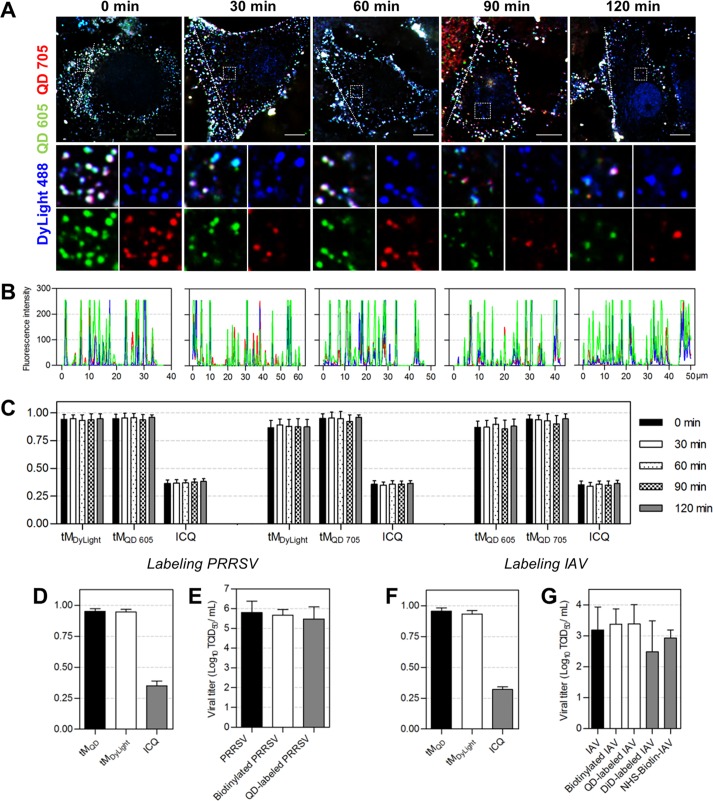
FIG 3.
Stability and universality of the QD labeling method. (A) JEV was dually labeled with SA-QD 605 (green) and SA-QD 705 (red). Vero cells infected by the double-labeled viruses for 0, 30, 60, 90, and 120 min were fixed and stained with anti-E-DyLight 488 (blue). Bars, 10 μm. (B) Line profiles showing distributions of the fluorescence signals on the lines in panel A. (C) The tMDyLight/tMQD 605/ICQ, tMDyLight/tMQD 705/ICQ, and tMQD 605/tMQD 705/ICQ values calculated from 30 randomly selected cells. (D to G) PRRSV and IAV were labeled with QDs using the lipid-specific method. (D and F) The tMQD, tMDyLight, and ICQ values calculated from 30 cells. (E and G) Titers of viruses, biotinylated viruses, QD-labeled viruses, DiD-labeled viruses, and viruses covalently biotinylated with NHS-biotin (n = 3 for PRRSV and 5 for IAV).