Summary
The osmolyte glycine betaine (GB) ranks among the few widespread biomolecules in all three domains of life. In corals, tissue concentrations of GB are substantially higher than in the ambient seawater. However, the synthetic routes remain unresolved, questioning whether intracellular GB originates from de novo synthesis or heterotrophic input. Here we show that the genomic blueprint of coral metaorganisms encode the biosynthetic and degradation machinery for GB. Member organisms also adopted the prokaryotic high-affinity carrier-mediated uptake of exogenous GB, rendering coral reefs potential sinks of marine dissolved GB. The machinery metabolizing GB is highly expressed in the coral model Aiptasia and its microalgal symbionts, signifying GB's role in the cnidarian-dinoflagellate symbiosis. We estimate that corals store between 106–109 grams of GB globally, representing about 16% of their nitrogen biomass. Our findings provide a framework for further mechanistic studies addressing GB's role in coral biology and reef ecosystem nitrogen cycling.
Subject Areas: Biological Sciences, Genomics, Phylogenetics, Bioinformatics, Genomic Analysis, Omics
Graphical Abstract
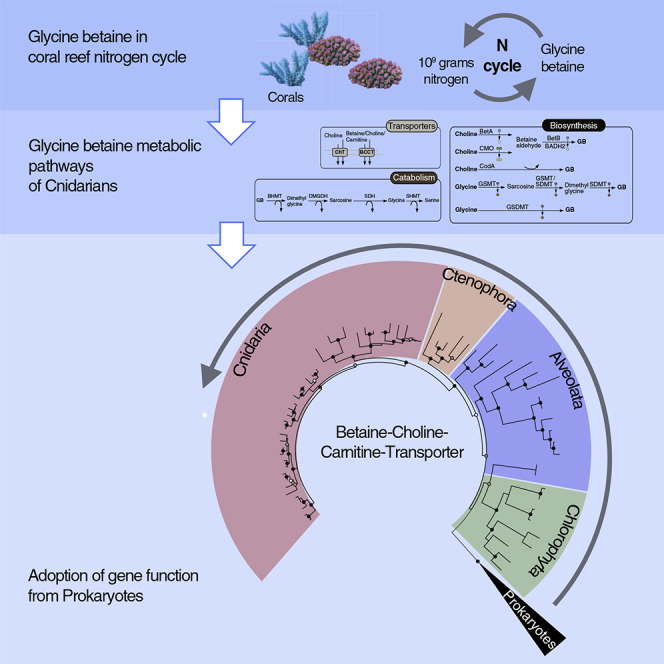
Highlights
-
•
Coral tissues contain high concentrations of the osmolyte glycine betaine
-
•
Corals and their microbial symbionts can produce and degrade glycine betaine
-
•
High gene expression patterns signifies role in coral-microbial symbiosis
-
•
Glycine betaine is estimated to encompass 16% of the coral's nitrogen biomass
Biological Sciences; Genomics; Phylogenetics; Bioinformatics; Genomic Analysis; Omics
Introduction
The quaternary ammonium compound glycine betaine (GB) represents one of the most ubiquitous osmolytes on Earth (Roesser and Müller, 2001, Welsh, 2000, Yancey et al., 1982). GB is widespread in the cells and tissues from organisms of all three domains of life (King, 1988, Roesser and Müller, 2001, Welsh, 2000, Yancey et al., 1982), where it generally serves as a compatible solute (osmolyte) to counteract high salinity effects and helps protect membranes and proteins against abiotic stressors (Burg and Ferraris, 2008, Chen and Murata, 2002). Although reliable estimates of dissolved GB in the ocean water are scarce, evidence suggests that dissolved concentrations of betaine analogs (e.g., proline betaine and alanine betaine) are in the picomolar range [∼3–482 pM; (Muslin, 2017)], whereas GB bound in seawater (cellular) particulates are in the nanomolar to lower micromolar range [up to 0.48 μM; (Airs and Archer, 2010, Beale and Airs, 2016, Cree, 2015, Keller et al., 2004)]. Paradoxically, the extensive knowledge of GB in marine systems stems from studies of cultured phytoplankton species, mostly microalgae/diatoms such as Emiliania huxleyi, Thalassiosira pseudonana, and Amphidinium carterae (Gebser and Pohnert, 2013, Keller et al., 1999a, Keller et al., 1999b, Spielmeyer et al., 2011, Spielmeyer and Pohnert, 2012). In the investigated phytoplankton cultures, maximum intracellular concentrations were higher under nitrogen-replete conditions [∼3–172 mM; (Keller et al., 1999b, Keller et al., 1999a)] than when nitrogen was limiting [4–15 mM; (Keller et al., 1999a)]. GB concentrations are also sensitive to elevated levels of temperature, CO2, and salinity (Gebser and Pohnert, 2013, Spielmeyer and Pohnert, 2012) and can be strain-/species-specific and growth-stage dependent (Keller et al., 1999b, Keller et al., 1999a, Spielmeyer et al., 2011). Crucially, GB can comprise up to 20% of the cellular nitrogen pool in phytoplankton (Keller et al., 1999a). Once excreted or released from lysed cells, this organic compound represents a ubiquitous and dynamic constituent of oceanic dissolved organic matter readily taken up by marine heterotrophic bacteria (Kiene et al., 1998, Lidbury et al., 2015, Sun et al., 2011).
Quantitatively significant levels of GB also accumulate in the tissues of tridacnid clams, sponges, and scleractinian corals (33–215 mmol kg−1 wet tissue); concentrations of up to 90-fold higher than the alternative sulfur-containing osmolyte dimethylsulfoniopropionate [DMSP; (Hagedorn et al., 2010, Hill et al., 2017, Hill et al., 2010, Yancey et al., 2010)], in turn, suggesting a major role for GB in coral physiology. However, the biosynthesis of GB has not been studied in corals or marine invertebrates in general [see for example (Stephens-Camacho et al., 2015)], limiting our understanding of the synthetic routes for de novo production and sequestration of exogenous GB. Hypothetically, the higher intracellular concentrations of GB in reef corals (Hagedorn et al., 2010, Hill et al., 2017, Hill et al., 2010, Yancey et al., 2010), relative to the nanomolar concentrations reported in seawater (Airs and Archer, 2010, Beale and Airs, 2016), suggest an intrinsic capacity of corals to synthesize GB, the retention of extracellular GB obtained via heterotrophic input [e.g., feeding; (Houlbreque and Ferrier-Pages, 2009)], or both.
