Abstract
Childhood trauma (CT) is a risk factor for schizophrenia spectrum disorders (SSDs), and cognitive impairment is a core feature and a vulnerability marker of SSDs. Studies of the relationship between CT and cognitive impairment in SSDs are inconclusive. In addition, few studies have examined differential effects of CT subtypes, e.g. physical, sexual or emotional abuse/neglect, on cognitive functioning. The present study therefore aimed to examine the effects of CT and CT subtypes on cognitive impairment in SSD. Participants (n = 78) with SSDs completed a comprehensive neuropsychological test battery and the Childhood Trauma Questionnaire Short-Form (CTQ-SF). We compared global cognitive performance as well as scores in seven subdomains (verbal abilities, visuospatial abilities, learning, memory, attention/working memory, executive abilities and processing speed) between participants reporting no CT and those reporting CT experiences using independent samples t-tests as well as linear regression analyses to control for possible confounders. CT subtype physical neglect was associated with attention and working memory after controlling for positive and negative psychosis symptoms, years of education, antipsychotics, gender and age, and adjustment of multiple testing. Our results indicate that the observed heterogeneity in cognitive impairment in SSDs, especially attention/working memory abilities, may in part be associated with childhood physical neglect.
Keywords: Neuropsychology, Adversity, Psychosis, Adverse childhood experiences
Highlights
-
•
Research on childhood trauma and cognitive impairment in SSDs is inconclusive
-
•
Few studies investigated if CT subtypes (abuse and neglect) could explain the heterogeneity in cognitive impairment in SSDs
-
•
CT subtype physical neglect was associated with impairment in attention/working memory abilities
-
•
The observed heterogeneity in cognitive impairment in SSDs may in part be associated with CT subtypes
Cognitive impairment is both a core feature of schizophrenia spectrum disorders (SSDs; Carrion et al., 2015), a vulnerability marker, and closely related to poor functional outcome and disability in SSDs (Kahn and Keefe, 2013). However, there is great variation in reported cognitive impairments in SSDs, and factors underlying this heterogeneity in cognitive functioning remain poorly understood. Risk factors influencing the development of SSDs may also potentially affect cognitive functioning directly or indirectly, such as illicit substance use which is a risk factor for psychosis, and has been found to influence cognitive vulnerability for psychosis (Løberg et al., 2014).
Childhood trauma (CT), e.g. physical, sexual, emotional abuse and physical and emotional neglect (Bernstein et al., 2003) is another risk factor for SSDs (Mørkved et al., 2017) which may be associated with cognitive impairment. The association between CT and SSDs is evident across study designs and populations, and CT has been found to increase the risk of psychosis with an odds ratio of 2.8 (Varese et al., 2012). CT have been shown to have a detrimental effect on brain development and cognitive functioning in non-psychotic individuals, attributed to disrupted neurodevelopment and stress-regulating brain systems (Pechtel and Pizzagalli, 2011). Understanding the relationship between CT and cognition in SSDs may thus aid both its etiological understanding as well as treatment models for psychosis.
A handful of studies have found negative effects of CT on cognition in SSD patients. Shannon et al. (2011) found that CT in SSD predicted greater impairments in working memory and episodic memory as compared to SSD with no history of CT. Quide et al. (2016) reported a negative association between CT and working memory performance in individuals with SSDs. However, some studies have failed to find an association between CT and cognitive impairment in SSDs (e.g. van Os et al., 2017). One study also indicated a positive effect of CT and cognitive abilities in SSDs (Ruby et al., 2017).
One possible explanation for the observed variance of cognitive impairment in SSDs might be differential effects of various types of CT (Schalinski et al., 2016). Li et al. (2017) reported negative effects of physical abuse, neglect and sexual abuse on language and attention. Ucok et al. (2015) found physical CT to have a negative impact on cognitive function in individuals at ultra-high risk of psychosis.
