Abstract
PURPOSE:
While patients with heart failure who achieve a peak oxygen uptake (peak ) of 10 mL·kg−1·min−1 or less are often considered for intensive surveillance or intervention, those achieving 14 mL·kg−1·min−1 or more are generally considered to be at lower risk. Among patients in the “intermediate” range of 10.1 to 13.9 mL·kg−1·min−1, optimally stratifying risk remains a challenge.
METHODS:
Patients with heart failure (N 1167) referred for cardiopulmonary exercise testing were observed for 21 ±13 months. Patients were classified into 3 groups of peak (≤10, 10.1–13.9, and ≥14 mL·kg−1·min−1). The ability of heart rate recovery at 1 minute (HRR1) and the minute ventilation/carbon dioxide output () slope to complement peak in predicting cardiovascular mortality were determined.
RESULTS:
Peak , HRR1 (16 beats per minute), and the / slope (>34) were independent predictors of mortality (hazard ratio 1.6, 95% CI: 1.2–2.29, P =.006; hazard ratio 1.7, 95% CI: 1.1–2.5, P .008; and hazard ratio 2.4, 95% CI: 1.6–3.4, P ≤.001, respectively). Compared with those achieving a peak ≥ 14 mL·kg−1·min−1, patients within the intermediate range with either an abnormal / slope or HRR1 had a nearly 2-fold higher risk of cardiac mortality. Those with both an abnormal HRR1 and / slope had a higher mortality risk than those with a peak 10 mL·kg−1·min−1. Survival was not different between those with a peak 10 mL·kg−1·min−1 and those in the intermediate range with either an abnormal HRR1 or / slope.
CONCLUSIONS:
HRR1 and the / slope effectively stratify patients with peak within the intermediate range into distinct groups at high and low risk.
Keywords: cardiopulmonary exercise test, heart failure, mortality
Despite recent improvements in medical therapy, the mortality rate in patients with heart failure (HF) remains high. The continued scarce availability of donor hearts makes the accurate selection of those with a poor prognosis and who may benefit most from intensive therapy or cardiac transplantation a major challenge.1,2 Since the landmark study by Mancini et al3 2 decades ago, peak oxygen uptake (peak ) has been considered one of the most important variables used to guide the selection of transplant recipients. While patients who achieve peak values 10 mL·kg−1·min−1 or less are generally considered to benefit most from transplantation, those with peak values 14 mL·kg−1·min−1 or more are generally considered to have an acceptable short-term survival, and surgical intervention is usually deferred.1,2
However, the limitations of peak in stratifying risk have been well-documented.4–7 Fundamental to these limitations is the fact that peak does not accurately reflect ventricular performance,7 or the level of circulatory, metabolic, or ventilatory dysfunction during exercise,6 and that it is dependent on subject effort. In part for this reason, evaluating risk in medically optimized patients who achieve a peak in the range of 10 to 14 mL·kg−1·min−1 has been debated. In recent years, cardiopulmonary exercise test (CPX) variables other than peak , in particular the slope of the relationship between minute ventilation and carbon dioxide production (/ slope) and heart rate recovery (HRR1), have been shown to powerfully predict mortality in HF.8–10 In many instances, these responses have been stronger predictors of adverse events than peak .8,9 Multivariate approaches, which combine CPX variables, may be an optimal method for estimating risk.11,12 However, few studies have investigated the prognostic value of such variables within the “intermediate range” of peak (10–14 mL·kg−1·min−1).13,14 Therefore, our objective was to test the hypothesis that the / slope and HRR1 would improve risk stratification in patients with HF who have peak values within this range.
METHODS
This was a multicenter analysis including patients with HF from the CPX laboratories at San Paolo Hospital, Milan, Italy; Virginia Commonwealth University, Richmond, Virginia; LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina; and the VA Palo Alto Health Care System, Palo Alto, California. A total of 1167 consecutive patients with chronic HF were included in the study. Inclusion criteria consisted of a diagnosis of HF, defined by symptoms compatible with HF, and evidence of left ventricular systolic dysfunction (left ventricular ejection fraction [LVEF] ≤ 45%) or abnormal filling pattern and ejection fraction greater than 45% by 2-dimensional echocardiography obtained within 1 month of exercise testing (those with HF symptoms and an ejection fraction ≥45% were defined as diastolic HF patients). Patients with pacemakers were excluded. All subjects completed a written informed consent, and institutional review board approval was obtained at each institution. All authors have read and approved the manuscript.
