1 above, which showed that live organisms were recovered from rectal swabs of mice infected with plasmid-free but not pGP3-deficient Chlamydia sp. The observation that no live organisms were detectable in the small intestine of mice orally inoculated with plasmid-free Chlamydia sp. is consistent with our previous report (41). We then compared the tissue yields of mice orally inoculated with 1 × 107 IFUs and found that significantly more live organisms were recovered from small intestinal tissues of mice inoculated with pGP3-deficient Chlamydia sp. than those with plasmid-free Chlamydia sp. Live organisms were consistently recovered from the small intestinal tissues of the pGP3-deficient Chlamydia-infected mice from days 7 to 21 while live organisms were minimally detectable from the small intestine of plasmid-free Chlamydia-infected mice. It seemed logical that pGP3-deficient Chlamydia sp. survived better than plasmid-free Chlamydia sp. in the small intestine. Nevertheless, the result was somewhat surprising to us since pGP3-deficient Chlamydia failed to colonize the GI tract based on the rectal swab recovery result (see Fig. 1). Despite the significant colonization in the small intestine by pGP3-deficient Chlamydia sp., no live organisms were recovered from the large intestinal tissues. This observation may provide an explanation for the lack of live pGP3-deficient Chlamydia sp. recovery from the rectal swabs (see Fig. 1 above) since chlamydial colonization in the colon is known to correlate with live organism recovery from rectal swabs (31, 39, 42, 43). However, it is still unclear why the small intestinal pGP3-deficient Chlamydia organisms failed to appear in the large intestine.
FIG 2.
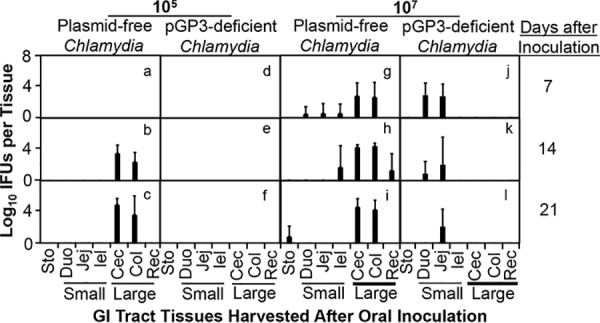
Comparison of live organism recovery from gastrointestinal tissues between mice orally inoculated with plasmid-free or pGP3-deficient Chlamydia sp. Groups of C57BL/6J mice (n = 3 to 5) were orally inoculated with 1 × 105 (left panels, a to f) or 1 × 107 (right panels, g to l) IFUs of plasmid-free (a to c and g to i) or pGP3-deficient (d to f and j to l) Chlamydia muridarum. Mice were then sacrificed for quantitating live chlamydial organism recovery in different regions of the gastrointestinal tract, including stomach (Sto); small intestinal tissues (Small), such as duodenum (Duo), jejunum (Jej) and ileum (Ile), and large intestinal tissues (Large), such as cecum (Cec), colon (Col), and rectum (Rec), as shown along the x axis on days 7, 14, and 21 after inoculation, as indicated on the right side of the figure. The live organisms recovered from different tissues were expressed as log10 IFUs as shown along the left y axis. No live pGP3-deficient Chlamydia organism was detected in any large intestinal tissues regardless of the inoculation dose (AUC, Wilcoxon signed-rank test, P < 0.05 for large intestinal tissue IFUs in j versus those in g, P < 0.01 for k versus h or l versus i).
The pGP3-deficient Chlamydia organisms are able to productively colonize the large intestine.
To determine whether the lack of live pGP3-deficient Chlamydia organisms in the large intestine is due to their failure to spread from small intestine to large intestine or their inability to colonize the large intestine after spreading, we monitored pGP3-deficient Chlamydia sp. colonization in the large intestine after directly delivering the organisms into the large intestine via an intracolon inoculation (Fig. 3). All Chlamydia organisms were able to productively colonize the GI tract following the intracolon inoculation regardless of their plasmid status when the inoculation dose was at 1 × 105 IFUs. All mice shed live organisms as early as day 3. The live organism shedding was constant and lasted for a long period of time. Both plasmid-free and pGP3-deficient Chlamydia organisms maintained stable colonization in the GI tract. However, when we reduced the inoculation dose to 1,000 IFUs, the plasmid-free Chlamydia organisms were no longer able to achieve any significant colonization, which is consistent with what we reported previously (41). Interestingly, the pGP3-deficient Chlamydia organisms were still able to productively colonize the large intestine as robustly as the wild-type Chlamydia sp. The above observations suggest that the lack of live pGP3-deficient Chlamydia organisms in the large intestine or rectal swabs following an oral inoculation may be caused by the failure of these organisms to spread to the large intestine.
