Abstract
Introduction
Observational data has suggested a link between vitamin D deficiency and coronary heart disease (CHD). However, randomized controlled trials (RCTs) have failed to show benefit. The objective of this study is to analyze the RCTs investigating vitamin D supplementation and the risk of CHD and stroke.
Methods
All RCTs that compared vitamin D supplementation to placebo and evaluated nonfatal myocardial infarction (MI), cardiac mortality, stroke and CHD events (a composite of cardiac mortality, MI, unstable angina and revascularization) were included. Rate ratios (RR) were calculated for each endpoint and to test for heterogeneity of treatment effect (HTE) the Chi2 and I2 tests were used for younger vs. older participants, shorter vs. longer trial duration, vitamin D supplements with vs. without calcium, and daily vs. monthly dosages of vitamin D. A meta-regression was performed with baseline vitamin D concentration as the covariate.
Results
22 RCTs were identified (n = 83,200). Vitamin D supplementation had no effect on nonfatal MI (RR 0.98, 95% confidence interval (CI) 0.89–1.08), cardiac death (RR 0.94, CI 0.84–1.06), CHD events (RR 1.00, CI 0.91–1.10), or stroke (RR, 0.97, CI 0.9–1.03). When performing the meta-regression with baseline circulating 25-hydroxyvitamin D (25(OH)D) concentrations as the covariate, vitamin D supplementation’s treatment effect on CVD outcomes was not associated with baseline 25(OH)D.
Conclusion
Vitamin D did not reduce CHD and stroke. A linear relationship does not exist between baseline 25(OH)D and vitamin D supplementation’s effect on CVD. Vitamin D levels should be checked and repleted in those with an absolute indication.
Keywords: Vitamin D supplementation, Cardiovascular Disease, Myocardial Infarction, Stroke
1. Introduction
Précis: Vitamin D supplementation does not alter the risk of coronary heart disease or stroke.
Vitamin D’s role in bone metabolism is well-known. More recently it has been suggested that vitamin D supplementation may have extra-skeletal benefits including beneficial effects on cardiovascular disease (CVD) [1]. This notion is supported by numerous observational and animal studies which suggest a link between vitamin D deficiency and CVD. As an example, among 18,000 healthy male participants in the Health Professionals Follow Up Study, those with 25-hydroxyvitamin D (25(OH)D) levels less than 15 ng/mL had a relative risk of 2.42 (95% CI, 1.53–3.84) compared to those with levels above 30 ng/ml for myocardial infarction (MI) after 10 years of follow-up [2]. Furthermore, the interest surrounding vitamin D supplementation is supported by biologic mechanisms which suggest that vitamin D may have beneficial effects on the cardiovascular system. The liganded vitamin D receptor (VDR) has been shown to inhibit vascular smooth muscle cell proliferation, [3] decrease blood pressure by a down regulation of the renin-angiotensin-aldosterone system, [4] and decrease inflammation through a downregulation of interleukin-6 and interleukin-8 [5]. Furthermore, the VDR has been found to be expressed in the cardiovascular system including the walls of coronary arteries and atherosclerotic coronary artery plaques [6]. It has also been suggested that low levels of 25(OH)D may lead to the development of vascular calcification [7]. Prior meta-analyses have studied the relationship between 25(OH)D and CVD [8], [9]. Zhang et al., found a significant inverse relationship between baseline 25(OH)D and CVD events and CVD mortality. The dose–response analysis revealed a J-shaped association between 25(OH)D and total CVD events suggesting that those with much lower 25(OH)D were at a greater risk of CVD [9]. This non-linear or J-shaped association between 25(OH)D and CVD has been observed in additional studies which suggests a threshold concentration of vitamin D for which CVD risk increases [10], [11].
Despite observational data suggesting a potential benefit and a link between vitamin D deficiency and CVD, to date there has been few randomized controlled trials (RCTs) which test vitamin D’s effect on CVD risk in a pre-specified manner. In addition, it is unknown if supplementing vitamin D in deficient patients improves CVD outcomes. Therefore, the objective of this study is to perform a systematic review and meta-analysis to analyze CVD outcomes in the available RCTs testing various doses of vitamin D supplementation in adults. In addition, the secondary aim of this study is to perform a meta-regression analysis using baseline 25(OH)D concentration as the covariate to test if the effect of vitamin D supplementation on coronary heart disease (CHD) and stroke is altered by varying plasma vitamin D concentrations in a linear fashion.