CPX Procedure and Data Collection
Symptom-limited CPX was performed on all patients using ramp protocols.15 A treadmill was used for testing in the American centers, while a cycle ergometer was used in the European center. Previous studies have demonstrated optimal peak , / slope and HRR1 prognostic threshold values are similar regardless of the mode of exercise in patients with HF.16 Ventilatory-expired gas analysis was performed with a metabolic system (MedGraphics CPX-D, Minneapolis, MN, or SensorMedics Vmax29, Yorba Linda, CA). Before each test, the equipment was calibrated in a standard fashion with reference gases. In addition, each center routinely performed validation procedures.17 A standard 12-lead electrocardiogram was obtained at rest, each minute during exercise, and for at least 5 minutes during the recovery phase; blood pressure was measured with a standard cuff sphygmomanometer. Minute ventilation ( ), oxygen uptake ( ), carbon dioxide output ( ), and other cardiopulmonary variables were acquired on a breath-by-breath basis and averaged over 10-or 15-second intervals. Peak and peak respiratory exchange ratio were expressed as the highest averaged samples obtained during the exercise test. and values, acquired from the initiation of exercise to peak, were used to calculate the / slope via least squares linear regression (y = mx + b, where m = slope). Previous work has shown that this method of calculating the / slope is prognostically optimal.18,19 Heart rate recovery was measured 1 minute post-CPX and termed HRR1.
End points
Subjects were followed for cardiac-related death for 3 years after CPX via hospital and outpatient medical chart review. Subjects were followed by the HF programs at their respective institutions, which provided a high likelihood that all major events were captured. Cardiac-related mortality was defined as death resulting from failure of the cardiac system. Individuals conducting the CPX were not involved in decisions regarding cause of death.
Statistical analysis
Continuous data are reported as mean SD. Oneway ANOVA was used to compare differences in continuous variables, whereas chi-square analysis was used to compare differences in categorical variables between peak subgroups. Bonferroni post hoc tests were used when appropriate. The optimal abnormal cut points for the / slope (>34), peak (≤14 mL·kg−1·min−1), and HRR1 (<16 beats per minute) were established by receiver operating characteristic curves. Univariate Cox regression analysis was used to assess the prognostic value of key baseline and CPX variables. Multivariate Cox regression analysis was used to determine the prognostic value of the / slope, peak , LVEF, HRR1, and etiology of HF. In addition, all variables retained in the multivariable model were included in the Cox regression model, which assessed the predictive power of / slope and HHR1 in the intermediate range group of peak . All analyses were adjusted for age, etiology, LVEF, and β-blocker use.
Patients were divided into 3 peak subgroups: 14 mL·kg−1·min−1, 10.1 to 13.9 mL·kg−1·min−1 (intermediate range), and 10 mL·kg−1·min−1. Kaplan-Meier analysis with log-rank testing was used to assess survival within each subgroup. Kaplan-Meier analyses were also used to assess the ability of the / slope, HRR1, or their combination to stratify those within the intermediate range into high-and low-risk mortality groups.
RESULTS
Demographic characteristics for all subjects and peak subgroups are presented in Table 1. Age was higher and male gender was less prevalent in patients in the 2 lower peak groups. Left ventricular ejection fraction was lower in those with peak values 10 mL·kg−1·min−1 or less. There were no differences in HF etiology, respiratory exchange ratio, and b-blocker or angiotensin-converting enzyme inhibitor use between groups.
Table 1 •.