In addition, the mixed findings on CT and cognitive impairment in SSDs could be attributed to discrepancies in the measurement of CT and the use of non-validated self-report questionnaires. The Childhood Trauma Questionnaire Short-Form (CTQ-SF; Bernstein et al., 1997) used in the present study is described as a reliable measure of CT in SSDs (Fisher et al., 2011). Finally, sample differences between studies may also have contributed to the equivocal findings. For example, antipsychotic drug use has been found to improve cognition in SSDs (Johnsen et al., 2013).
In sum, findings on the relation between CT and cognitive impairment in SSDs are inconclusive, and few studies to date have examined whether CT subtypes might differentially affect cognitive functioning in SSDs. The aim of the present study is therefore to investigate possible effects of CT and CT subtypes on global cognitive performance and specific cognitive domains in a clinically representative sample of patients with SSDs.
1. Methods and material
The present study is based on cross-sectional data from the Bergen Psychosis project 2 (BP2), Haukeland University Hospital, Bergen, Norway. The BP2 is an independently funded multi-site prospective study including a randomized, rater-blind, head-to-head comparison of amisulpride, aripiprazole, and olanzapine, approved by the Regional Committee for Medical Research Ethics (2010–3387) and registered as a clinical trial 10/03/2011 (www.clinicaltrials.gov: NCT01446328). Inclusion/exclusion criteria for the BP2 have been described elsewhere (Mørkved et al., 2018). The current sample consisted of 78 patients with SSDs, 49 (63.6%) male, mean age 29.8 years (SD = 12.4 years; Table 1).
Table 1.
Mean (SD) clinical and demographic characteristics by CT/no CT group.
| No CT (n = 37) | CT (n = 41) | Total (n = 78) | t/χ2 | p-Value | |
|---|---|---|---|---|---|
| Age | 29.46 (11.97) | 30.20 (12.87) | 29.84 (12.37) | −0.26 | 0.795 |
| Gender | |||||
| Male | 28 (57.14%) | 21 (42.86%) | 49 (62.80%) | 4.98 | 0.026* |
| Female | 9 (31.03%) | 20 (68.97%) | 29 (37.20%) | ||
| Duration of illness (n = 70) | 5.99 (10.71) | 5.30 (5.99) | 5.63 (8.55) | 0.33 | 0.737 |
| Duration of untreated psychosis (n = 58) | 26 (36.87) | 83.06 (132.35) | 55.52 (101.90) | −2.20 | 0.032 |
| Antipsychotics DDD | 1.18 (0.51) | 1.13 (0.80) | 1.30 (0.75) | 0.34 | 0.736 |
| Years of education | 13 (2.79) | 11.88 (2.67) | 12.41 (2.76) | 1.82 | 0.073 |
| Education | |||||
| Primary | 14 (42.42%) | 19 (57.58%) | 33 (42.3%) | 0.73 | 0.392 |
| Further | 23 (52.27%) | 21 (47.73%) | 44 (57.14%) | ||
| Civil status | |||||
| Single | 30 (49.18%) | 31 (50.82%) | 61 (91%) | 0.67 | 0.414 |
| Married/divorced | 4 (66.67%) | 2 (33.33%) | 6 (9%) | ||
| Living situation | |||||
| Independently | 20 (47.62%) | 22 (52.38%) | 42 (54.55%) | 1.09 | 0.578 |
| Supported housing/institution | 16 (42.11%) | 18 (47.37%) | 34 (44.16%) | ||
| No residence | 1 (100%) | 0 (0%) | 1 (1.30%) | ||
| PANSS baseline (n = 77) | |||||
| Positive symptoms | 18.54 (5.59) | 21.38 (5.30) | 20.01 (5.59) | −2.28 | 0.025* |
| Negative symptoms | 15.84 (6.38) | 19.05 (6.33) | 17.51 (6.51) | −2.22 | 0.029* |
| General psychopathology scale | 36.41 (11.34) | 39.40 (7.66) | 37.96 (9.66) | −1.37 | 0.175 |