2. Methods:
The Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement was followed [11], [12]. A search was performed to identify all prospective RCTs which tested vitamin D supplementation and analyzed various health outcomes. Relevant English language articles were found by searching Medline and Embase with search terms corresponding to “vitamin D supplementation”, “cardiovascular disease”, and “stroke”. In addition to searching the above databases, previously published meta-analyses were searched as a source for RCTs. The references of all articles were also searched. RCTs testing either daily or monthly doses of vitamin D (25(OH)D) supplementation were considered for inclusion. Both 25(OH)D2 and 25(OH)D3 were considered for inclusion. RCTs which compared vitamin D supplementation to placebo were eligible for inclusion. If one of the primary endpoints of this meta-analysis were reported, the trial was considered for inclusion. The primary endpoints for this study included CHD events (a composite of cardiac mortality, nonfatal myocardial infarction and revascularization procedure (CABG or PCI), nonfatal MI, stroke (either hemorrhagic or ischemic), and cardiac death (sudden cardiac death, any death presumed due to a cardiovascular cause).
Two authors (MN, GK) searched all titles and abstracts. All articles were evaluated by both authors to assess if the study meets inclusion criteria. Data were independently extracted for the RCTs in a standardized manner. Regardless of the pre-specified outcome of the trial, if one of the primary endpoints of this meta-analysis is reported, the trial was included. The average age and number of participants at trial baseline was collected. The average follow-up time and formulation of 25(OH)D was recorded. All studies were assessed for bias using the Cochrane Handbook for Systematic Review of Interventions by two authors [13]. Bias was assessed on predetermined criteria including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other (i.e. predetermined outcome of trial, financial consideration) (Supplemental Table 1).
The primary analysis was conducted with the Mantel-Haenszel method. Summary rate ratios (RR) with 95% confidence interval (CI) will be calculated using a random effects model. Examination of heterogeneity across all studies will be performed using Q statistics and I2. The 95% CIs will be estimated using binominal distribution. Multiple subgroup analyses were performed. A subgroup analysis was performed based on trial duration (less than 6 months versus >6 months). Also, a subgroup analysis based on average age at trial baseline was performed (less than 60 years vs > 60 years). Publication bias was visually assessed using funnel plots. Statistical analyses were conducted with Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. A random effects meta-regression was performed for the CHD events, nonfatal MI and stroke outcome using mean baseline plasma vitamin D concentration as the covariate. A meta-regression was not performed on the cardiac death outcome as only 2 RCTs which reported a cardiac death also reported mean baseline trial plasma vitamin D concentrations. Meta-regression linear graphs were created by plotting our moderator variable (baseline plasma vitamin D concentration) on the x-axis and the treatment effect size of vitamin D on the y-axis (the log of the rate ratio of vitamin D’s treatment effect on each outcome for each RCT). Each circle on the figure represents an included RCT and the size of the circle is proportional to the weight of each study in the regression model. The darker line in the center represents the regression line and the outer lighter colored lines represent the 95% CI. The following statistical tests were utilized in the meta-regression: Tau2 which estimates the true variance among RCTs, I2 which represents the ratio of heterogeneity to total observed variation in the RCTs and R2 index which is the proportion of between study variance explained by the moderator. Also, regression coefficients were calculated and describe how vitamin D’s treatment effect on each outcome will change with a unit change in the moderator variable. The meta-regression was performed using Comprehensive Meta-Analysis Version 3, Biostat, Englewood, NJ, 2013 Table 1.
Table 1.
Baseline Characteristics Table. This table includes the baseline characteristics of the included randomized controlled trials which includes trial design, follow up time, number of participants, mean age, dose of vitamin D, frequency of vitamin D, primary outcome and if reported baseline plasma vitamin D concentration of participants.