Demographics and Clinical Characteristics (M ± SD Unless Otherwise Indicated)
| Peak (mL·kg −1·min−1) |
||||
|---|---|---|---|---|
| Variable | All Subjects | (A) ≥14 | (B) 10.1–13.9 | (C) ≥ 10 |
| Subjects, n | 1167 | 671 | 330 | 166 |
| Age, y | 58 ± 13 | 57 ± 13 | 59 ± 13a | 60 ± 14a |
| Male gender, n (%) | 854 (73) | 550 (82) | 203 (61)a | 101 (61)a |
| Etiology, % nonischemic/ischemic | 54.1/45.9 | 53.4/46.6 | 56.1/43.9 | 54.1/45.9 |
| Systolic/diastolic HF, % | 79/21 | 78/22 | 78/22 | 87/13a,b |
| NYHA functional class | 2.4 ± 0.8 | 2.1 ± 0.8 | 2.6 ± 0.7a,b | 2.9 ± 0.6a |
| Ejection fraction, % | 32.6 ±14.2 | 33.8 ± 13.7 | 32.4 ± 14.8b | 28.4 ± 13.9a |
| Peak o2, mL·kg−1·min−1 | 15.7 ± 5.9 | 19.4 ± 5.1 | 12.1 ± 1.1 a,b | 8.1 ± 1.4a |
| / slope | 35.1 ± 9 | 31.8 ± 6.7 | 37.6 ± 8.4a,b | 43.7 ± 13.0a |
| Heart rate recovery at 1 min, bpm | 19 ± 12 | 21 ± 12 | 16 ± 10a | 14 ± 12a |
| Peak respiratory exchange ratio | 1.08 ± 0.15 | 1.09 ± 0.14 | 1.08 ± 0.13 | 1.07 ± 0.18 |
| Prescribed ß-blocker, % | 64 | 62 | 64 | 68 |
| Prescribed angiotensin-converting enzyme inhibitor, % | 72 | 74 | 68 | 72 |
| Prescribed diuretic, % | 71 | 63 | 81a | 84a |
| Cardiac mortality, n (%) | 204 (17) | 86 (13) | 74 (22)a | 44 (26)a |
Abbreviations: HF, heart failure; NYHA, New York Heart Association class; , oxygen uptake; / slope, minute ventilation/carbon dioxide production slope.
P <.05 (A) vs others.
P <.05 (B) vs C.
Table 2 shows the results of univariate and multivariate Cox regression analyses. Univariately, the / slope, HRR1, peak , and LVEF were all independent predictors of mortality. In the multivariate analysis, those with an abnormal / slope response had 2.4 times higher risk of cardiac mortality when compared with patients with a normal response. HRR1 (1.7-fold higher risk) and peak (1.6-fold higher risk) were also multivariate predictors of cardiac mortality, while LVEF was removed from the regression. When added to the multivariate model as a continuous variable, peak was also an independent predictor of mortality with an 11% reduction in risk of mortality per unit increment in mL·kg−1·min−1.
Table 2 •.
Univariate and Multivariate Cox Regression Analysisa
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Univariate | |||
| Ejection fraction | 1.75 | 1.26–2.43 | <.001 |
| Peak o2 < 14 mL· | 2.52 | 1.83–3.47 | <.001 |
| kg−1·min−1 | |||
| / slope | 3.19 | 2.28–4.46 | <.001 |
| HRR1 | 2.29 | 1.57–3.34 | <.001 |
| Multivariate | |||
| Ejection fraction | 1.28 | 0.91–1.81 | .15 |
| Peak | 1.62 | 1.15–2.29 | .006 |
| / slope | 2.36 | 1.64–3.40 | <.001 |
| HHR1 | 1.70 | 1.14–2.51 | .008 |
Abbreviations: , oxygen uptake; / slope, minute ventilation/carbon dioxide production slope; HRR1, heart rate recovery at the first minute.
Adjusted for ejection fraction, age, and etiology.