| Total | 70.78 (20.89) | 79.83 (14.79) | 75.48 (18.43) | −2.21 | 0.029* |
| DUDIT (n = 54) | 12.73 (12.57) | 9.34 (11.92) | 10.97 (12.24) | 1.02 | 0.313 |
| AUDIT (n = 68) | 9.10 (6.46) | 8.19 (6.43) | 8.63 (6.41) | 0.59 | 0.559 |
| CTQ-SF | |||||
| Emotional abuse | 6.46 (1.94) | 12.85 (5.24) | 9.82 (5.13) | −7.00 | 0.001* |
| Physical abuse | 5.22 (0.53) | 7.24 (3.63) | 6.28 (2.83) | −3.37 | 0.001* |
| Sexual abuse | 5.05 (0.33) | 7.34 (4.25) | 6.28 (3.28) | −3.26 | 0.001* |
| Emotional neglect | 7.73 (2.62) | 14.95 (5.58) | 11.52 (5.71) | −7.18 | 0.001* |
| Physical neglect | 6.24 (1.46) | 9.48 (3.67) | 9.95 (3.26) | −5.03 | 0.001* |
| Sum | 30.70 (3.99) | 51.88 (14.21) | 41.83 (15.02) | −8.75 | 0.001* |
| Cognitive domains | |||||
| Verbal abilities (n = 76) | 49.35 (9.54) | 45.64 (9.13) | 47.39 (9.45) | 1.73 | 0.087 |
| Visuospatial abilities | 46.73 (10.18) | 44.39 (9.78) | 45.50 (9.97) | 1.03 | 0.306 |
| Learning | 43.63 (7.35) | 42.21 (7.50) | 42.88 (7.42) | 0.85 | 0.400 |
| Memory | 46.03 (6.99) | 43.15 (8.84) | 44.52 (8.10) | 1.58 | 0.116 |
| Attention/working memory | 44.20 (6.47) | 42.39 (8.84) | 43.25 (7.81) | 1.02 | 0.309 |
| Executive abilities (n = 75) | 48.97 (10.99) | 45.29 (12.09) | 47.05 (11.65) | 1.38 | 0.173 |
| Processing speed | 43.59 (8.05) | 40.75 (10.03) | 42.10 (9.19) | 1.37 | 0.175 |
| Global cognitive performance | 46.20 (6.39) | 43.45 (7.59) | 44.76 (7.13) | 1.69 | 0.095 |
Note. N = 78 if not stated otherwise. Continuous variables analyzed using independent samples t-test, and categorical variables analyzed using χ2. Duration of untreated psychosis in weeks, and duration of illness in years. DDD = defined daily dose, PANSS = The Positive and Negative Syndrome Scale, CTQ-SF = Childhood Trauma Questionnaire Short Form, AUDIT = Alcohol Use Disorder Identification Test, DUDIT = Drug Use Disorder Identification Test. * significant at p < .05. Verbal abilities: Wechsler Adult Intelligence Scale III (WAIS III; Wechsler, 1997) subtests vocabulary and similarities subtests, and the D-KEFS verbal fluency test (Delis et al., 2001). Visuospatial abilities: Block design and digit symbol-coding subtests of WAIS III, as well as the Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Learning: California verbal learning test (CVLT; Delis et al., 1987) trials 1–5, and the digit span subtest of the WAIS III. Memory: CVLT (subtests short delay free and cued recall, long delay free and cued recall, and delayed recognition) and Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Attention/working memory: Digit vigilance test (Lewis and Rennick, 1979), the CalCAP Continuous performance test subtests sequential reaction time and choice reaction time (Conners, 2002), Trail Making Test (Part B) (Reitan, 1986), the WAIS III subtests digit span and letter-number sequencing, and the Wechsler Memory Scale (Wechsler, 1997). Executive abilities: Wisconsin Card Sorting test (Heaton, 1981) and the Stroop test (Stroop, 1935). Processing speed: Trail Making Test (Part A) (Reitan, 1986), the digit symbol-coding subtest of the WAIS III, the Grooved Pegboard Test (Bryden and Roy, 2005), and the CalCAP subtest simple reaction time (Conners, 2002).