| Name, Year | Design | Follow- Up (years) | Number of Participants (mean age in years) | Dose and Compound | Primary Outcome | Baseline 25-hydroxyvitamin D Concentration |
|---|---|---|---|---|---|---|
| Baron 2016 | Double-blind, placebo controlled | 3 or 5 years | 2259 patients with prior colonic adenoma (58 years) | 1000 IU of vitamin D daily | Adenoma occurrence | 24.4 ng/mL |
| Brazier 2005 | Double-blind, placebo controlled | 1 year | 192 women (74.6 years) | 500 mg calcium carbonate and vitamin D 400 IU taken twice daily | Renal function | 7.2 ng/mL |
| Ford 2014 | Double-blind, placebo controlled | 2 years | 5292 participants with prior low trauma fracture (77.5 years) | 800 IU of vitamin D daily | Fracture | n/a |
| Gallagher 2012 | Double-blind placebo controlled | 1 year | 163 postmenopausal women (67 years) | 400, 800, 1600, 240, 3200, 4000, 4800 IU/ daily or placebo | 25(OH)D and PTH levels | 7.6 ng/mL |
| Gulseth 2017 | Double-blind, placebo controlled | 0.5 year | 62 men and women with type 2 diabetes and vitamin D deficiency (55.6 years) | 400,000 IU oral vitamin D3 or placebo. Those randomized to the vitamin D group received an additional 200,000 IU if 25(OH)D level was less than 100 nmol/L after 4 weeks. | Insulin sensitivity | 15.2 ng/ml |
| Inkovaara 1983 | Double-blind, placebo controlled | 9 months | 87 patients (79.5 years) | 1000 IU/day of vitamin D or placebo | Bone fracture | n/a |
| Jackson 2006 | Double-blind, placebo controlled | 7 years | 36,282 postmenopausal women (62.4 years) | 400 IU of vitamin D daily or placebo | Hip Fracture | n/a |
| Jin 2016 | Double-blind, placebo controlled | 2 years | 413 patients with symptomatic knee osteoarthritis (63.2 years) | 50,000 IU of vitamin D per month vs. placebo | Change in tibial cartilage volume, change in the Western Ontario and McMaster Universities Arthritis Index pain score | 17.48 ng/ml |
| Jorde 2016 | Double-blind, placebo controlled | 5 years | 511, patients with prediabetes (62 years) | 20,000 IU/week or placebo | Progression to type 2 diabetes | 24.0 ng/ml |
| Komulainen 1999 | Double-blind, placebo controlled | 5 years | 343 women (52.6 years) | 300 IU daily and 100 IU daily or placebo | Bone Mineral Density | n/a |
| Matrineau 2015 (ViDiAs) | Double-blind, placebo controlled | 1 year | 250 adults with asthma (48 years) | 3 mg of vitamin D3 or placebo | Time to first asthma exacerbation and time to first upper respiratory tract infection | 19.8 ng/mL |
| Matrineau 2015 (ViDiFlu) | Double blind, placebo controlled | 1 year | 240 patients (67.1 years) | 2.4 mg of vitamin D every 2 months + 10 µg daily or 3 mg every 2 months vs. placebo | Time to first acute respiratory infection | 17.2 ng/mL |
| Miskulin 2016 | Double-blind, placebo controlled | 0.5 year | 276 vitamin D deficient patients with end-stage renal disease (61.1 years) | 50,000 IU weekly for 6 months | Change in epotien dose | 16.4 ng/mL |
| Prince 2008 | Double-blind, placebo controlled | 1 year | 302 vitamin D deficient women with a history of fall in the previous year (77.2 years) | 1000 IU per day of ergocalciferol | Falls | 17.9 ng/mL |