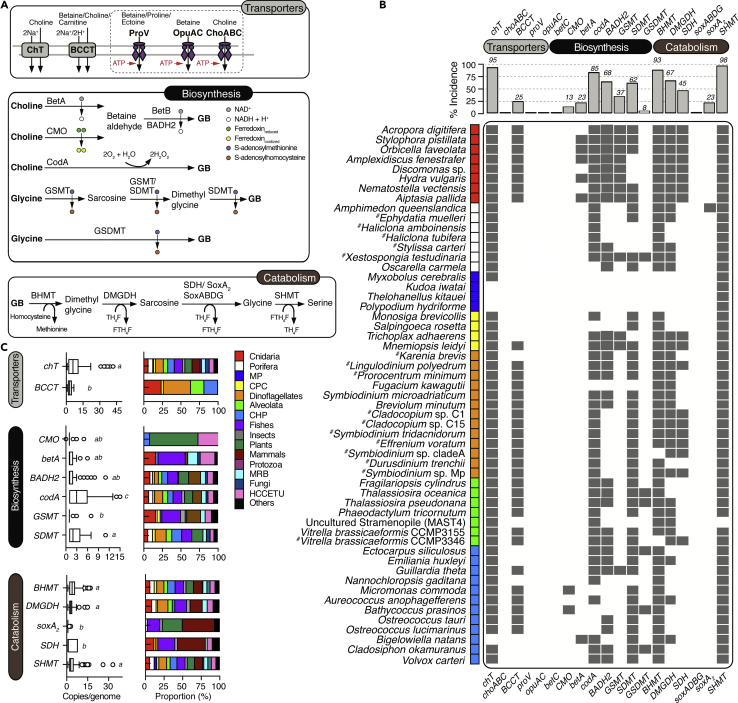
A two-enzyme oxidation pathway generally synthesizes GB from choline via the unstable intermediate betaine aldehyde in higher plants and bacteria (Figure 1A). The first oxidation step is catalyzed by an NAD+-dependent choline dehydrogenase (BetA) in prokaryotes and by a flavin-dependent choline monooxygenase (CMO) in plants, whereas the second reaction is catalyzed by an NAD+-dependent betaine aldehyde dehydrogenase (BetB) in all organisms (Chen and Murata, 2002). In some microorganisms, choline (and betaine aldehyde) is directly oxidized by choline oxidase (CodA) to GB with molecular oxygen as the electron acceptor (Lambou et al., 2013). GB can also be generated from glycine via a three-step methylation pathway involving glycine-sarcosine methyltranferase (GSMT) and sarcosine-dimethylglycine methyltransferase (SDMT) (Nyyssölä et al., 2000), with S-adenosylmethionine serving as the methyl group donor (Figure 1A). However, in a few organisms, all three successive methylation steps are catalyzed by a single enzyme called glycine sarcosine dimethylglycine N-methyltransferase (GSDMT), essentially a polypeptide with GSMT and SDMT domains (Kageyama et al., 2018, Lai et al., 2006). GB can be catabolized to serine via dimethylglycine, sarcosine, and glycine (Figure 1A) in reactions catalyzed by the enzymes betaine-homocysteine methyltransferase (BMHT), dimethylglycine dehydrogenase (DMGDH), sarcosine dehydrogenases (SoxA2, SoxABCDG, or SDH), and serine hydroxymethyltransferase (SHMT), respectively. Here, we leveraged on available eukaryotic genomes and transcriptomes to elucidate the genetic basis for GB accumulation in marine invertebrates, with a focus on corals, where GB is postulated to play a role in thermotolerance and photoprotection (Hagedorn et al., 2010, Hill et al., 2017, Hill et al., 2010, Yancey et al., 2010).
Figure 1.
Incidence of Glycine Betaine (GB) Metabolic Pathways in Eukaryotes
(A) Schematic view of pathways for the uptake, biosynthesis (via oxidation and methylation), and catabolism of GB.
(B) Incidence of key genes in eukaryotic genomes (n = 114) and transcriptomes (n = 16) in the upper panel and diverse marine invertebrate lineages (and their close relatives) harboring BCCT carriers (lower panel). Data from transcriptomes are designated with “#”. Additional information is provided in Tables S1 and S2.
(C) Copy numbers of predicted genes in each genome (excluding transcriptomes) and the taxonomic distribution of abundant metabolic pathways based on the presence-absence of key genes in the sampled genomes and transcriptomes. Statistically significantly different average copy numbers per genome are denoted by different letters as deduced by one-way ANOVA tests (p< 0.001) with two-stage step-up Benjamin-Krieger-Yekutieli's multiple comparison test. Enzymes: ChT, choline transporter; BCCT, betaine-choline-carnitine transporters; BetA, choline dehydrogenase; CMO, choline monooxygenase; BetB/BADH2, betaine aldehyde dehydrogenase; CodA, choline oxidase; GSMT, glycine-sarcosine methyltransferase; SDMT, sarcosine dimethyltransferase; GSDMT, glycine sarcosine dimethylglycine N-methyltransferase; BHMT, betaine-homocysteine methyltransferase; DMGDH, dimethylglycine dehydrogenase; SDH, eukaryotic sarcosine dehydrogenase; SoxA2, monomeric sarcosine oxidase; SoxABDG, heterotetrameric sarcosine oxidase. Abbreviations: Na+, sodium ion; H+, proton; NAD+ and NADH, oxidized and reduced nicotinamide adenine dinucleotide; TH4F, tetrahydrofolate; FTH4F, formyl-tetrahydrofolate; MP, Myxozoa and Polypodiozoa; CPC, Choanoflagellates, Placozoa and Ctenophora; CHP, Cryptophytes, Haptophytes, and Plantae (Algae); MRB, Mollusca, Rotifera, and Brachiopoda; HCCETU, Hemichordates, Cyclosteomes, Cephalochordates, Echinoderms, Tunicates, and Urochordates. Species indicated as “Others” are listed in Table S2.
Results and Discussion
Eukaryotic Genomes Encode Multiple Synthetic Routes for GB Production
Bioinformatic analyses revealed that the genomes (n = 114) and transcriptomes (n = 16; Table S1) of an overwhelming majority of ecologically important marine invertebrates encode the biosynthetic pathway for GB via choline oxidation (CodA, CMO, BetA, and BADH2), glycine methylation (GSMT/SDMT and GSDMT), or both (Figures 1A and 1B). Genomes of eukaryotes inhabiting terrestrial and freshwater ecosystems exhibited a similar trend (Table S2). Crucially, 85% of the analyzed eukaryotic genomes possessed a putative codA gene (Figure 1B). Subject to further experimental validation, these findings suggest that the complementary pathway oxidizing choline directly to GB is widespread in marine eukaryotes.
Putative enzymes of the alternative, dual-enzyme pathways for oxidizing choline to GB using choline monooxygenase (CMO) or choline dehydrogenase (BetA)—for the first reaction—and betaine aldehyde dehydrogenases (BetB or BADH2)—for the second reaction were also present in the genomes/transcriptomes (Figure 1B; Table S2). Potential CMO, betA, and BADH2 homologs with 40%–92% identity to validated proteins (Figure S1) were present in 13%, 23%, and 68% of the studied eukaryotes, respectively (Figure 1B). However, only two genomes (Acanthaster planci and Sonneratia caseolaris) possessed a putative betB homolog (Table S2). In-depth phylogenetic analyses showed that 88% of the predicted BADH2 genes were homologous (>50% amino acid identity; Figure S1) to functionally characterized animal BADHs within aldehyde dehydrogenase (ALDH) family 9 (Julián-Sánchez et al., 2007), whereas the remainder were affiliated to validated plant BADH2s (Figure S2) within the ALDH family 10 proteins (Kirch et al., 2004). Co-occurrence analysis of predicted CMO, betA, and BADH2 homologs in individual genomes/transcriptomes further showed that 11% and 43% of the analyzed eukaryotes possessed the complete CMO/BADH2- and BetA/BADH2-catalyzed pathways, respectively. However, the complete CMO/BADH2 pathway was predicted absent in Symbiodiniaceae and cnidarian genomes/transcriptomes (Figure 1B; Table S2). Notably, the putative “CMO”-like genes in both groups of organisms showed greater homology to carnitine monooxygenases (30%–40% identity) involved in carnitine metabolism than to ratified eukaryotic CMO proteins (≤25% identity). Crucially, these results indicate the possibility to oxidize choline to GB using the BetA/BADH2-catalyzed pathway in five out of eight cnidarians, which is absent in all Symbiodiniaceae genomes/transcriptomes analyzed (Figure 1B).