Participants were recruited at the Medical University in Innsbruck, Innsbruck, Austria (n = 10); Stavanger University Hospital, Stavanger, Norway (n = 8); and Haukeland University Hospital, Bergen, Norway (n = 60), and gave informed consent to participate.
Participants were required to meet diagnostic criteria for SSDs in the range F20–29 of the ICD-10 (WHO, 1992): F20 Schizophrenia (n = 44), F21 Schizotypal disorder (n = 2), F22 Persistent delusional disorder (n = 7), F23 Acute and transient psychotic disorders (n = 11), F25 Schizoaffective disorder (n = 5), or F29 Unspecified nonorganic psychosis (n = 9), as determined by the Structural Clinical Interview for Axis I Disorders (SCID; Spitzer et al., 1992), be >18 years of age, be able to read, understand and speak the native language, and score ≥ 4 on at least one of the following items on the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987): Delusions (P1), hallucinatory behavior (P3), grandiosity (P5), suspiciousness/persecution (P6) or unusual thought content (G9). Exclusion criteria were organic psychosis or psychosis due to substance use.
2. Measurement
2.1. Childhood trauma
The CTQ-SF is a 28-item self-report questionnaire screening for five subtypes of childhood trauma: childhood sexual, physical and emotional abuse, and physical and emotional neglect (Bernstein et al., 2003). Each subscale consists of five items scored on a five-point Likert scale ranging from 1 (never true) to 5 (very often true), summarized into an overall CTQ-SF sum score ranging from 25 to 125. Three items make up the Minimization-denial subscale. The CTQ-SF has shown good internal consistency, test-retest reliability, and excellent internal reliability, as well as good sensitivity and specificity (Dovran et al., 2013). For the present study, the overall reliability estimate for the CTQ-SF was high: Cronbach's α = 0.91. Subscale Cronbach's α were: Emotional abuse = 0.88, physical abuse = 0.80, sexual abuse = 0.91, emotional neglect = 0.92, and physical neglect = 0.60.
2.2. Cognitive assessment
Trained research nurses performed the cognitive assessments: a three-hour comprehensive test battery. The following seven domains of cognition were assessed: 1) verbal abilities; 2) visuospatial abilities; 3) verbal learning; 4) memory (long-term memory and recognition); 5) attention/working memory; 6) executive abilities and 7) processing speed.
Verbal abilities were assessed by the Wechsler Adult Intelligence Scale III (WAIS III; Wechsler, 1997) subtests vocabulary and similarities subtests, and the Delis-Kaplan Executive Function System (D-KEFS) verbal fluency test (Delis et al., 2001). Visuospatial abilities were assessed by the WAIS III subtests block design and digit symbol-coding, as well as the Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Learning was assessed by the California verbal learning test (CVLT; Delis et al., 1987) i.e. trials 1–5, and the digit span subtest of the WAIS III. Memory was assessed by the CVLT (subtests short delay free and cued recall, long delay free and cued recall, and delayed recognition) and Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Attention and working memory were assessed by the Digit vigilance test (Lewis and Rennick, 1979), the CALCAP Continuous Performance Test subtests sequential reaction time and choice reaction time (Miller, 1990), Trail Making Test (TMB; Reitan, 1986), the WAIS III subtests digit span and letter-number sequencing, and the Wechsler Memory Scale (Wechsler, 1997). Executive abilities were measured using the Wisconsin Card Sorting test (Heaton, 1981) and the Stroop test (Stroop, 1935). Processing speed was measured using the TMA (Reitan, 1986), the digit symbol-coding subtest of the WAIS III, the Grooved Pegboard Test (Bryden and Roy, 2005), and the CALCAP subtest simple reaction time (Conners, 2002).