| Sanders 2010 | Double-blind, placebo controlled | 3–5 years | 2256 women (76 years) | 500,000 IU yearly of vitamin D | Falls and fractures | 19.6 ng/mL |
| Scragg 2017 | Double-blind, placebo controlled | 3.3 years | 5108 patients (65.9 years) | 200,000 IU initial dose followed by 100,000 IU q4 weeks | CVD and death | 25.4 ng/mL |
| Trivedi 2003 | Double-blind, placebo controlled | 5 years | 2686 participants (74.8 years) | 100,000 IU of vitamin D every 4 months over 5 years | Fracture incidence and total mortality | n/a |
| VITAL Trial | Double-blind, placebo controlled | 5.3 years | 25,871 patients (67.1 years) | 2000 IU/day of vitamin D3 versus placebo | Major cardiovascular events and invasive cancer of any kind | 30.8 mg/mL (measured in 61% of baseline population) |
| Witham 2013 | Double-blind, placebo controlled | 1 year | 159 participants with hypertension (77 years) | 100,000 IU of vitamin D every 3 months for 1 year | Change in office blood pressure | 18 ng/mL |
| Witham 2010 | Double-blind, placebo controlled | 0.38 year | 105 patients with systolic heart failure (79.8 years) | 100,000 IU at baseline at and at 10 weeks vs. placebo | 6 min walk test | 8.8 ng/mL |
| Zitterman 2017 | Double blind, placebo controlled | 3 year | 400 patients with heart failure (55 years) | 4000 IU/day of vitamin D versus placebo | All-cause mortality | 13.2 ng/mL |
3. Results
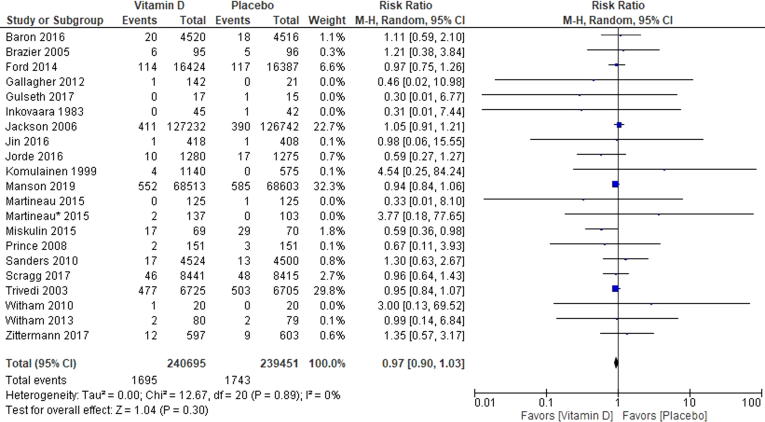
3.1. All coronary heart disease events
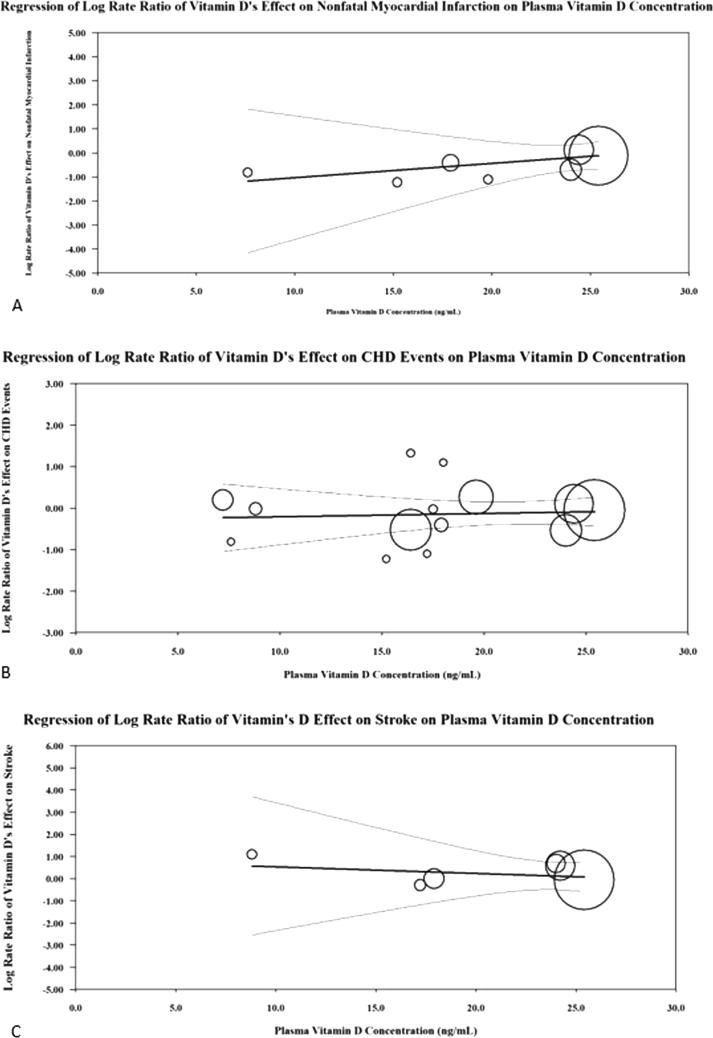
Among trials that reported a CHD event (n = 21) there were 83,093 participants [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. The weighted mean age was 65.7 years. The mean follow-up duration was 2.6 years. Among trials that reported mean baseline 25(OH)D concentrations (n = 14) the mean concentration among all trials was 17.5 ng/ml with a range between 7.2 and 25.4 ng/ml [14], [15], [17], [20], [21], [23], [24], [25], [26], [27], [28], [31], [32], [33]. There was no evidence of statistical heterogeneity among all of the RCTs (Chi2 = 12.67, p = 0.89, I2 = 0%) (Fig. 1). There was no visual evidence of publication bias when viewing the funnel plot (Supplemental Fig. 3). Among participants randomized to vitamin D 2203/41,664 (5.29%) experienced a CHD event and 2194/41,429 (5.3%) in the control group experienced a CHD event (RR 0.97 [95%CI, 0.90–1.03]) (Fig. 1). When performing the test for subgroup differences there is no heterogeneity of treatment effect based on mean age at trial baseline (Chi2 = 0.66, p = 0.42, I2 = 0%), longer vs. shorter trial duration (Chi2 = 1.22, p = 0.27, I2 = 18%), daily vs. nondaily vitamin D supplements (Chi2 = 3.02, p = 0.08, I2 = 66.9%). However, when comparing trials which test vitamin D supplements with (RR 1.09, 95%CI 0.99–1.19) versus without calcium (RR 0.94, 95%CI 0.87–1.01) there was statistically significant heterogeneity of treatment effect (Chi2 = 6.86, p = 0.02, I2 = 82.9%) (Supplemental Figure 4). When performing the random effects meta-regression for CHD events, there was no relationship between plasma vitamin D concentration and the log rate ratio of vitamin D’s treatment effect on CHD events [coefficient = 0.008 (95% CI −0.05–0.06), Tau2 = 0.00, I2 = 0%, and R2 = 0.00] (Fig. 3).