FIG 3.

Comparison of live organism shedding between mice infected via intracolon inoculation with different Chlamydia organisms. Groups of C57BL/6J mice (n = 4 to 6) inoculated via intracolon inoculation with 1 × 103 (top panels, a, c, and e) or 1 × 105 (bottom panels, b, d, and f) IFUs of wild-type Chlamydia sp. (a and b), plasmid-free Chlamydia sp. (c and d) or pGP3-deficient Chlamydia sp. (e and f) were monitored for live chlamydial organism shedding in rectal swabs on days 3 and 7 and weekly thereafter, as indicated along x axis. The live organisms recovered from rectal swabs were expressed as log10 IFUs, as shown along the y axis. At an inoculation dose of 1 × 103 IFUs, the level of colonization by pGP3-deficient Chlamydia sp. was significantly higher than that of plasmid-free Chlamydia sp. (AUC, Wilcoxon signed-rank test, P < 0.01, e versus c).
The pGP3-deficient Chlamydia organisms still fail to spread to the large intestine after direct delivery into small intestine.
To determine whether the failure of the orally inoculated pGP3-deficient Chlamydia sp. to spread to the large intestine is caused by the gastric barrier effects (for example, gastric acidic shock may condition the chlamydial organisms to become defective in spreading to the large intestine), we delivered pGP3-deficient Chlamydia sp. directly into the small intestine via an intrajejunal inoculation and monitored live organisms in rectal swabs (Fig. 4). Both plasmid-positive and plasmid-free chlamydial organisms were recovered from rectal swabs after intrajejunal inoculation, which is consistent with what we recently reported (41). By bypassing the gastric barrier, intrajejunal inoculation seemed to enhance the colonization of plasmid-free Chlamydia sp. since live organisms were detected in rectal swabs as early as day 7, while the earliest positive detection was day 14 when the same of amount of plasmid-free Chlamydia sp. was inoculated orally (see Fig. 1) (see references 36 and 41). However, no live pGP3-deficient Chlamydia was detected in the rectal swabs regardless of the inoculation doses, suggesting that the gastric barrier may not have a significant effect on the spreading of pGP3-deficient Chlamydia sp. to the large intestine. This conclusion was confirmed when individual GI tract tissues were analyzed (Fig. 5). At an intrajejunal inoculation dose of 105 IFUs per mouse, significant numbers of live pGP3-deficient Chlamydia sp. were detected in the small intestinal tissues on both days 3 and 7, while plasmid-free Chlamydia sp. was barely detectable at any time points. At the inoculation dose of 106 IFUs, live organisms were detected in the small intestinal tissues of mice inoculated with either plasmid-free or pGP3-deficient Chlamydia sp. However, plasmid-free, but not pGP3-deficient, Chlamydia organisms reached the large intestine. Thus, with or without the potential gastric barrier effects, pGP3-deficient Chlamydia sp. consistently failed to spread from the small intestine to large intestine.
FIG 4.

Comparison of live organism shedding from mice inoculated via intrajejunal inoculation with different Chlamydia organisms. Groups of C57BL/6J mice (n = 3 to 5) infected via intrajejunal inoculation with 1 × 105 (top panels, a, c, and e) or 1 × 106 (bottom panels, b, d, and f) IFUs of wild-type Chlamydia sp. (a and b), plasmid-free Chlamydia sp. (c and d), or pGP3-deficient Chlamydia sp. (e and f) were monitored for live chlamydial organism shedding in rectal swabs on days 3 and 7 and weekly thereafter, as indicated along x axis. The live organisms recovered from rectal swabs were expressed as log10 IFUs, as shown along the y axis. No live pGP3-deficient Chlamydia organism was detected in the rectal swabs regardless of the inoculation dose (AUC, Wilcoxon signed-rank test, P < 0.01, e versus c or f versus d).