Glycine methylation pathways (Figure 1A) were generally underrepresented in the studied genomes/transcriptomes (Figure 1B); the complete GSMT/SDMT- and GSDMT-catalyzed pathways occurred in 18% and ∼8% of the genomes respectively. Interestingly, the GSMT/SDMT-catalyzed pathway was also predicted in five coral genomes but found lacking in Symbiodiniaceae (Figure 1B). Although further biochemical evidence is warranted to establish activities of all these pathways, the possession of the GSMT gene putatively encoding glycine-sarcosine methyltransferase by cnidarians, a demosponge (Amphimedon queenslandica), and many of the investigated mammalian genomes (Figure 1B; Table S2), suggests the potential to turnover glycine to dimethylglycine (Lai et al., 2006, Nyyssölä et al., 2001), which also counteracts osmotic and thermal stresses (Bashir et al., 2014). In summary, corals and their dinoflagellate endosymbionts alongside other marine invertebrates are deduced to possess at least one pathway for producing GB de novo, which points to the significance of GB in coral biology and underscores reef-building corals as a potential source of GB in the ocean.
The Ability to Scavenge Exogenous GB Is Taxonomically Constrained but Present in the Coral Host, Microalgae, and Bacteria of Coral Metaorganisms
Intriguingly, genomic profiling of transporters involved in GB metabolism demonstrates that the ability to scavenge extracellular GB in higher organisms is constrained to very few eukaryotic lineages, namely cnidarians, ctenophore, diatoms, dinoflagellates, ciliates, and cryptophytes (Figure 1B). The vast majority of eukaryotic genomes/transcriptomes investigated here encode the putative sodium-dependent high-affinity choline carrier ChT (30%–50% identity to validated proteins; Figure S1), which can take up choline for GB biosynthesis (Figures 1B and 1C; Table S2). However, only 25.4% of the eukaryotes harbored a potential GB uptake system (Figures 1B and 1C). Crucially, the eukaryotes that do were predicted to possess numerous copies of genes putatively encoding the betaine-choline-carnitine-transporter (BCCT) family (30%–40% identity to validated carriers; Figure S1). BCCT carriers are sodium- or proton-coupled transporters that are well characterized exclusively in prokaryotes, where they canonically mediate high-affinity uptake of betaines, choline, or carnitine (Ziegler et al., 2010). Homologs of BCCT carriers were also found in the genomes/transcriptomes of diverse Symbiodiniaceae species (Figures 1B and 2A), the intracellular dinoflagellate symbionts of cnidarians, suggesting the potential capacity to accumulate betaine, choline, or carnitine in hospite. Of evolutionary significance is the fact that BCCT homologs were found only in a few animals hosting microalgal endosymbionts (LaJeunesse et al., 2018) or close photosynthetic relatives, suggesting an important role in the cnidarian-dinoflagellate symbiosis.
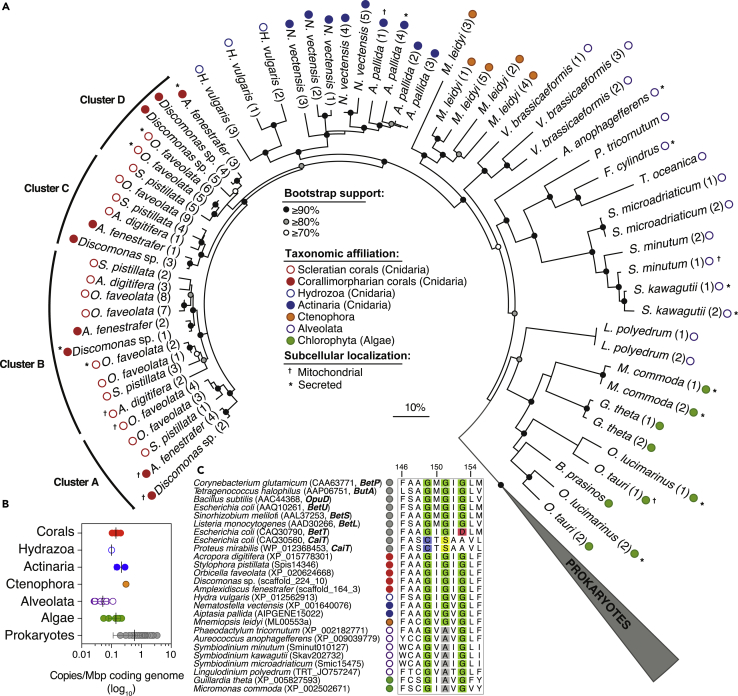
Figure 2.
Evolutionary History of Predicted Eukaryotic BCCT Carriers
(A) An unrooted maximum-likelihood phylogenetic tree showing the affiliation of predicted coral BCCT proteins (red circles) next to their closest cnidarian relatives (blue circles) and the basal prokaryotic BCCT carriers (wedged gray symbol). Colored circles highlight taxonomic affiliation of eukaryotic BCCT proteins. Note that the coral BCCTs are divided into four clusters (≥80% bootstrap support), with some putatively located in the mitochondria.
(B) The average density of predicted BCCT genes in marine invertebrates and marine prokaryotes with fully sequenced genomes (n = 125; Table S6); difference in copy number per mega-base pair (Mbp) of coding genome was only significant (one-way ANOVA, p = 0.0012) for Prokaryotes (gray circles) relative to Alveolata (green circles).
(C) Multiple aminoacid sequence alignment showing the G-x-G-x-G motif (highlighted in green) found in validated prokaryotic BCCT carriers specific for GB (BetP, ButA, OpuD, BetU, and BetL), choline (BetT), and carnitine (CaiT). Glycine residues lacking in choline and carnitine carriers are colored in red and blue (and yellow) respectively, whereas those missing in Symbiodiniaceae proteins are shown in gray. The amino acid positions reflect those in BetP from C. glutamicum. For brevity, only representative eukaryotic proteins are shown; Figure S6 provides the complete data.
It is noteworthy that the alternative ABC-type transport systems commonly found in prokaryotes for GB (OpuA and OpuC) or choline (OpuB, ProU, and ChoABC) (Ziegler et al., 2010) are lacking in the eukaryotes studied here. Also, only the diatom Thalassiosira pseudonana, the cryptomonad alga Guillardia theta and the coccolithophore Emiliania huxleyi encoded putative homologs of the choline and GB exporter EmrE (Tables S3 and S4), which exports naturally occurring quaternary cation compounds (Bay and Turner, 2012). Taken together, the systematic evaluation of potential GB uptake systems across genomes/transcriptomes of model and non-model metazoans depicts a confined distribution of BCCT and EmrE carriers to globally important marine invertebrates such as cnidarians and microalgae.
Cnidarians host a diverse suite of prokaryotes (Rohwer et al., 2002). Therefore, we sought to explore whether their bacterial associates also contribute to GB metabolism. Focusing on the ubiquitous Endozoicomonas species (n = 13), encompassing bacteria that seem to commonly colonize tissues of diverse cnidarians (Neave et al., 2016, Neave et al., 2017a, Neave et al., 2017b), our data show that not all species of these “obligate” endosymbionts have the putative machinery to synthesize and export GB though all possessed the ability to scavenge GB (Figure S3). These findings are also consistent with the incidence of profiled synthetic and uptake routes for GB across 52 metagenome-assembled prokaryotic genomes [MAGs; (Robbins et al., 2019)], representing the most abundant bacterial and archaeal taxa in the environmentally resilient reef-building coral Porites lutea (Table S5).
The majority of the genomes queried (56 out of 64) encoded BHMT proteins that can potentially convert GB to dimethylglycine, but most—all Endozoicomonas species and one-third of MAGs from P. lutea—lack putative enzymes for catabolizing dimethylglycine to sarcosine (Figure S3; Table S5). Thus, we speculate that hosting bacteria with reduced degradative capacity for GB maintains a high intracellular concentration of its precursor dimethylglycine—also an osmolyte, in coral tissues. Considering the ubiquity of Endozoicomonas (and other microbes) in coral microbiomes (Neave et al., 2016, Neave et al., 2017a, Neave et al., 2017b), we infer that the production of osmotically active compounds might be a cooperative mechanism supporting coral holobiont physiology (Ochsenkühn et al., 2017). Although speculative at this point, we propose that the coral host and its associated microbes—that is, Symbiodiniaceae and some bacteria—produce GB from choline or glycine and facilitate the exchange of GB scavenged from prey or the ambient seawater (by the host) or generated in situ (by the entire metaorganism) within the holobiont. This hypothesized model, however, requires experimental validation.