Fig. 1.
Forest Plot for Coronary Heart Disease Events. This Forrest plot represents the rate ratio of coronary heart disease outcomes in those participants randomized to vitamin D supplementation and placebo. Coronary heart disease events in a composite outcome including cardiac mortality, nonfatal myocardial infarction and coronary artery revascularization procedure.
Fig. 3.
This figure represents the meta-regression analysis performed for coronary heart disease events (A), nonfatal myocardial infarction (B) and stroke (C) with mean baseline vitamin D concentration (ng/mL) as the covariate.
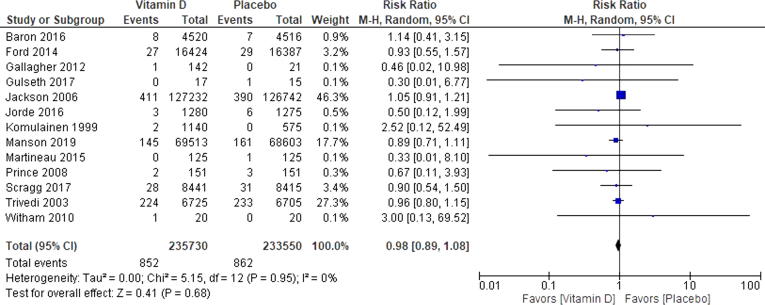
3.2. Nonfatal myocardial infarction
Among trials that reported a nonfatal MI (n = 13) there were 79,234 participants [14], [16], [17], [19], [26], [34], [21], [22], [23], [28], [29], [30], [31]. The weighted mean age was 65.5 years. The mean follow-up duration was 3.2 years. Among trials that reported mean baseline 25(OH)D concentrations the mean among all trials was 19 ng/ml with a range between 8.8 and 25.4 ng/mL [14], [17], [21], [23], [26], [28], [31], [34]. There was no evidence of statistical heterogeneity among all of the RCTs (Chi2 = 5.15, p = 0.95, I2 = 0%) (Fig. 2). There was no visual evidence of publication bias when viewing the funnel plot (Supplemental Figure 5). Among participants randomized to vitamin D 852/39,773 (2.14%) experienced a CHD event and 862/39,461 (2.18%) in the control group experienced a nonfatal MI (RR 0.98 [95%CI, 0.89–1.08]) (Fig. 2). When performing the test for subgroup differences there is no heterogeneity of treatment effect based on mean age at trial baseline (Chi2 = 0.00, p = 0.97, I2 = 0%), longer vs. shorter trial duration (Chi2 = 0.24, p = 0.62, I2 = 0%), daily vs. nondaily dosages of vitamin D (Chi2 = 0.35, p = 0.55, I2 = 0%), and vitamin D supplements with versus without calcium (Chi2 = 1.53, p = 0.22, I2 = 34.8%). When performing the random effects meta-regression for nonfatal MI, there was no relationship between plasma vitamin D concentration and the log rate ratio of vitamin D’s treatment effect on nonfatal MI [coefficient = 0.06 (95% CI −0.12–0.02), Tau2 = 0.00, I2 = 0%, and R2 = 0.00] (Fig. 3).
Fig. 2.
Forest plot for Nonfatal Myocardial Infarction. This Forrest plot represents the rate ratio of a nonfatal myocardial infarction in those participants randomized to vitamin D supplementation or placebo.
3.3. Cardiac death
Among trials that reported a cardiac death there were 75,726 participants [16], [18], [19], [33], [28], [29], [30]. The weighted mean age of participants was 65.7 years. The mean follow-up duration was 3.8 years. Among the two trials that reported mean baseline 25(OH)D concentrations the mean among all trials was 19.3 ng/ml [28], [33]. There was some evidence of nonsignificant heterogeneity among all of the RCTs (Chi2 = 6.18, p = 0.40, I2 = 3%) (Supplemental Fig. 2). There was no visual evidence of publication bias when viewing the funnel plot (Supplemental Figure 6). Among participants randomized to vitamin D 589/37,899 (1.6%) experienced a cardiac death and 623/37,827 (1.7%) in the control group experienced a cardiac death (RR 0.94 [95%CI, 0.84–1.06]). When performing the test for subgroup differences there is no heterogeneity of treatment effect based on vitamin D supplements with versus without calcium (Chi2 = 1.10, p = 0.29, I2 = 9.1%). A meta-regression for the cardiac death outcome was unable to be performed due to only 2 RCTs reporting mean baseline vitamin D concentrations.