FIG 5.

Comparison of live organism recovery from gastrointestinal tissues between mice intrajejunally inoculated with plasmid-free or pGP3-deficient Chlamydia. Groups of C57BL/6J mice (n = 3 to 5) were intrajejunally inoculated with 1 × 105 (left panels, a to d) or 1 × 106 (right panels, e to i) IFUs of plasmid-free (a, b, e, and f) or pGP3-deficient (c, d, h, and i) Chlamydia sp. Mice were then sacrificed for quantitating live chlamydial organism recovery in different regions of gastrointestinal tract, including stomach (Sto); small intestinal tissues (Small) such as duodenum (Duo), jejunum (Jej), and ileum (Ile); and large intestinal tissues (Large), such as cecum (Cec), colon (Col), and rectum (Rec), as shown along the x axis on days 3 and 7 after inoculation, as indicated on the right side of the figure. The live organisms recovered from different tissues were expressed as log10 IFUs, as shown along the left y axis. No live pGP3-deficient Chlamydia sp. was detected in any large intestinal tissues regardless of the inoculation dose (AUC, Wilcoxon signed-rank test, P < 0.05, for large intestinal tissue IFUs in i versus f).
Intrajejunal coinoculation of plasmid-free and pGP3-deficient Chlamydia sp. prevented both types of organisms from spreading to the large intestine.
The above-described experiments demonstrated that the less invasive plasmid-free Chlamydia sp. was able to spread to the large intestine, while the more invasive pGP3-deficient Chlamydia sp. failed to do so. We then hypothesized that pGP3-deficient but not plasmid-free Chlamydia might be able to activate an intestinal resistance and that this resistance might be responsible for preventing the spread of the pGP3-deficient Chlamydia organisms. If this hypothesis was true, the pGP3-activated intestinal resistance should have also been able to prevent the spread of the plasmid-free Chlamydia organisms. Indeed, we found that when both plasmid-free and pGP3-deficient Chlamydia organisms were intrajejunally coinoculated into the small intestine of the same mouse, the plasmid-free Chlamydia sp. was no longer able to spread to the large intestine (Fig. 6). The blockade of plasmid-free Chlamydia sp. from spreading in the coinoculated mice was unlikely caused by the increased total number of chlamydial organisms coinoculated into the same mouse small intestine. This is because plasmid-free Chlamydia sp. by itself was able to spread at an inoculation dose of either 1 × 105 or 1 × 106 IFUs. However, when coinoculated with either 1 × 103 or 1 × 105 IFUs of pGP3-deficient Chlamydia sp., the plasmid-free Chlamydia organisms were prevented from spreading, regardless of the inoculation doses. These new observations have demonstrated that pGP3-deficient Chlamydia sp. at an inoculation dose as low as 1 × 103 IFUs is able to activate an intestinal resistance for preventing the spread of plasmid-free Chlamydia sp. with an inoculation dose as high as 1 × 106 IFUs.
FIG 6.

Comparison of live organism recovery from rectal swabs of mice intrajejunally inoculated with plasmid-free or pGP3-deficient Chlamydia sp. or both (coinoculation). Groups of C57BL/6J mice (n = 4) were intrajejunally inoculated with 1 × 105 (a to c) or 1 × 106 (d to f) IFUs of plasmid-free Chlamydia sp. (abbreviated as CMpf) without (a and d) or with pGP3-deficient Chlamydia sp. (CMpGP3S) at 1 × 105 (b and e) or 1 × 103 (c and f) IFUs, as listed at the left side. Mice shown in g were inoculated with 1 × 105 IFUs of plasmid-free Chlamydia sp. alone. All mice were monitored for live chlamydial organism shedding in rectal swabs on days 3 and 7 and weekly thereafter, as indicated along the x axis. The live organisms recovered from rectal swabs were expressed as log10 IFUs, as shown along the y axis. No live chlamydial organism was detected in any rectal swabs as long as the mice were inoculated or coinoculated with pGP3-deficient Chlamydia sp. (AUC, Wilcoxon signed-rank test, P < 0.05, b or c versus a, e or f versus d).