Eukaryotic BCCT Homologs Are Distant Relatives of Prokaryotic GB Carriers
The narrow distribution of putative BCCT genes in diverse eukaryotes studied here (Figure 1B) warrants elucidating the evolutionary history of the deduced transport system. Phylogenetic analyses indicate that the predicted eukaryotic BCCT carriers are distantly related to bona fide BCCT transporters from prokaryotes (Figure 2A). Moreover, these carriers group into discrete sequence clusters that reflect the metazoan evolutionary history (Derelle et al., 2015). Cnidarian sequences also form multiple protein clusters (A to D; Figure 2A), which are interspersed by proteins containing signal peptides or mitochondrial-like sequence motifs (Figure 2A), suggesting multiple subcellular localization of the carrier in coral tissues. Collectively, these results imply that the acquisition of BCCT carriers likely occurred independently sensu stricto before the diversification of metazoans.
Further analyses comparing copy numbers of predicted BCCT genes across different species indicate that the average copies (± SD) scaled by the coding genome size (in mega base pairs, Mbp), were only significantly different (one-way ANOVA; p = 0.0012; Figure 2B) between marine prokaryotes (0.59 ± 0.48 copies Mbp−1; n = 125; Table S6) and alveolates (0.03–0.11 copies Mbp−1; n = 8). The genomic density of predicted BCCT genes is also similar among coral species with different life histories (∼0.5 ± 0.2 copies Mbp−1; n = 4–5 replicates per species) as deduced from metagenomic coding sequences of ten Red Sea scleractinian coral species (Figure S4; Table S7). However, the clustering of similar sequences at 95% nucleotide identity over 80% of the length of the shorter sequence reveals specificity of predicted BCCT copies to individual coral species (Figure S5). Taken together, this suggests that BCCT copy number and gene isoforms are highly conserved in cnidarians but evolved species specifically.
Coral BCCT Homologs Likely Transport GB as Their Cargo
Additional bioinformatic analyses strongly support the annotation of the predicted eukaryotic BCCT genes as GB transporters. Multiple sequence alignments of the predicted amino acid sequences and functionally characterized BCCT proteins with varied substrate specificity (Figures 2C, S6, and S7) revealed conservation of key active site glycine residues (G-x-G-x-G; Figures 2C and S6) contributing to sodium and substrate binding, as well as tryptophan and tyrosine residues (Figure S7) that catalyze GB binding in sodium-coupled betaine symporters (Perez et al., 2011, Ressl et al., 2009). Interestingly, the central glycine residue in the case of Symbiodiniaceae and some other algal species is substituted by alanine (G-x-A-x-G; Figures 2C and S6), in a region that renders the substrate-binding site accessible (Perez et al., 2011). Although further experimental validation is required, we hypothesize altered kinetics and reduced affinity for GB in Symbiodiniaceae BCCT carriers relative to host BCCT carriers, based on point mutation studies of the GB carrier of Corynebacterium glutamicum (Perez et al., 2011). It is noteworthy that betaine carriers can also transport small quantities of structurally related organic solutes such as proline betaine, dimethylsulfoniopropionate (DMSP), and dimethylglycine (Tandon et al., 2020, Ziegler et al., 2010) and that transportation is also fully reversible (Farwick et al., 1995).
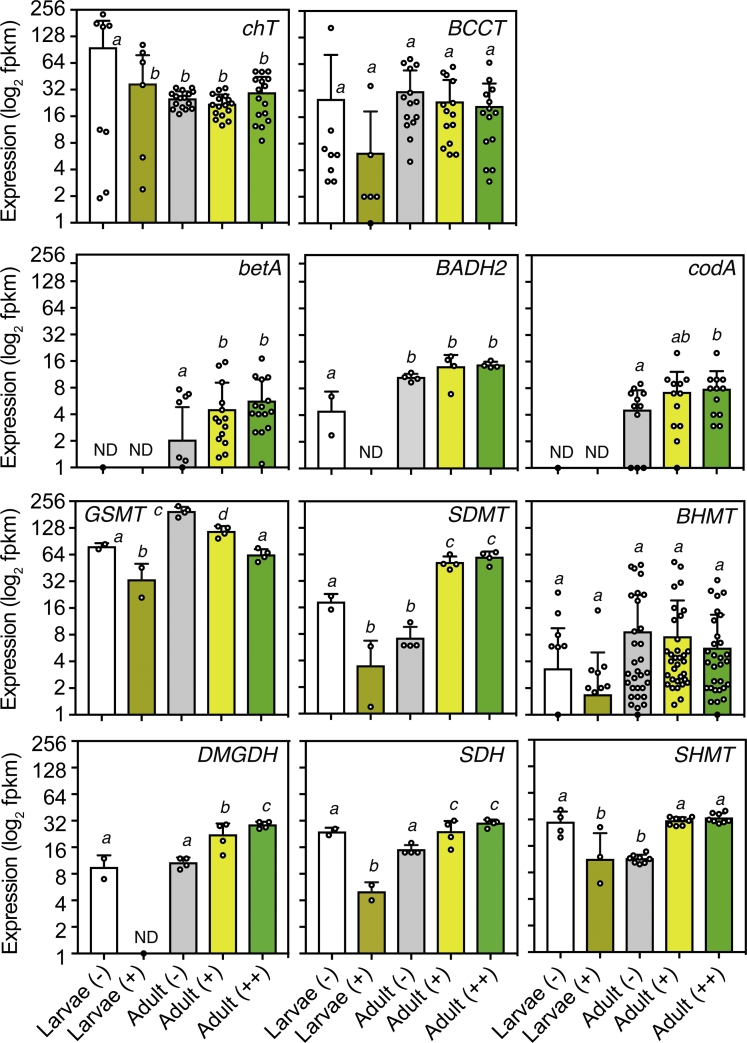
High Expression of GB Metabolism in Aiptasia and Pelagic Symbiodiniaceae
To gain further insight on the relevance of the predicted synthetic and transport pathways for GB in coral physiology, we analyzed previously published transcriptomic data from the coral model system Aiptasia, encompassing different developmental and symbiotic states (Baumgarten et al., 2015). Gene expression analyses indicated that the pathways involved in the metabolism of GB were highly expressed in both symbiotic and aposymbiotic states (i.e., with and without microalgal symbionts) across larval and adult stages (Figure 3). For instance, the choline transporter ChT was highly expressed in aposymbiotic larvae, whereas the BCCT carrier was expressed similarly in larvae and adult stages. Genes putatively involved in the production of GB from choline oxidation (betA/BADH2 and codA) were significantly highly expressed (one way ANOVA, p > 0.001) in adult anemones, especially in symbiotic colonies (e.g., betA and codA). In contrast, the glycine methylation pathway catalyzed by GSMT and SDMT was differentially highly expressed in symbiotic and aposymbiotic anemones, respectively (Figure 3). Crucially, transcription was in both cases, four-fold higher than choline oxidation pathways catalyzed by BetA/BADH2 and CodA. The degradative pathway catalyzed by BHMT, DMGDH, SDH, and SHMT, however, followed a trend of higher expression in symbiotic adult anemones (Figure 3), suggesting a role of Symbiodiniaceae in GB catabolism. Our results therefore suggest a greater contribution of GSMT-/SDMT-catalyzed pathway to GB production and the turnover of intracellular GB by Symbiodiniaceae.
Figure 3.