3.4. Stroke
Among trials that reported a stroke (n = 13) there were 79,245 participants [14], [16], [18], [19], [21], [22], [24], [26], [28], [29], [30], [31], [32]. The weighted mean age was 65.6 years. The mean follow-up duration was 3.1 years. Among trials that reported mean baseline 25(OH)D concentrations the mean among all trials was 19.4 ng/ml with a range of 8.8–25.4 ng/mL [14], [21], [24], [26], [28], [31], [32]. There was no evidence of statistical heterogeneity among all of the RCTs (Chi2 = 6.56, p = 0.89, I2 = 0%) (Supplemental Fig. 3). There was no visual evidence of publication bias when viewing the funnel plot (Supplemental Figure 7). Among participants randomized to vitamin D 825/39,735 (2.1%) experienced a stroke and 818/39,510 (2.1%) in the control group experienced a stroke (RR 1.00 [95%CI, 0.91–1.10]) (Supplemental Fig. 3). When performing the test for subgroup differences there is no heterogeneity of treatment effect based on mean age at trial baseline (Chi2 = 1.42, p = 0.23, I2 = 29.8%), longer vs. shorter trial duration (Chi2 = 1.08, p = 0.30, I2 = 7.4%), daily vs. nondaily dosages of vitamin D (Chi2 = 0.20, p = 0.66, I2 = 0%), and vitamin D supplements with versus without calcium (Chi2 = 0.00, p = 0.96, I2 = 0%). When performing the random effects meta-regression for stroke, there was no relationship between plasma vitamin D concentration and the log rate ratio of vitamin D’s treatment effect on stroke [coefficient = -0.03 (95% CI −0.23–0.17), Tau2 = 0.00, I2 = 0%, and R2 = 0.00] (Fig. 3).
4. Discussion
This meta-analysis found no reduction in nonfatal MI, cardiac death, CHD events and stroke in participants randomized to vitamin D supplementation of varying doses. When performing the test for subgroup differences for each outcome studied the only statistically significant finding was found when comparing trials testing vitamin D alone versus those testing vitamin D plus calcium for CHD events. However, the reduction in CHD events among participants randomized to vitamin D alone did not meet statistical significance. Furthermore, the meta-regression using baseline 25(OH)D concentration as the covariate did not find any relationship between 25(OH)D and vitamin D's effect on CHD events, nonfatal MI or stroke. There was no effect of vitamin D supplementation on CVD risk as mean baseline vitamin D levels decreased in the individual RCTs. This could have been determined based on the lack of heterogeneity in the primary analysis; however, the meta-regression was performed to highlight this finding.
Our results are consistent with a 2014 meta-analysis from Ford et al. Among twenty-one RCTs with 13,033 participants, vitamin D supplementation was not found to reduce the risk of cardiac failure (hazard ratio (HR) 0.82, 95% CI 0.58–1.15), MI (HR 0.96, 95% CI 0.83–1.10) or stroke (HR 1.07, 95% CI 0.91–1.29) [35]. A more recent meta-analysis of RCTs by Barbarawi et al., including > 83,000 participants found that vitamin D supplementation compared to placebo did not reduce major adverse cardiovascular events (RR 1.00, 95%CI 0.95–1.06) or all-cause mortality (RR 0.97, 95%CI 0.93–1.02) [36]. This was consistent with an additional meta-analysis of RCTs which found no reduction in MI, stroke, MI mortality and total CVD among participants randomized to vitamin D [37]. Furthermore, the recent publication of the VITAL trial which is included in this meta-analysis has assisted in our understanding of vitamin D’s role in the primary prevention of CVD. This RCT tested in 2000 IU/day of vitamin D in 25,871 relatively healthy adults. After on average of 5 years of follow up there was no reduction in the primary outcome which was a composite outcome and included myocardial infarction, stroke, or death from cardiovascular causes [30]. Sixty-one percent of participants had a baseline vitamin D level drawn and the mean concentration was 30.8 ng/mL. Of these participants, 12.7% had a vitamin D level below 20 ng/mL [30].
Why is there no benefit with vitamin D supplementation despite observational data showing a link? The observational data is unable to establish a casual relationship between vitamin D deficiency and CVD and caution should be applied when analyzing nonrandomized studies. Many confounding variables exist in these populations which are difficult to control for and can lead to both vitamin D deficiency and CVD. These include ability for participants to perform outdoor physical activity which leads to more ultraviolet-B exposure from the sun and more vitamin D production. Obesity and increased age also result in decreased vitamin D concentrations and an increased risk of CVD. Furthermore, chronic diseases which lead to malnutrition can result and be linked to both vitamin D deficiency and CVD.
In 2015, the U.S Preventative Services Task Force (USPTF) released a recommendation statement in regard to vitamin D deficiency screening in which they found insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic patients [38]. In particular, no studies were identified that studied the direct benefits or harms of screening for vitamin D deficiency in adults. Also, the USPTF found no evidence to recommend vitamin D supplementation in asymptomatic patients for the prevention of skeletal or nonskeletal outcomes. These findings are consistent with an Institute of Medicine report which found insufficient evidence to recommend vitamin D supplementation for the purpose of extra-skeletal benefits and set forth the recommended dietary allowance (RDA) for vitamin D as 600 IU/day for individuals aged 1–70 years and 800 IU/day for those over the age of 71 years [39].