The study included well-validated and reliable cognitive measures commonly used in studies of cognitive functioning in individuals with SSDs: The Wechsler Memory Scale (Wechsler, 1997) was found to be a reliable measure of memory deficits in schizophrenia (Gold et al., 1992). The WAIS III is described as having good psychometric properties (Silva, 2008). The Delis Kaplan Executive Function System (D-KEFS) (Delis et al., 2001) was found to have good psychometric properties (Shunk et al., 2006), as did the TMT Part A and B (Bowie and Harvey, 2006; Delis et al., 2001), the Grooved Pegboard Test (Erdodi et al., 2018) and the Rey-Osterrieth Complex Figure Test (Shin et al., 2006). The CVLT (Delis et al., 1987) is described as reliable and valid (Woods et al., 2006). The CALCAP Continuous performance test was found to possess adequate psychometric properties (Miller, 1990). Kopp et al. (2019) report promising reliability data for the Wisconsin Card Sorting test (Heaton, 1981).
Raw scores from cognitive tests were converted to standardized t-scores based on the best available norms (corrected for age, but not for gender and education). Cognitive domain t-scores were calculated as the mean t-score across tests for each domain. A global cognitive performance t-score was calculated by averaging the t-scores from every test.
2.3. Other measurements
The use of antipsychotic drugs at the time of neurocognitive testing was converted to Defined Daily Doses (DDD) as given by the World Health Organization Collaborating Centre for Drug Statistics Methodology at the Norwegian Institute of Public Health (www.whocc.no). The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. Adherence with medication was assessed by means of serum level measurements of antipsychotic drugs.
2.4. Procedure
Patients included in BP 2 were assessed at baseline, week 1, 3, 6, 12, 26, 39 and 52. The CTQ-SF was administered at the 6-weeks. The PANSS was measured at week 1, and the cognitive test battery at the 12-week follow-up.
2.5. Statistical analyses
All analyses were carried out using STATA. Measures are presented as means (M) and standard deviations (SD), or as number (n) and percentages (%). A p-level of <.05 was considered statistically significant, except for in the regression analyses where we corrected for multiple testing by means of a Bonferroni adjustment (0.05/40 = p < .00125). Missing data were handled through imputation based on expectation maximation, and the amount of missing data in the CTQ-SF scale was 0.73%.
CTQ-SF scores were categorized into none, low, moderate and severe abuse or neglect, based on threshold scores in the CTQ-SF manual. A dichotomous variable was created, grouping none and low levels of CT (meaning CT absent) on the one hand, and moderate and severe levels (CT present) on the other (Bernstein and Fink, 1998). The sample was divided into two groups: Participants reporting CT (n = 41), and participants with no CT experiences (n = 37). The relation between demographic variables and CT/no CT-groups was investigated using independent sample t-tests, non-parametric Mann-Whitney U test, or χ2 tests. Significant results in these tests determined the inclusion of that variable in the regression models to control for confounders. Antipsychotics was included based on previous research showing effect on cognition in SSDs receiving antipsychotic treatment (Johnsen et al., 2013).
For the linear regression analyses, CTQ-SF subscale scores (5–25) were used as predictors for the cognitive performance scores. The first analyses used CTQ-SF subscales as predictors for the cognitive domains. Then, we included gender, PANSS positive and negative symptom scales, and antipsychotic medication (DDD) as confounders. Due to multicollinearity, the PANSS total score was omitted from the analyses. In the last model, we controlled for years of education if this was not already corrected for in the test scoring norms. All scoring norms were corrected for age.
The goodness of fit as measured by adjusted R2 (R2a) is assessed as small if ≤0.09, moderate between 0.1 and 0.3 and large effect if ≥0.3 (Mehmetoglu and Jakobsen, 2017). We visually inspected frequency distributions of variables for normality. All regression models were tested for, and adhered to, the assumptions underlying linear multiple regression.
3. Results
3.1. Demographic and clinical data
When examining the CTQ-SF, we found that 21 of 78 (26.9%) patients with SSDs reported emotional abuse, 8 of 78 (10.3%) reported physical abuse, 12 of 78 (15.4%) reported sexual abuse, 23 of 78 (29.5%) reported emotional neglect and 20 of 78 (25.6%) reported physical neglect (according to the cut-off of moderate to severe level of abuse and neglect). Further, 37 (47.4%) patients reported no CT, and 41 (52.6%) patients reported 1–5 CT.