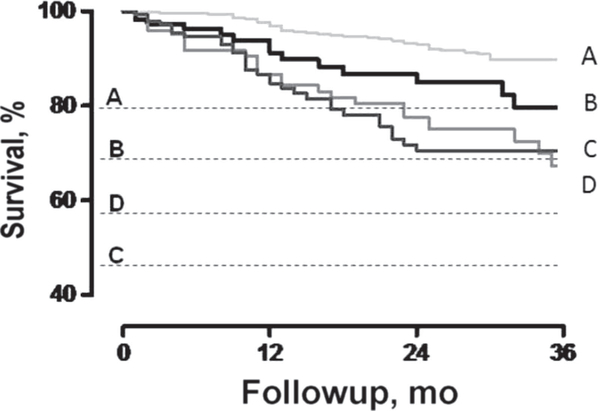
Kaplan-Meier curves, illustrating survival rates between the peak tertiles, are shown in Figure 1; survival was progressively lower as peak was reduced. Compared with those with a peak ≥14 mL·kg−1·min−1, patients within the intermediate range with either an abnormal / slope or HRR1 had a nearly 2-fold higher risk of cardiac mortality (Figure 2). Importantly, patients within the intermediate range with both an abnormal / slope and an abnormal HRR1 response had an even higher mortality risk than those with a peak ≤10 mL·kg−1·min−1 (hazard ratio [HR] 3.51, 95% CI: 2.33–5.29, P <.001, and HR 3.11, 95% CI: 2.07–4.68, P <.001, respectively) (Table 3). Survival was not different between those with a peak 10 mL·kg−1·min−1 or less and those within the intermediate range with either an abnormal HRR1 or / slope.
Figure 1.
Kaplan-Meier survival curves for peak oxygen uptake ( ) subgroups.
Figure 2.
Kaplan-Meier survival curves for peak oxygen uptake ( ) subgroups adding the minute ventilation/carbon dioxide production ( /) slope and heart rate recovery at first minute (HRR1) for subjects in the intermediate range.
(A) Peak ≥ 14 mL·kg−1·min−1.
(B) Peak 10.1–13.9 mL·kg−1·min−1 and abnormal / slope or HRR1.
(C) Peak ≤ 10 mL·kg−1·min−1.
(D) Peak 10.1–13.9 mL·kg−1·min−1 and abnormal / slope and HRR1.
Table 3 •.
Relative Risks Associated With Peak , / Slope, HHR1, and Their Combination
| Groups | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Peak ≥ 14 mL·kg−1·min−1 | 1 (Ref) | … | … |
| Peak 10.1–13.9 with | |||
| / ≥ 34 or HRR1 <16 | 1.81 | 1.07–3.05 | .02 |
| Peak 10.1–13.9 mL·kg−1·min−1 with | |||
| / ≥ 34 and HRR1 < 16 | 3.51 | 2.33–5.29 | <.001 |
| Peak ≤ 10 mL·kg−1·min−1 | 3.11 | 2.07−4.68 | <.001 |
Abbreviations: , oxygen uptake; / slope, minute ventilation/carbon dioxide production () slope; HRR1, heart rate recovery at the first minute.
DISCUSSION
Traditionally, HF patients who achieve a peak > 14 mL·kg−1·min−1 have been considered to have an acceptable prognosis and generally do not benefit from surgical or other intensive intervention.1 Conversely, patients with a peak < 10 mL·kg−1·min−1 have a poor short-term prognosis and interventions such as transplantation have been shown to significantly improve outcomes.1–3 However, classifying risk in patients who achieve a peak in what has been termed the intermediate range (10–14 mL·kg−1·min−1) has been less clear. A potential application of CPX variables other than peak would likely be in the specific group of patients with peak values in this intermediate range, among whom peak alone is often limited in differentiating high and low mortality risk.
To our knowledge, this study is the first to focus on the prognostic value of the / slope and HRR1 in patients with HF who fall within the intermediate range of peak . The present results suggest that the / slope and HRR1, markers of ventilatory efficiency, and autonomic function, respectively, are strong predictors of cardiovascular mortality among HF patients in this subgroup. Importantly, these responses stratify patients with peak in the range of 10.1 to 13.9 mL·kg−1·min−1 into distinct groups with high and low risk for cardiovascular mortality. Patients within this range who exhibit abnormal responses for both the / slope and HRR1 had an even higher mortality risk than those with peak values 10 mL·kg−1·min−1 or less, underscoring their utility in identifying high-risk patients in this important category.