Transcriptional Activity of GB Metabolism in the Coral Model Aiptasia
Gene expression was determined from published whole tissue transcriptomes (Baumgarten et al., 2015) of aquacultured Aiptasia larvae and adults fed regularly on brine shrimps (n = 2–4 replicates per experiment). Transcriptomes encompass various developmental and symbiotic states. Animals without dinoflagellate endosymbionts are indicated with “–”, whereas adults with intermediate or full endosymbiont levels are denoted with “+” and “++”, respectively. Barplots indicate average (± SD) expression in replicated samples and all predicted gene homologs (denoted by circular symbols). Statistically significantly different expression levels are denoted by different letters as deduced by one-way ANOVA tests (p > 0.05) with two-stage step-up Benjamin-Krieger-Yekutieli's multiple comparison test. ND, average expression was below ~1 FPKM.
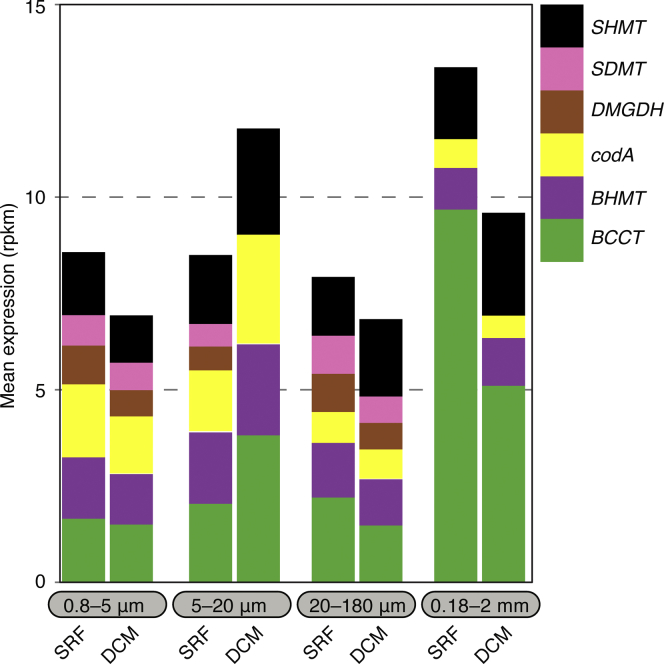
We next evaluated whether free-living populations of the microalgal symbionts actively transcribed the metabolic pathway for GB in the global ocean by interrogating a catalog of 116 million expressed eukaryotic genes of the Tara Oceans Expedition (Carradec et al., 2018) available at http://www.genoscope.cns.fr/tara/. We retrieved several unigenes with significant homology to Symbiodiniaceae genes (≥80% identity and alignment length of ≥60%) involved in GB metabolism, including BCCT (33), codA (1), DMGDH (3), SDMT (1), SHMT (6), and BHMT (31). The genes encoding for BCCT uptake and exchange system for GB were the most highly expressed among the six examined genes (Figure 4). Expression was highest in the mesoplankton size fraction (180–2000 μm)—suggested to correspond to Symbiodiniaceae in symbiosis with their host organisms (Decelle et al., 2018), especially in subsurface samples (Figure 4). In contrast, the codA gene encoding the one-step enzymatic route of GB production was highly expressed in the smaller (0.8–20 μm) size fractions (i.e., free-living Symbiodiniaceae). The expression levels of transport (BCCT) and synthesis (CodA) machineries suggest differences in the metabolism of GB between free-living and symbiotic lifestyles, with higher GB uptake potential inferred in symbiotic Symbiodiniaceae although experimental validation is needed.
Figure 4.
Expression of GB Metabolic Pathways in Free-Living Symbiodiniaceae
Bars shown mean expression in different size fractions and euphotic zone (subsurface, SRF and deep chlorophyll maximum, DCM) based on the eukaryotic unigene catalog of Tara Ocean from (Carradec et al., 2018). RPKM (reads per kilo base covered per million mapped reads) denotes normalized expression values.
A Putative Role for GB in Stress Resilience and Nitrogen Storage of Coral Metaorganisms
The “omics” evidence presented here strongly supports the metabolism of GB as a genetic facet of coral physiology and symbiotic interactions, especially given our estimates that GB comprises about 16% of the total nitrogen biomass of corals [based on the predicted biomass of corals of about 30 g C m−2 (Crossland et al., 1991), a median GB concentration in coral biomass of 6 μmol cm−2 (Hill et al., 2010), and a C:N ratio of 6.5 in coral tissues]. Despite its osmolytic properties, GB may have additional properties that supersede other osmolytes, such as DMSP. For instance, in plants GB inhibits thermal and photon stress by stabilizing extrinsic proteins vital for the photosynthetic and oxygen-evolving complex of photosystem II (Chen and Murata, 2002). A similar role is hypothesized in microalgae and corals (Hill et al., 2010), because corals located at shallower depths and locations exposed to high irradiance possess elevated pools of GB relative to colonies living in deeper waters and shaded areas (Hill et al., 2010). Moreover, although corals are osmoconformers (i.e., in osmotic equilibrium with their environment), their partnership with Symbiodiniaceae that formed at least 160 million years ago (LaJeunesse et al., 2018) probably generated osmotic constraints, whereby the coral host had to devise a suitable intracellular environment for its endosymbionts (Ochsenkühn et al., 2017).
In addition, we postulate that the thermodynamic constraints imposed by salinity (Oren, 1999) coupled with the inherent biochemical properties of GB rendered this osmolyte particularly suitable for a number of reasons. Firstly, it is among the “cheapest” osmolytes to produce in nature (Oren, 1999). Secondly, the strategy of scavenging this ubiquitous marine osmolyte using ATP-independent carriers is also metabolically less costly than de novo biosynthesis. Thirdly, it is superior to other osmolytes, including successively methylated derivatives of glycine, regarding the degree and effectiveness of offsetting salt-induced stress on enzymes (Courtenay et al., 2000, Yancey et al., 1982). Fourthly, it represents a readily available, albeit significant internal nitrogen stock, which can be degraded or transformed into other N-containing compounds such as amino acids. Likely, GB can be replaced by DMSP under nitrogen limitation (Keller et al., 1999a), so that nitrogen is utilized for essential processes such as amino acid and protein synthesis. Lastly, the metabolism of GB might be a key determinant of one-carbon metabolism supporting methyl-dependent processes (such as DNA methylation and protein synthesis). Of note, the reaction catalyzed by BHMT (Figure 1) is also not compromised by the lack of B-type vitamins (Holm et al., 2007)—which are often-limiting nutrients in the ocean (Sañudo-Wilhelmy et al., 2014). Because carbon and nitrogen metabolism are generally closely linked, we hypothesize that the symbiosis between corals and their carbohydrate-producing microalgal symbionts may have selectively enhanced the sequestration and storage of GB to support metabolic activity by maintaining a suitable osmotic environment, while serving as a reservoir for nitrogen to accommodate the growth requirements of the endosymbionts.
Corals and Reef Ecosystems Are a Sink for Dissolved GB in the Sea
It is worthwhile to note that the uptake of GB by the high-affinity membrane carriers deduced here might be effective at the ambient dissolved (picomolar range) concentrations reported in the ocean (Muslin, 2017), especially given the high affinity for GB (Km of up to 8.6 μM) in prokaryotic BCCT carriers (Farwick et al., 1995, Peter et al., 1996). However, the ingestion of plankton (Houlbreque and Ferrier-Pages, 2009) most likely comprises the main source of GB in coral tissues since heterotrophic intake (i.e., feeding) can deliver three to four times more nitrogen than dissolved sources in model coral species (Houlbreque and Ferrier-Pages, 2009). The fact that intracellular concentrations of GB make up a significant fraction (7%–23%) of the organic N in marine phytoplankton (Keller et al., 1999a, King, 1988) implies that incidental GB acquired from feeding might be important in the nitrogen economy of coral reefs. Following this notion, we reasoned that, if heterotrophic input via feeding is vital for a coral's nutritional ecology, then GB pool sizes in corals have to be placed in the context of their planktonic prey, which likely includes GB producers.