There are limitations to performing a meta-analysis of this kind. There was wide variability in baseline characteristics of the included RCTs. Few trials included the endpoint of interest for this meta-analysis (nonfatal MI, cardiac mortality, CHD events, and stroke) in a primary pre-specified manner. Some included RCTs test a combined vitamin D and calcium supplement with fracture or bone mineral density as the primary outcome. Therefore, it is likely that these outcomes were not ascertained through rigorous adjudication methods thus predisposing them to significant bias (including recall and reporting bias). Most trials are of small size and were not designed or adequately powered to detect differences in hard CVD outcomes. Another limitation is the wide variability in dose and frequency of vitamin D tested. Given the wide range of follow up observed (0.38–7 years), a hazard ratio may have been the more appropriate measure of risk. Another limitation of the analysis is the interlaboratory variation which has been observed with vitamin D measurement [40]. Measurement of vitamin D was not standardized across all RCTs. Also, the meta-regression tested a linear association between 25(OH)D concentrations and vitamin D’s effect on CVD outcomes. Therefore, this analysis is unable to answer the question of “Does vitamin D supplementation improve CVD outcomes in deficient populations?” One way to answer this question would be to perform a RCT powered to detect differences in CVD outcomes, in deficient populations testing vitamin D supplementation versus placebo or to perform an individual participant data meta-analysis of RCTs. Meta-regressions are observational in nature and are therefore also subject to confounding variables and bias.
In conclusion, this meta-analysis found no reduction in CHD outcomes or stroke outcomes among the available RCTs testing vitamin D supplementation. Despite there being numerous observational studies and biologic mechanisms suggesting vitamin D may improve CVD, this meta-analysis including the available randomized data has failed to show benefit. Furthermore, a linear relationship between baseline 25(OH)D concentrations and vitamin D’s effect on CVD outcomes was not observed. In line with the recent USPTF statement, clinicians should avoid overdiagnosis and overtreatment of vitamin D deficiency in adults. Vitamin D levels should only be checked and repleted in those with an absolute indication.
CRediT authorship contribution statement
Matthew Nudy: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation. George Krakowski: Conceptualization, Methodology, Formal analysis. Mehrdad Ghahramani: Conceptualization, Methodology, Formal analysis. Mohammed Ruzieh: Conceptualization, Methodology, Formal analysis. Andrew J. Foy: Conceptualization, Methodology, Formal analysis.
Footnotes
Presentation: The following data was presented as an oral presentation at the American Heart Association’s annual scientific meeting on November 12, 2018 in Chicago, IL.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100537.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Schnatz P.F., Nudy M., Jiang X., Demko J., Appt S. Vitamin D deficiency and cardiovascular disease in postmenopausal women: contributions from human and nonhuman primate studies. Menopause. 2015;22(5):554–563. doi: 10.1097/GME.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E., Liu Y., Hollis B.W., Rimm E.B. 25-Hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu-Wong J.R., Nakane M., Ma J., Ruan X., Kroeger P.E. Effects of vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.C., Qiao G., Uskokovic M., Xiang W., Zheng W., Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Rostkowska-Nadolska B., Sliupkas-Dyrda E., Potyka J. Vitamin D derivatives: calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv Med Sci. 2010;55(1):86–92. doi: 10.2478/v10039-010-0012-9. [DOI] [PubMed] [Google Scholar]
- 6.Schnatz P.F., Nudy M., O’Sullivan D.M., Jiang X. The quantification of vitamin D receptors in coronary arteries and their association with atherosclerosis. Maturitas. 2012;73(2):143–147. doi: 10.1016/j.maturitas.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zittermann A, Schleithoff SS, Koerfer. Vitamin D and vascular calcification. Current Opinion in Lipidology 2007;18:41-46. [DOI] [PubMed]
- 8.Wang L., Song Y., Manson J.E. Circulating levels of 25hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R., Li B., Gao X. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:810–819. doi: 10.3945/ajcn.116.140392. [DOI] [PubMed] [Google Scholar]
- 10.Crowe F.L., Thayakaran R., Gittoes N. Non-linear associations of 25-hydroxyvitamin D concentrations with risk of cardiovascular disease and all-cause mortality: results from the Health Improvement Network (THIN) database. J Steroid Biochem Mol Biol. 2019;195 doi: 10.1016/j.jsbmb.2019.105480. [DOI] [PubMed] [Google Scholar]