Over the last decade, there have been numerous studies demonstrating the prognostic significance of an abnormal / slope in HF patients.5,8–11,13,16,18,19 An elevated / slope has been linked to poor pulmonary perfusion,20,21 an impaired cardiac output both at rest22 and during exercise,23 early lactate accumulation, heightened skeletal muscle, and chemoreceptor sensitivity,24,25 and deconditioning. The optimal cut point for the / slope in our population was 34, which is in accordance with previous studies.8,10,11 We observed that the addition of the / slope in patients within the intermediate range of peak stratified patients into distinct groups of high and low cardiovascular mortality (Table 3, Figure 2). Corrá et al13 reported that the / slope discriminated high-and low-risk patients within a wider peak range (10–18 mL·kg−1·min−1); HF patients with a / slope greater than 35 had a mortality rate that was similar to that in patients with a peak < 10 mL·kg−1·min−1. Conversely, we observed that patients in the intermediate range for peak who had both an abnormal / slope and HRR1 had a higher mortality risk than those with a peak 10 mL·kg−1·min−1 (Figure 2, Table 3).
While HRR1 has been shown to have prognostic value in patients with coronary disease for some time,26,27 such studies in patients with HF are more recent.9,11,28 We previously observed that HF patients with an HRR1 < 6 beats per minute had a high mortality risk; the combination of an abnormal / slope and impaired HRR1 resulted in an approximate 90% event rate 1 year after CPX.29 In subsequent analyses, we observed that HRR1 was a powerful prognostic marker when adjusted for β-blocker use, EF, HF etiology, and mode of exercise, and that HRR1 consistently added prognostic value to the / slope and other CPX responses.9,11 The present findings confirm these earlier results in a larger population of patients with HF. HRR1, together with / slope, provided powerful risk prediction for cardiac mortality in both univariate and multivariate analyses. Among patients specifically within the intermediate range of peak , we observed that an abnormal HRR1 was particularly helpful in estimating risk, providing a doubling of the HR in patients with a heightened / slope. Thus, these results suggest that the / slope and HRR1 should be routinely measured when using CPX to estimate risk in patients with HF.
Previous studies have observed that patients with both an abnormal HRR1 and a high / slope have particularly high mortality rates.9,11,28,29 The current results confirm the value of these responses among patients who fall within the intermediate range for peak . Because the physiopathology of HF is multifactorial, variables that incorporate different mechanisms that may be underlying exercise intolerance in these patients may provide better risk stratification. The improved estimates of risk using these variables in the current study might be explained by the fact that they represent distinct manifestations of HF, including autonomic imbalance (HRR1) and ventilation-perfusion mismatching in the lungs ( / slope).30,31
This study has several limitations. Our sample represents a generalized group of HF patients, among whom few were being evaluated specifically for transplantation. Had the analysis been performed in patients with more severe disease, the prognostic characteristics of the CPX responses would likely have differed. The fact that we only assessed heart rate in the first minute of recovery is another limitation since the second minute in recovery and other time points have been shown to provide improved risk stratification in some studies.32,33 Our exercise tests included an active cool-down phase, which differs from some studies in measuring HRR1 in which patients were placed in the supine position immediately. Further research is needed to determine the optimal recovery protocol for estimating risk in patients with HF.
SUMMARY
The application of 2 easily derived variables from CPX, the / slope and HRR1, effectively stratifies risk among HF patients who fall into the intermediate range for peak . The inclusion of these responses in the risk paradigm, when evaluating patients with HF, may help to better select candidates for transplantation or identify those who require closer clinical followup and/or device therapy.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Luiz Eduardo Ritt, Hospital Santa Izabel, Salvador, BA, and São Paulo Federal University, São Paulo, Brazil.
Ricardo Brandão Oliveira, Rio de Janeiro State University, Rio de Janeiro, Brazil.
Jonathan Myers, VA Palo Alto Health Care System, Cardiology Division, Stanford University, Palo Alto, California.
Ross Arena, Physical Therapy Program, Department of Orthopaedics and Division of Cardiology, Department of Internal Medicine, University of New Mexico, Albuquerque.
Mary Ann Peberdy, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Daniel Bensimhon, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina.
Paul Chase, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina.
Daniel Forman, Brigham and Women’s Hospital, Cardiovascular Division, and VA Boston Healthcare System, Geriatric Research, Education, and Clinical Center, Boston, Massachusetts.
Marco Guazzi, University of Milano, San Paolo Hospital, Cardiology Division, Milano, Italy..