Therefore, as a first step, we profiled the biosynthetic pathways of GB in 189 completely sequenced microbial genomes [mean (±SD) completeness of 99 ± 1%] retrieved from the marine microbial reference (MarRef) genome database (Klemetsen et al., 2018) encompassing diverse species from various oceanic provinces (Table S8). The inventory of genes encoding putative synthetic enzymes for GB showed a high incidence of choline-oxidizing pathways (in 6%–∼55% of the genomes) and low occurrence of glycine-methylation-based pathways (up to ∼17%; Figure S8A), possibly reflecting high energetic costs of methylation reactions, particularly the production of the methyl donor/acceptor S-adenosylmethionine (Luka et al., 2009, Nyyssölä et al., 2000). The co-occurrence of choline-oxidizing enzyme complements in individual genomes (Figure S8B) further revealed the predominance of the BetA/BADH2 and BetA/BetB-catalyzed pathways (in ∼36% and 13%, respectively) relative to the pathway involving choline monooxygenase (CMO). Also, a low-average copy number per genome (Figure S8C) was observed for betA (1 copy; n = 71 genomes), betB (1 copy; n = 26), and BADH2 (1 copy; n = 72) in comparison to codA (2.3 copies; n = 104), where the high coefficient of variation of 67% (versus 11% in betA) around the mean suggested significant species-dependent copy variations. Considering the high incidence of co-occurrence of single-copy genes for the BetA/BADH2-catalyzed pathway in reference marine microbial genomes (Figure S8B; Table S8) implies that betA and BADH2 homologs are feasible proxies for estimating the relative abundance of GB producers from environmental metagenomes, because they have a greater likelihood of co-occurring in individual genomes. It is also noteworthy that two-thirds of the genomes with codA (55% of the genomes) also possessed the complete BetA/BADH2 pathway.
On this basis, we subsequently probed the abundance of betA and BADH2 protein-coding genes by interrogating global ocean metagenomic sequence datasets and normalized counts of retrieved genes using the mean abundance of four conserved single-copy housekeeping genes present in microbial genomes (Table S9). The two respective genes were predicted to be present in 2.4% and 1.2% of prokaryotes sampled in the Tara Oceans marine metagenomes (Sunagawa et al., 2015), which encompass picoplankton-sized microorganisms (0.22–3 μm) from the photic and epipelagic layers (Table S9). The mean of roughly 2% suggests that the environmental abundance of picoplankton producing GB is twice that of DMSP-producing microbes (Curson et al., 2017) in the pelagic ocean; however, the value could be higher, if we consider picoplankton encoding the one-step CodA-catalyzed pathway.
Based on the above estimates [i.e., a 2% proportion of GB-producing picoplankton, a cellular concentration of GB between 0.9 and 483 nM in seawater particulates >0.2 μm (Beale and Airs, 2016, Cree, 2015, Keller et al., 2004), ∼105 cells per ml, and a total biomass of microbial picoplankton in the pelagic ocean of 3.6 × 1028 cells (Whitman et al., 1998)], we project a global GB stock in pelagic marine picoplankton in the range of ∼1 to 410 × 1012 grams. Scaling these estimates to the areal cover of reefs of ∼0.1% of the world's ocean surface (Spalding et al., 2001), and assuming the global average water depth in areas occupied by reefs of 20 m, suggests that between 0.1 to 41 × 109 grams of GB is potentially distributed within picoplanktonic particulates in the waters overlaying corals.
These estimates are similar to the predicted global stock of GB in corals of about 18 × 106 to 4.64 × 109 grams, based on the GB concentration in coral tissues of 0.05–13.2 μmol cm−2 (Hill et al., 2010), the molecular weight of GB (117.15 g mol−1), and a mean areal coral reef cover of 0.3 × 106 km2 (Spalding et al., 2001). However, the predicted global stock of GB present in corals may be significantly underestimated considering that much of the GB is likely catabolized by corals and their symbionts (e.g., via dimethylglycine to serine) or translocated through coral mucus ropes into the adjacent sediments and pore waters. Thus, at the global scale, coral reef ecosystems and their reef-building corals represent a rare, albeit significant and dynamic “hot spot” of microbial-derived GB in the pelagic ocean.
Conclusions
The systematic bioinformatic framework applied here combined with existing biochemical data strongly suggests a role of marine invertebrates in the biogeochemical cycling of GB in the ocean, adding corals to the roster of sinks and organisms producing GB. Because GB is a major reservoir of nitrogen and considering the general scarcity of nitrogen in the pelagic ocean (Bristow et al., 2017), we propose that GB serves both as an osmolyte and as a potent nitrogen reserve in marine environments hosting coral reefs. Moreover, given its photoprotective role in plants including alleviating oxidative stress (Chen and Murata, 2008), we speculate further that GB serves thermo- and photoprotective roles in the coral holobiont. This in turn implicates GB in the integrity of the coral-microalgal symbiosis and the resilience of coral reef ecosystems, further reiterating the notion that coral reefs are biological hotspots, acting as sinks for dissolved organic nitrogen in oligotrophic seas. Our findings open a path for a number of testable hypotheses requiring in-depth biochemical and experimental analysis for (1) the many uncharacterized genes and pathways we elucidated and postulated to play a role in regulating GB uptake, storage, and cycling, as well as (2) the hypothesized protective and nutritional roles of GB in the metabolism of coral holobionts.
Limitations of the Study
In the current study, we examined the genetic potential of corals to metabolize GB, which is present in their tissues at high concentrations. Most of the bioinformatic analyses are based on genomic/transcriptomic data, questioning whether the inference for GB production based on homologs is sufficient in the absence of biochemical validation of the deduced enzymes. A second limiting factor in robust interpretation of our results relates to the localization of the enzyme activities within the coral holobiont and the concentrations of GB in the studied animals species. These limitations call for ratification of the pathways that we deduced to function in GB metabolism in corals and their microbial symbionts and elucidating the contribution of coral holobiont partners in shaping the dynamics of intracellular GB pools.
Resource Availability
Lead Contact
Materials Availability
All data needed to evaluate the results and conclusions are presented in the main text and/or are available in the Supplementary Materials. Additional data or scripts related to this paper are available from the corresponding authors.
Data and Code Availability
The Raw sequence data for the coral metagenomes have been deposited in NCBI under BioProject number PRJNA437202 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA437202).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dorothee Huchon (Tel Aviv University) for kindly availing the myxosporian genomes and transcriptomes and Eric Pelletier (Genoscope) for assistance with the eukaryotic gene catalog data. Additionally, we are grateful to Craig Michell for assistance with metagenomic library construction and the technical personnel at KAUST's Bioscience Core Lab for sequencing. Furthermore, we thank the Coastal & Marine Resources core laboratory team for their help in undertaking the sampling and members of the Thurber lab (Rebecca Vega Thurber, Jerome Payet, and Ryan McMinds) at Oregon State University for sampling assistance. The metagenomic sequencing project was funded through the KAUST SEED funding scheme to C.R. Voolstra.
Author Contributions
D.K.N. conceived and designed the study and performed the bioinformatic analyses. M.Z. and C.R.V. determined and provided the metagenomic data. C.M.D. provided the bioinformatic resources for the analyses. D.K.N. wrote the manuscript with contributions from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101120.
Contributor Information
David K. Ngugi, Email: david.ngugi@dsmz.de.