- 11.Gaksch M., Jorde R., Grimnes G. Vitamin D and mortality individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLos ONE. 2017;12(2) doi: 10.1371/journal.pone.0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7) [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P., Altamn D.G., Gotzsche P.C. The Cochrane collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron J.A., Barry E.L., Mott L.A. A trial of calcium and vitamin D for the prevention of colorectal adenomas. NEJM. 2015;373(16):1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazier M., Grados F., Kamel S. Clinical and laboratory safety of one year’s use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clinical Therapeutics. 2005;27(12):1885–1893. doi: 10.1016/j.clinthera.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Ford J.A., MacLennan G.S., Avenell A. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 18.Gulseth H.L., Wium C., Angel K., Eriksen E.F., Birkeland K.I. Effects of vitamin D supplementation on insulin sensitivity and insulin secretion in subjects with type 2 diabetes and vitamin D deficiency: a randomized controlled trial. Diabetes Care. 2017;40(7):872–878. doi: 10.2337/dc16-2302. [DOI] [PubMed] [Google Scholar]
- 19.Inkovaara J., Gothoni G., Halttula R., Heikinheimo R., Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age and Ageing. 1983;12(2):124–130. doi: 10.1093/ageing/12.2.124. [DOI] [PubMed] [Google Scholar]
- 20.Jackson R.D., LaCroix A.Z., Gass M. Calcium plus vitamin D supplementation and the risk of fractures. NEJM. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 21.Jin X., Jones G., Cicuttini F., Wluka A., Zhu Z., Han W., Antony B., Wang X., Winzenberg T., Blizzard L., Ding C. Effect of Vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. 2016;315(10):1005–1013. doi: 10.1001/jama.2016.1961. [DOI] [PubMed] [Google Scholar]
- 22.Jorde R., Sollid S.T., Svartberg J., Schirmer H., Joakimsen R.M., Njølstad I., Fuskevåg O.M., Figenschau Y., Hutchinson M.Y. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. 2016;101(4):1647–1655. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 23.Komulainen M., Kroger H., Tuppurainen M.T. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin d in early postmenopausal women: a population-based 5-year randomized trial. J Clin Endocrinol Metab. 1999;84(2):546–552. doi: 10.1210/jcem.84.2.5496. [DOI] [PubMed] [Google Scholar]
- 24.Martineau A.R., MacLaughlin B.D., Hooper R.L. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs) Thorax. 2015;70(5):451–457. doi: 10.1136/thoraxjnl-2014-206449. [DOI] [PubMed] [Google Scholar]
- 25.Martineau A.R., Hanifa Y., Witt K.D. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu) Thorax. 2015;70(10):953–960. doi: 10.1136/thoraxjnl-2015-206996. [DOI] [PubMed] [Google Scholar]
- 26.Miskulin D.C., Majchrzak K., Tighiouart H., Muther R.S., Kapoian T., Johnson D.S., Weiner D.E. Ergocalciferol supplementation in hemodialysis patients with vitamin D deficiency: a randomized clinical trial. J Am Soc Nephrol. 2016;27(6):1801–1810. doi: 10.1681/ASN.2015040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince R.L., Austin N., Devine A. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168(1):103–108. doi: 10.1001/archinternmed.2007.31. [DOI] [PubMed] [Google Scholar]
- 28.Sanders K.M., Stuart A.L., Williamson E.J. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA. 2010;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 29.Scragg R., Stewart A.W., Waayer D. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cadiol. 2017;2(6):608–616. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trivedi D.P., Doll R., Khaw T. Effect of four monthly oral vitamin D (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson J.E., Cook N.R., Lee I. Vitamin D supplements and prevention of cancer and cardiovascular disease. NEJM. 2019;380(1):33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witham M.D., Crighton L.J., Gillespie N.D. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3(2):195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 33.Witham MD, Price JG Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: The VitDISH randomized controlled trial. JAMA Intern Med 2013;173(18):1672-1679. [DOI] [PubMed]
- 34.Zittermann A., Ernst J.B., Prokop S. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3 year randomized clinical trial with 4000 IU vitamin D daily. European Heart Journal. 2017;38(29):2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher J.C., Sai A., Templin T., Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156(6):425–437. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 36.Barbarawi M., Kheiri B., Zayed Y. Vitamin D supplementation and cardiovascular disease risks in more than 83,000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiology. 2019;4(8):765–775. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins D.J., Spence J.D., Giovannucci E.L. Supplemental vitamins and minerals for CVD prevention and treatment. JACC. 2018;71(21):2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 38.LeFevre M.L. on behalf of the U.S. Preventive Services Task Force. Screening for Vitamin D Deficiency in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;162(2):133–140. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 39.Ross A.C., Manson J.E., Abrams S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2010;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lips P., Chapuy M.C., Dawson-Hughes B. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.