DISCUSSION
Chlamydia muridarum is known to colonize the mouse GI tract for long periods of time (3, 44, 45), and the plasmid, a known pathogenic determinant in the genital tract, is more important for Chlamydia sp. to colonize the GI tract than the genital tract (36). Plasmid-free Chlamydia sp. displays different levels of deficiencies in colonizing different segments of the GI tract (41). Nevertheless, the plasmid-free Chlamydia organisms were still able to reach the large intestine following an oral inoculation. The plasmid-encoded pGP3 has been shown to promote chlamydial resistance to gastric barriers (38, 40). However, the role of pGP3 in chlamydial colonization in the intestinal tissues remains unknown. We then carefully compared plasmid-free Chlamydia sp. versus pGP3-deficient Chlamydia sp. for colonizing different mouse GI tract tissues under a wide range of inoculum doses. We found that pGP3-deficient Chlamydia sp. (that misses only one plasmid-encoded protein) was more defective than plasmid-free Chlamydia sp. (that lacks the entire plasmid) in colonizing the GI tract regardless of the inoculation doses, confirming a conflicting observation noted in our previous studies (38). Thus, our initial motivation was to investigate the mechanisms of the conflicting observation.
We have demonstrated that a major reason for the failure of pGP3-deficient Chlamydia sp. to colonize the GI tract is due to its inability to disseminate from the small intestine to large intestine. First, orally inoculated pGP3-deficient Chlamydia sp. was able to productively colonize the small intestine since live pGP3-deficient Chlamydia organisms were consistently recovered from the jejunum for 21 days after oral inoculation. However, no live pGP3-deficient Chlamydia sp. was ever recovered from the large intestine of the same mice. Second, intrajejunally inoculated pGP3-deficient Chlamydia organisms also failed to reach the large intestine, although these organisms, initially inoculated at jejunum, spread to both the ileum and duodenum. Finally, direct delivery of pGP3-deficient Chlamydia organisms into the colon resulted in robust colonization of the organisms in the GI tract. Thus, the failure of pGP3-deficient Chlamydia organisms to reach the large intestine is not due to their inability to colonize the colon tissue but due to a lack of spreading from the small intestine to large intestine.
The next question is why pGP3-deficient Chlamydia sp. fails to spread to the large intestine while plasmid-free Chlamydia sp. is able to do so. The failure of pGP3-deficient Chlamydia organisms to spread to the large intestine is unlikely due to its own deficiency. First, plasmid-free Chlamydia sp. that lacks the entire plasmid still maintained its ability to spread to the large intestine, suggesting that pGP3-deficient Chlamydia sp. should possess the same ability. Second, pGP3-deficient Chlamydia sp. was more competent than plasmid-free Chlamydia sp. in colonizing the small intestinal tissues following oral or intrajejunal inoculation, confirming that pGP3-deficient Chlamydia sp. is more capable of overcoming small intestinal mucosal barriers than plasmid-free Chlamydia sp. Third, pGP3-deficient Chlamydia sp. colonized the colon significantly better than plasmid-free Chlamydia sp. following an intracolon inoculation, validating that pGP3-deficient Chlamydia sp. is more capable of overcoming large intestinal mucosal barriers. Finally, the same trend also holds true in the genital tract since the pGP3-deficient Chlamydia organisms developed more robust infection than plasmid-free Chlamydia sp. following an intrabursal inoculation (15). pGP3-deficient Chlamydia sp. appears to be more efficient in overcoming mucosal barriers in general. Thus, the failure of pGP3-deficient Chlamydia sp. to spread to the large intestine was not due to the deficiency of the pGP3-deficient organisms. A more likely explanation for the failure is that pGP3-deficient Chlamydia sp. is prevented from spreading to the large intestine by an intestinal resistance or immunity. This intestinal immunity may regulate chlamydial spreading from the small to large intestine and it may be activated by Chlamydia sp. that is deficient only in pGP3 but not by Chlamydia sp. that lacks the entire plasmid. In the absence of the plasmid, the organisms may be able to sneak from the small intestine into large intestine without activating the intestinal immunity. We tested the above hypothesis using an intrajejunal coinoculation approach. We found that when both plasmid-free and pGP3-deficient Chlamydia organisms were intrajejunally coinoculated into the small intestine of the same mouse, the plasmid-free Chlamydia sp. was no longer able to spread to the large intestine. Thus, it is highly likely that plasmid-free Chlamydia sp. is blocked from spreading in the coinoculated mice by an intestinal resistance induced by pGP3-deficient Chlamydia sp. Efforts are under way to further define and characterize the intestinal resistance, and the current study has laid a foundation for us to do so.