Christian R. Voolstra, Email: christian.voolstra@uni-konstanz.de.
Supplemental Information
The genomes are from (Robbins et al., 2019).
References
- Airs R.L., Archer S.D. Analysis of glycine betaine and choline in seawater particulates by liquid chromatography/electrospray ionization/mass spectrometry. Limnol. Oceanogr. Methods. 2010;8:499–506. [Google Scholar]
- Bashir A., Hoffmann T., Smits S.H.J., Bremer E. Dimethylglycine provides salt and temperature stress protection to Bacillus subtilis. Appl. Environ. Microbiol. 2014;80:2773–2785. doi: 10.1128/AEM.00078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten S., Simakov O., Esherick L.Y., Liew Y.J., Lehnert E.M., Michell C.T., Li Y., Hambleton E.A., Guse A., Oates M.E. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc. Natl. Acad. Sci. U S A. 2015;112:11893–11898. doi: 10.1073/pnas.1513318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay D.C., Turner R.J. Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants. J. Bacteriol. 2012;194:5941–5948. doi: 10.1128/JB.00666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale R., Airs R. Quantification of glycine betaine, choline and trimethylamine N-oxide in seawater particulates: minimisation of seawater associated ion suppression. Anal. Chim. Acta. 2016;938:114–122. doi: 10.1016/j.aca.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Bristow L.A., Mohr W., Ahmerkamp S., Kuypers M.M.M. Nutrients that limit growth in the ocean. Curr. Biol. 2017;27:R474–R478. doi: 10.1016/j.cub.2017.03.030. [DOI] [PubMed] [Google Scholar]
- Burg M.B., Ferraris J.D. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 2008;283:7309–7313. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carradec Q., Pelletier E., Da Silva C., Alberti A., Seeleuthner Y., Blanc-Mathieu R., Lima-Mendez G., Rocha F., Tirichine L., Labadie K. A global ocean atlas of eukaryotic genes. Nat. Commun. 2018:1–13. doi: 10.1038/s41467-017-02342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.H.H., Murata N. Glycine betaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13:499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Chen T.H.H., Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002;5:250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- Courtenay E.S., Capp M.W., Anderson C.F., Record M.T. Vapor pressure osmometry studies of osmolyte−protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry. 2000;39:4455–4471. doi: 10.1021/bi992887l. [DOI] [PubMed] [Google Scholar]
- Cree C. University of Plymouth; 2015. Distributions of glycine Betaine and the Methylamines in Coastal Waters: Analytical Developments and a Seasonal Study. [Google Scholar]
- Crossland C.J., Hatcher B.G., Smith S.V. Role of coral reefs in global ocean production. Coral Reefs. 1991;10:55–64. [Google Scholar]
- Curson A.R.J., Liu J., Martínez A.B., Green R.T., Chan Y., Carrión O., Williams B.T., Zhang S.-H., Yang G.-P., Page P.C.B. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2017;2:17009. doi: 10.1038/nmicrobiol.2017.9. [DOI] [PubMed] [Google Scholar]
- Decelle J., Carradec Q., Pochon X., Henry N., Romac S., Mahé F., Dunthorn M., Kourlaiev A., Voolstra C.R., Wincker P., de Vargas C. Worldwide occurrence and activity of the reef-building coral symbiont Symbiodinium in the open ocean. Curr. Biol. 2018;28:3625–3633.e3. doi: 10.1016/j.cub.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Derelle R., Torruella G., Torruella G., Klimeš V., Klimeš V., Brinkmann H., Brinkmann H., Kim E., Kim E., Vlček Č. Bacterial proteins pinpoint a single eukaryotic root. Proc. Natl. Acad. Sci. U S A. 2015;112:E693–E699. doi: 10.1073/pnas.1420657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwick M., Siewe R.M., Kramer R. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J. Bacteriol. 1995;177:4690–4695. doi: 10.1128/jb.177.16.4690-4695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebser B., Pohnert G. Synchronized regulation of different zwitterionic metabolites in the osmoadaption of phytoplankton. Mar. Drugs. 2013;11:2168–2182. doi: 10.3390/md11062168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M., Carter V.L., Ly S., Andrell R.M., Yancey P.H., Leong J.A.C., Kleinhans F.W. Analysis of internal osmolality in developing coral larvae, Fungia scutaria. Physiol. Biochem. Zool. 2010;83:157–166. doi: 10.1086/648484. [DOI] [PubMed] [Google Scholar]
- Hill R.W., Armstrong E.J., Florn A.M., Li C., Walquist R.W., Edward A. Abundant betaines in giant clams (Tridacnidae) and western Pacific reef corals, including study of coral betaine acclimatization. Mar. Ecol. Prog. Ser. 2017;576:27–41. [Google Scholar]
- Hill R.W., Li C., Jones A.D., Gunn J.P., Frade P.R. Abundant betaines in reef-building corals and ecological indicators of a photoprotective role. Coral Reefs. 2010;29:869–880. [Google Scholar]
- Holm P.I., Hustad S., Ueland P.M., Vollset S.E., Grotmol T., Schneede J. Modulation of the homocysteine-betaine relationship by methylenetetrahydrofolate reductase 677 C->T genotypes and B-vitamin status in a large-scale epidemiological study. J. Clin. Endocrinol. Metab. 2007;92:1535–1541. doi: 10.1210/jc.2006-1471. [DOI] [PubMed] [Google Scholar]
- Houlbreque F., Ferrier-Pages C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009;84:1–17. doi: 10.1111/j.1469-185X.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- Julián-Sánchez A., Riveros-Rosas H., Martinez-Castilla L.P., Velasco-Garcia R., Muñoz-Clares R.A. Phylogenetic and structural relationships of the betaine aldehyde dehydrogenases. In: Henry W., Edmund M., Ronald L., Bryce P., editors. Enzymology and Molecular Biology of Carbonyl Metabolism. Purdue University Press; 2007. pp. 64–76. [Google Scholar]
- Kageyama H., Tanaka Y., Takabe T. Biosynthetic pathways of glycine betaine in Thalassiosira pseudonana; functional characterization of enzyme catalyzing three-step methylation of glycine. Plant Physiol. Biochem. 2018;127:248–255. doi: 10.1016/j.plaphy.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Keller M.D., Kiene R.P., Matrai P.A., Bellows W.K. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. I. Batch cultures. Mar. Biol. 1999;135:237–248. [Google Scholar]
- Keller M.D., Kiene R.P., Matrai P.A., Bellows W.K. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. II. N-limited chemostat cultures. Mar. Biol. 1999;135:249–257. [Google Scholar]
- Keller M.D., Matrai P.A., Kiene R.P., Bellows W.K. Responses of coastal phytoplankton populations to nitrogen additions: dynamics of cell-associated dimethylsulfoniopropionate (DMSP), glycine betaine (GBT), and homarine. Can. J. Fish. Aquat. Sci. 2004;61:685–699. [Google Scholar]
- Kiene R.P., Williams L., Walker J.E. Seawater microorganisms have a high affinity glycine betaine uptake system which also recognizes dimethylsulfoniopropionate. Aquat. Microb. Ecol. 1998;15:39–51. [Google Scholar]
- King G.M. Distribution and metabolism of quaternary amines in marine sediments. In: Blackburn T.H., Sorensen J., editors. Nitrogen Cycling in Coastal Marine Environments. John Wiley and Sons Ltd; 1988. pp. 143–173. [Google Scholar]