We tested for gender differences between CT/no CT groups, and found that the majority of male participants reported no CT, whereas the majority of the female participants reported CT experiences. This difference was statistically significant (Table 1). We also found that the CT group reported significantly higher levels of positive and negative psychosis symptoms compared to the no CT group (Table 1). There were no other significant effects of demographic and clinical data on CT/no CT groups. Further, serum levels generally corresponded well with the antipsychotic drug doses (DDD), indicating satisfactory adherence with medication.
Mean (SD) median, skewness and kurtosis was the following for cognitive domains: Global cognitive performance 44.76 (7.13) 45.26, −0.24 and 2.55; verbal abilities 47.39 (9.45) 46.12, 0.41 and 2.76; visuospatial abilities 45.50 (9.98) 45.88, −0.30 and 2.40; learning 42.89 (7.41) 41.88, −0.02 and 3.51; memory 44.52 (8.10) 43.84, −0.77 and 4.25; attention/working 43.25 (7.81) 42.89, −0.03 and 2.77; executive abilities 47.05 (11.65) 47.5, −0.51 and 3.23; processing speed 42.10 (9.19) 43.25, −0.34 and 3.13. The values were assessed as satisfactory.
3.2. Comparison of cognitive performance in SSDs by CT/no CT groups
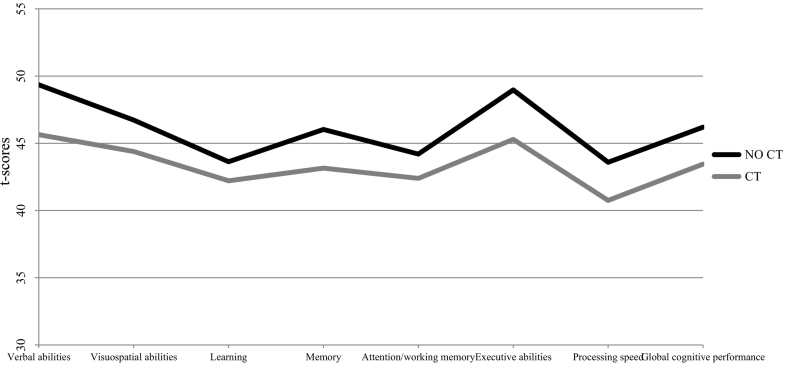
We compared cognitive performance in two groups of SSDs patients; one group with no CT, compared to those reporting CT experiences There were no statistically significant differences in global cognitive performance between CT/no CT groups (p = .095), nor in verbal abilities (p = .087), visuospatial abilities (p = .306), learning (p = .400), memory (p = .116), attention/working memory (p = .309), executive abilities (p = .173) or processing speed (p = .175; Table 1 and Fig. 1).
Fig. 1.
Cognitive performance by cognitive domain grouped by CT.
Note. N = 78, except verbal abilities (n = 76) and executive abilities (n = 75). CT = moderate to severe childhood trauma. Bonferroni adjusted p-level of .00125. No significant results. Verbal abilities: Wechsler Adult Intelligence Scale III (WAIS III; Wechsler, 1997) subtests vocabulary and similarities subtests, and the D-KEFS verbal fluency test (Delis et al., 2001). Visuospatial abilities: Block design and digit symbol-coding subtests of WAIS III, as well as the Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Learning: California verbal learning test (CVLT; Delis et al., 1987) trials 1–5, and the digit span subtest of the WAIS III. Memory: CVLT (subtests short delay free and cued recall, long delay free and cued recall, and delayed recognition) and Rey-Osterrieth Complex Figure Test (Shin et al., 2006). Attention/working memory: Digit vigilance test (Lewis and Rennick, 1979), the CalCAP Continuous performance test subtests sequential reaction time and choice reaction time (Conners, 2002), Trail Making Test (Part B) (Reitan, 1986), the WAIS III subtests digit span and letter-number sequencing, and the Wechsler Memory Scale (Wechsler, 1997). Executive abilities: Wisconsin Card Sorting test (Heaton, 1981) and the Stroop test (Stroop, 1935). Processing speed: Trail Making Test (Part A) (Reitan, 1986), the digit symbol-coding subtest of the WAIS III, the Grooved Pegboard Test (Bryden and Roy, 2005), and the CalCAP subtest simple reaction time (Conners, 2002).