There are limitations of this study. First, the current study is solely based on the mouse model. The knowledge learned from the current study may not be necessarily applicable to C. trachomatis infection in humans. Nevertheless, C. trachomatis is also frequently detected in the GI tracts of humans (4–8). Neither the significance nor the mechanisms of human GI tract Chlamydia colonization are known. Thus, investigating chlamydial colonization in the GI tracts of animals is still necessary. Second, the current study was focused only on the GI tract lumenal spreading. However, Chlamydia sp. may spread via the hematogenous route (45), and Chlamydia sp. is known to spread systemically (46). Thus, there may be multiple pathways for chlamydial organisms to spread between the host tissues in addition to the lumenal spreading. It is possible that plasmid-free Chlamydia sp. is able to spread to the large intestine via a pathway that is not available for pGP3-deficient Chlamydia sp. or the different spreading abilities of these two types of organisms may be regulated by different microbiota compositions in the small versus large intestines. Efforts are also under way to determine the contribution of different pathways to chlamydial spreading to the colon.
MATERIALS AND METHODS
Chlamydia muridarum organisms.
All chlamydial organisms used in the current study were produced previously (15, 18, 47). Briefly, Chlamydia muridarum strain Nigg3 (GenBank accession number CP009760.1) was used to derive a plasmid-free clone, CMUT3.G5 (designated plasmid-free Chlamydia or CMpf for short in the current study). The CMUT3.G5 clone was then used as a recipient strain for transformation with a complete recombinant plasmid expressing gfp pCMgfp to produce CMpGFP (designated wild-type Chlamydia sp.) or the plasmid that carries a premature stop codon in pgp3 gene to produce the pGP3-deficient clone CMpGP3S (designated pGP3-deficient Chlamydia sp. or CMpGP3S for short). All organisms were amplified in HeLa cells (human cervical carcinoma epithelial cells; ATCC number CCL-2) and purified as elementary bodies (EBs) using discontinuous density centrifugation as previously described (48, 49). The purified EBs were stored in aliquots of sucrose-phosphate-glutamic acid (SPG) buffer (0.2 M sucrose, 20 mM sodium phosphate at pH 7.4, and 5 mM glutamic acid) at −80°C.
Mouse infection.
All animal experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Six- to 7-week-old female C57BL/6J (stock number 000664; Jackson Laboratories, Inc., Bar Harbor, ME.) mice were inoculated with purified EBs at different doses (measured in inclusion-forming units [IFUs]) via different routes, as indicated in individual experiments. After inoculation, mice were monitored for rectal shedding of live organisms or sacrificed for monitoring live organisms in different tissues at designated time points after infection, as indicated in individual experiments. The oral or intragastric inoculation was carried out by intragastric intubation of chlamydial organisms in a total volume of 200 μl SPG using a straight, ball-tipped needle (N-PK 020; Braintree Scientific, Inc., Braintree, MA) as described previously (39). Intrajejunal inoculation was carried out as described previously (50, 51). Briefly, mice anesthetized under isoflurane underwent laparotomy (0.5-cm incision). Chlamydial organisms in a total volume of 50 μl SPG were injected into the middle region of jejunum lumen with a 27-gauge needle. The jejunum middle region was identified and pulled closer to the abdominal wall to aid the injection by using curved forceps. After gently pulling out the needle, the jejunum was returned to abdominal cavity and the abdominal wall was closed with two or three 9-mm autoclips using an autoclip applier (both from Braintree Scientific, Inc.). When two different chlamydial organisms were coinoculated into the same mouse, the desired organisms were mixed in the same SPG solution with corresponding inoculation doses, as indicated in individual experiments, and delivered into the mouse small intestine via a single injection. For intracolon inoculation, chlamydial organisms were diluted in 50 μl of SPG buffer containing the desired numbers of IFUs, as indicated in individual experiments, and delivered to the colon using a straight ball-tipped needle designed for mouse oral gavage (N-PK 020; Braintree Scientific, Inc.). After inoculation, mice were monitored for rectal live organism shedding or sacrificed for titrating live organisms in corresponding samples.