- Kirch H.-H., Bartels D., Wei Y., Schnable P.S., Wood A.J. The ALDH gene superfamily of Arabidopsis. Trends Plant Sci. 2004;9:371–377. doi: 10.1016/j.tplants.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Klemetsen T., Raknes I.A., Fu J., Agafonov A., Balasundaram S.V., Tartari G., Robertsen E., Willassen N.P. The MAR databases: development and implementation of databases specific for marine metagenomics. Nucleic Acids Res. 2018;46:D692–D699. doi: 10.1093/nar/gkx1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Wang C.-C., Chuang M.-J., Wu Y.-C., Lee Y.-C. Effects of substrate and potassium on the betaine-synthesizing enzyme glycine sarcosine dimethylglycine N-methyltransferase from a halophilic methanoarchaeonMethanohalophilus portucalensis. Res. Microbiol. 2006;157:948–955. doi: 10.1016/j.resmic.2006.08.007. [DOI] [PubMed] [Google Scholar]
- LaJeunesse T.C., Parkinson J.E., Gabrielson P.W., Jeong H.J., Reimer J.D., Voolstra C.R., Santos S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018;28:2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Lambou K., Pennati A., Valsecchi I., Tada R., Sherman S., Sato H., Beau R., Gadda G., Latge J.P. Pathway of glycine betaine biosynthesis in Aspergillus fumigatus. Eukaryot. Cell. 2013;12:853–863. doi: 10.1128/EC.00348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidbury I., Kimberley G., Scanlan D.J., Murrell J.C., Chen Y. Comparative genomics and mutagenesis analyses of choline metabolism in the marine Roseobacter clade. Environ. Microbiol. 2015;17:5048–5062. doi: 10.1111/1462-2920.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka Z., Mudd S.H., Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J. Biol. Chem. 2009;284:22507–22511. doi: 10.1074/jbc.R109.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin O. Oregon State University; 2017. Quantification of Osmolytes in the Sargasso Sea Surface Layer Water Column. [Google Scholar]
- Neave M.J., Apprill A., Ferrier-Pages C., Voolstra C.R. Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl. Microbiol. Biotechnol. 2016;100:8315–8324. doi: 10.1007/s00253-016-7777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave M.J., Michell C.T., Apprill A., Voolstra C.R. Endozoicomonas genomes reveal functional adaptation and plasticity in bacterial strains symbiotically associated with diverse marine hosts. Sci. Rep. 2017;7:40579. doi: 10.1038/srep40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave M.J., Rachmawati R., Xun L., Michell C.T., Bourne D.G., Apprill A., Voolstra C.R. Differential specificity between closely related corals and abundant Endozoicomonas endosymbionts across global scales. ISME J. 2017;11:186–200. doi: 10.1038/ismej.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyyssölä A., Kerovuo J., Kaukinen P., vonWeymarn N., Reinikainen T. Extreme halophiles synthesize betaine from glycine by methylation. J. Biol. Chem. 2000;275:22196–22201. doi: 10.1074/jbc.M910111199. [DOI] [PubMed] [Google Scholar]
- Nyyssölä A., Reinikainen T., Leisola M. Characterization of glycine sarcosine N-methyltransferase and sarcosine dimethylglycine N-methyltransferase. Appl. Environ. Microbiol. 2001;67:2044–2050. doi: 10.1128/AEM.67.5.2044-2050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenkühn M.A., Röthig T., D’Angelo C., Wiedenmann J., Voolstra C.R. The role of floridoside in osmoadaptation of coral-associated algal endosymbionts to high-salinity conditions. Sci. Adv. 2017;3:e1602047. doi: 10.1126/sciadv.1602047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999;63:334–348. doi: 10.1128/mmbr.63.2.334-348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C., Koshy C., Ressl S., Nicklisch S., mer R.K.A., Ziegler C. Substrate specificity and ion coupling in the Na+/betaine symporter BetP. EMBO J. 2011;30:1221–1229. doi: 10.1038/emboj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H., Burkovski A., Kramer R. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J. Bacteriol. 1996;178:5229–5234. doi: 10.1128/jb.178.17.5229-5234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressl S., Terwisscha van Scheltinga A.C., Vonrhein C., Ott V., Ziegler C. Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- Robbins S.J., Singleton C.M., Chan C.X., Messer L.F., Geers A.U., Ying H., Baker A., Bell S.C., Morrow K.M., Ragan M.A. A genomic view of the reef-building coral Porites lutea and its microbial symbionts. Nat. Microbiol. 2019;4:2090–2100. doi: 10.1038/s41564-019-0532-4. [DOI] [PubMed] [Google Scholar]
- Roesser M., Müller V. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 2001;3:743–754. doi: 10.1046/j.1462-2920.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Rohwer F., Seguritan V., Azam F., Knowlton N. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 2002;243:1–10. [Google Scholar]
- Sañudo-Wilhelmy S.A., Gómez-Consarnau L., Suffridge C., Webb E.A. The role of B vitamins in marine biogeochemistry. Ann. Rev. Mar. Sci. 2014;6:339–367. doi: 10.1146/annurev-marine-120710-100912. [DOI] [PubMed] [Google Scholar]
- Spalding M.D., Ravilious C., Green E.P. University of California Press; 2001. World Atlas of Coral Reefs. [Google Scholar]
- Spielmeyer A., Gebser B., Pohnert G. Dimethylsulfide sources from microalgae: improvement and application of a derivatization-based method for the determination of dimethylsulfoniopropionate and other zwitterionic osmolytes in phytoplankton. Mar. Chem. 2011;124:48–56. [Google Scholar]
- Spielmeyer A., Pohnert G. Influence of temperature and elevated carbon dioxide on the production of dimethylsulfoniopropionate and glycine betaine by marine phytoplankton. Mar. Environ. Res. 2012;73:62–69. doi: 10.1016/j.marenvres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Stephens-Camacho N.A., Muhlia-Almazan A., Sanchez-Paz A., Rosas-Rodiguez J.A. Surviving environmental stress: the role of betaine aldehyde dehydrogenase in marine crustaceans. J. Invertebr. Pathol. 2015;12:66–74. [Google Scholar]
- Sun J., Steindler L., Thrash J.C., Halsey K.H., Smith D.P., Carter A.E., Landry Z.C., Giovannoni S.J. One carbon metabolism in SAR11 pelagic marine bacteria. PLoS One. 2011;6:e23973. doi: 10.1371/journal.pone.0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa S., Coelho L.P., Chaffron S., Kultima J.R., Labadie K., Salazar G., Djahanschiri B., Zeller G., Mende D.R., Alberti A. Structure and function of the global ocean microbiome. Science. 2015;348:1261359. doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]
- Tandon K., Lu C.-Y., Chiang P.-W., Wada N., Yang S.-H., Chan Y.-F., Chen P.-Y., Chang H.-Y., Chiou Y.-J., Chou M.-S. Comparative genomics: dominant coral-bacterium Endozoicomonas acroporae metabolizes dimethylsulfoniopropionate (DMSP) ISME J. 2020;55:1290–1303. doi: 10.1038/s41396-020-0610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D.T. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 2000;24:263–290. doi: 10.1111/j.1574-6976.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Whitman W.B., Coleman D.C., Wiebe W.J. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. U S A. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P.H., Clark M.E., Hand S.C., Bowlus R.D., Somero G.N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yancey P.H., Heppenstall M., Ly S., Andrell R.M., Gates R.D., Carter V.L., Hagedorn M. Betaines and dimethylsulfoniopropionate as major osmolytes in Cnidaria with endosymbiotic dinoflagellates. Physiol. Biochem. Zool. 2010;83:167–173. doi: 10.1086/644625. [DOI] [PubMed] [Google Scholar]
- Ziegler C., Bremer E., Krämer R. The BCCT family of carriers: from physiology to crystal structure. Mol. Microbiol. 2010;78:13–34. doi: 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The genomes are from (Robbins et al., 2019).
Data Availability Statement
The Raw sequence data for the coral metagenomes have been deposited in NCBI under BioProject number PRJNA437202 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA437202).