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guide-line update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Circulation. 2005;112:e154–e235. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006;25:1024–1042. [DOI] [PubMed] [Google Scholar]
- 3.Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 4.Myers J, Gullestad L, Vagelos R, et al. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J. 2000;139:78–84. [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. [DOI] [PubMed] [Google Scholar]
- 6.Wilson JR, Rayos G, Yeoh TK, Gothard P, Bak K. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation. 1995;92:47–53. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Progress Cardiovasc Dis. 1995;38:1–22. [DOI] [PubMed] [Google Scholar]
- 8.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak and / slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354–360. [DOI] [PubMed] [Google Scholar]
- 9.Arena R, Myers J, Abella J, et al. The prognostic value of heart rate response during exercise and recovery in patients with heart failure: influence of beta-blockade. Int J Cardiol. 2010; 138:166–173. [DOI] [PubMed] [Google Scholar]
- 10.Ingle L. Theoretical rationale and practical recommendations for cardiopulmonary exercise testing in patients with chronic heart failure. Heart Fail Rev. 2007;12:12–22. [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156:1177–1183. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson KD, Schwartz SJ, Chen TM, Kar-Lai W, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. [DOI] [PubMed] [Google Scholar]
- 13.Corra U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J. 2002;143:418–426. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira RB, Myers JN, Araujo CG S, et al. Does peak oxygen pulse complement peak V˙ o2 in risk stratifying patients with heart failure? Am J Cardiol. 2009;104:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arena R, Humphrey R, Peberdy MA, Madigan M. Predicting peak oxygen consumption during a conservative ramping protocol: implications for the heart failure population. J Cardiopulm Rehabil. 2003;23:183–189. [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic characteristics of cardiopulmonary exercise testing in heart failure: comparing American and European models. Eur J Cardiovasc Prev Rehabil. 2005;12:562–567. [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Arena R, Franklin B, et al. Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation. 2009;119:3144–3161. [DOI] [PubMed] [Google Scholar]
- 18.Arena R, Myers J, Aslam S, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–727. [DOI] [PubMed] [Google Scholar]
- 19.Bard RL, Gillespie BW, Clarke NS, Egan TG, Nicklas JM. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant. 2006;25:589–595. [DOI] [PubMed] [Google Scholar]
- 20.Wada O, Asanoi H, Miyagi K, et al. Importance of abnormal lung perfusion in excessive exercise ventilation in chronic heart failure. Am Heart J. 1993;125:790–798. [DOI] [PubMed] [Google Scholar]
- 21.Banning AP, Lewis NP, Northridge DB, Hendersen AH. Perfusion/ventilation mismatch during exercise in chronic heart failure: an investigation of circulatory determinants. Br Heart J. 1995;74:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reindl I, Wernecke KD, Opitz C, et al. Impaired ventilatory efficiency in chronic heart failure: possible role of pulmonary vasoconstriction. Am Heart J. 1998;136:778–785. [DOI] [PubMed] [Google Scholar]
- 23.Myers J, Dziekan G, Goebbels U, Dubach P. Influence of highintensity exercise training on the ventilatory response to exercise in patients with reduced ventricular function. Med Sci Sports Exerc. 1999;31:929–937. [DOI] [PubMed] [Google Scholar]
- 24.Chua TP, Clark AL, Amadi AA. The relationship between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27:650–657. [DOI] [PubMed] [Google Scholar]
- 25.Ponikowski P, Francis DP, Piepoli MF, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103:967–972. [DOI] [PubMed] [Google Scholar]
- 26.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. [DOI] [PubMed] [Google Scholar]
- 27.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 28.Kubrychtova V, Olson TP, Bailey KR, Thapa P, Allison TG, Johnson BD. Heart rate recovery and prognosis in heart failure patients. Eur J Appl Physiol. 2009;105:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151:151. [DOI] [PubMed] [Google Scholar]
- 30.Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–H141. [DOI] [PubMed] [Google Scholar]
- 31.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. [DOI] [PubMed] [Google Scholar]
- 32.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. [DOI] [PubMed] [Google Scholar]
- 33.Gorelik DD, Hadley D, Myers J, Froelicher V. Is there a better way to predict death using heart rate recovery? Clin Cardiol. 2006;29:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]