Titrating live chlamydial organisms from rectal swabs and gastrointestinal tissues.
To quantitate live chlamydial organisms in rectal swabs, each swab was vortexed with glass beads in 0.5 ml of SPG. For titrating live chlamydial organisms recovered from mouse tissues, different GI tract tissues were harvested on designated days after inoculation, as specified in individual experiments. Each tissue segment was transferred to a vial containing 2 ml cold SPG buffer, including stomach (Sto), duodenum (Duo), jejunum (Jej), and ileum (Ile), cecum (Cec), colon (Col), and anorectum (Rec), for homogenization and sonication (43). The chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate and counted using an immunofluorescence assay (52). The total number of IFUs per swab was calculated based on the mean IFUs per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean IFU/swab was derived from the serially diluted samples for any given swab. The total number of IFUs/swab was converted into log10 for calculating group mean and standard deviation.
Immunofluorescence assay.
An immunofluorescence assay was used for titrating live organisms as described previously (32). Briefly, HeLa cells grown on coverslips were fixed with paraformaldehyde (Sigma) and permeabilized with saponin (Sigma). After being washed and blocked, the cell samples were subjected to a combination of antibody and chemical staining. Hoechst (blue; Sigma) was used to visualize nuclear DNA. A rabbit anti-chlamydial antibody (raised by immunization with serovar D EBs [data not shown]) plus a goat anti-rabbit IgG conjugated with Cy2 (green; Jackson ImmunoResearch Laboratories, Inc.) were used to visualize chlamydial inclusions. The immunolabeled cultures were used for counting inclusions under a IX-81 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY).
Statistical analyses.
The numbers of live organisms in IFUs and genome copies either at individual data points or over a time course were compared using the Wilcoxon signed-rank test. Area under the concentration-time curve (AUC) was used for comparing time course or clusters of tissue sample data.
ACKNOWLEDGMENT
This work was supported, in part, by grants (to G.Z.) from the U.S. National Institutes of Health.
REFERENCES
- 1.Yang R, Jacobson C, Gardner G, Carmichael I, Campbell AJ, Ryan U. 2014. Longitudinal prevalence and faecal shedding of Chlamydia pecorum in sheep. Vet J 201:322–326. doi: 10.1016/j.tvjl.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Pospischil A, Borel N, Chowdhury EH, Guscetti F. 2009. Aberrant chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet Microbiol 135:147–156. doi: 10.1016/j.vetmic.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig AP, Kong FY, Yeruva L, Hocking JS, Rank RG, Wilson DP, Donovan B. 2015. Is it time to switch to doxycycline from azithromycin for treating genital chlamydial infections in women? Modelling the impact of autoinoculation from the gastrointestinal tract to the genital tract. BMC Infect Dis 15:200. doi: 10.1186/s12879-015-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters RPH, Dubbink JH, van der Eem L, Verweij SP, Bos MLA, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morré SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 6.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 7.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 8.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 9.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Invest Drugs 3:980–986. [PubMed] [Google Scholar]
- 11.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/iai.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. doi: 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. doi: 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell CM, Ingalls RR, Andrews CW, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 20.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Cheng W, Shivshankar P, Lei L, Zhang X, Wu Y, Yeh IT, Zhong G. 2009. Distinct roles of CD28- and CD40 ligand-mediated costimulation in the development of protective immunity and pathology during Chlamydia muridarum urogenital infection in mice. Infect Immun 77:3080–3089. doi: 10.1128/IAI.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. 2010. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol 184:2602–2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- 23.Dai J, Tang L, Chen J, Yu P, Chen Z, Zhong G. 2016. The p47phox deficiency significantly attenuates the pathogenicity of Chlamydia muridarum in the mouse oviduct but not uterine tissues. Microbes Infect 18:190–198. doi: 10.1016/j.micinf.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. 2011. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manam S, Nicholson BJ, Murthy AK. 2013. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis 67:221–224. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas NS, Lusher M, Storey CC, Clarke IN. 1997. Plasmid diversity in Chlamydia. Microbiology 143:1847–1854. doi: 10.1099/00221287-143-6-1847. [DOI] [PubMed] [Google Scholar]
- 28.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res 28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Zhang Q, Yang Z, Conrad T, Liu Y, Zhong G. 2015. Plasmid-encoded Pgp5 is a significant contributor to Chlamydia muridarum induction of hydrosalpinx. PLoS One 10:e0124840. doi: 10.1371/journal.pone.0124840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. doi: 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2018. Nonpathogenic colonization with chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeruva L, Melnyk S, Spencer N, Bowlin A, Rank RG. 2013. Differential susceptibilities to azithromycin treatment of chlamydial infection in the gastrointestinal tract and cervix. Antimicrob Agents Chemother 57:6290–6294. doi: 10.1128/AAC.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract—a two-hit hypothesis. Trends Microbiol 26:611–623. doi: 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. doi: 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao L, Zhang T, Liu Q, Wang J, Zhong G. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-17. doi: 10.1128/IAI.00321-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao L, Zhang T, Melero J, Huang Y, Liu Y, Liu Q, He C, Nelson DE, Zhong G. 2018. The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 86:e00429-17. doi: 10.1128/IAI.00429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koprivsek JJ, Zhang T, Tian Q, He Y, Xu H, Xu Z, Zhong G. 2019. Distinct roles of chromosome- versus plasmid-encoded genital tract virulence factors in promoting Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 87:e00265-19. doi: 10.1128/IAI.00265-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Huo Z, Ma J, He C, Zhong G. 2019. The plasmid-encoded pGP3 promotes Chlamydia evasion of acidic barriers in both stomach and vagina. Infect Immun 87:e00844-18. doi: 10.1128/IAI.00844-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, He C, Huo Z, Xu Y, Arulanandam B, Liu Q, Zhong G. 2019. The cryptic plasmid improves Chlamydia fitness in different regions of the gastrointestinal tract. Infect Immun 88:e00860-19. doi: 10.1128/IAI.00860-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koprivsek JJ, He Y, Song C, Zhang N, Tumanov A, Zhong G. 2019. Evasion of innate lymphoid cells-regulated IFNgamma responses by Chlamydia muridarum to achieve long-lasting colonization in mouse colon. Infect Immun 88:e00798-19. doi: 10.1128/IAI.00798-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin H, He C, Koprivsek JJ, Chen J, Zhou Z, Arulanandam B, Xu Z, Tang L, Zhong G. 2019. Antigen-specific CD4(+) T cell-derived gamma interferon is both necessary and sufficient for clearing chlamydia from the small intestine but not the large intestine. Infect Immun 87:e00055-19. doi: 10.1128/IAI.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. doi: 10.1128/IAI.67.7.3686-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S, Clark AP, Sullivan ED, Miller RD, Summersgill JT. 2004. Detailed protocol for purification of Chlamydia pneumoniae elementary bodies. J Clin Microbiol 42:3288–3290. doi: 10.1128/JCM.42.7.3288-3290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meitin CA, Bender BS, Small PA Jr. 1994. Enteric immunization of mice against influenza with recombinant vaccinia. Proc Natl Acad Sci U S A 91:11187–11191. doi: 10.1073/pnas.91.23.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Etchart N, Wild F, Kaiserlian D. 1996. Mucosal and systemic immune responses to measles virus haemagglutinin in mice immunized with a recombinant vaccinia virus. J Gen Virol 77:2471–2478. doi: 10.1099/0022-1317-77-10-2471. [DOI] [PubMed] [Google Scholar]
- 52.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]