3.3. The association of CT subtypes on cognitive performance
In the first linear regression models, we tested for the effect of CT subtypes on cognitive performance in SSDs. The analyses showed statistically significant effects for the regression models (CT subtypes as predictors) on global cognitive performance, F(5, 69) = 3.14, p = .013, adjusted R2 (R2a) = 0.13, visuospatial abilities, F(5, 72) = 2.99, p = .016, R2a = 0.11, learning, F(5, 72) = 2.76, p = .024, R2a = 0.10, memory, F(5, 72) = 3.32, p = .009, R2a = 0.13, attention and working memory, F(5, 72) = 4.90, p < .001, R2a = 0.20, and processing speed, F(5, 72) = 2.61, p = .031, R2a = 0.10. Goodness of fit for the models was small to moderate. No significant effects were found for the CT subtypes and verbal abilities (p = .131) and executive functioning (p = .419).
After correcting for multiple comparisons (Bonferroni adjustment 0.05/40 = p < .00125), the results indicated that the association between the predictors and cognitive impairment in SSDs is mainly driven by physical neglect in predicting impairment in global cognitive performance (p < .001), visuospatial abilities (p < .001), attention/working memory (p < .001) and memory (p < .001; Table 2).
Table 2.
The effects of CTQ-SF subtypes on cognition by cognitive domain.
| Global cognitive performance | Verbal abilities | Visuospatial abilities | Learning | Memory | Attention/working memory | Executive abilities | Visuomotor processing speed | |
|---|---|---|---|---|---|---|---|---|
| Emotional abuse | −0.134 | 0.096 | 0.026 | −0.143 | 0.088 | −0.227 | −0.222 | −0.444 |
| (−0.58) | (0.30) | (0.08) | (−0.61) | (0.35) | (−0.98) | (−0.57) | (−1.53) | |
| Physical abuse | 0.007 | −0.483 | 0.254 | 0.096 | −0.146 | 0.065 | 0.747 | 0.280 |
| (0.02) | (−0.77) | (0.43) | (0.21) | (−0.31) | (0.15) | (0.99) | (0.50) | |
| Sexual abuse | 0.152 | −0.0265 | 0.014 | −0.084 | 0.233 | 0.021 | −0.191 | 0.242 |
| (0.48) | (−0.06) | (0.03) | (−0.27) | (0.69) | (0.07) | (−0.36) | (0.62) | |
| Emotional neglect | 0.348 | −0.0281 | 0.343 | 0.416⁎ | 0.261 | 0.511⁎⁎ | 0.334 | 0.444 |
| (1.84) | (−0.11) | (1.35) | (2.18) | (1.28) | (2.70) | (1.05) | (1.88) | |
| Physical neglect | −1.288⁎⁎⁎ | −1.013⁎ | −1.560⁎⁎⁎ | −1.027⁎⁎ | −1.243⁎⁎⁎ | −1.342⁎⁎⁎ | −1.182⁎ | −1.142⁎⁎ |
| (−4.12) | (−2.21) | (−3.72) | (−3.27) | (−3.70) | (−4.31) | (−2.13) | (−2.92) | |
| Constant | 50.86 | 58.40 | 52.01 | 47.58 | 50.01 | 49.71 | 51.22 | 47.14 |
| (20.21) | (15.91) | (15.40) | (18.82) | (18.50) | (19.81) | (11.57) | (14.97) | |
| N | 75 | 76 | 78 | 78 | 78 | 78 | 75 | 78 |
Note. Numbers are regression coefficients, and t-statistics in parenthesis. Constant = The value of the dependent variable holding all predictors constant. PANSS = The Positive and Negative Syndrome Scale. CTQ-SF = Childhood trauma questionnaire short-form. Unstandardized coefficients are reported due to the independent variables being measured in the same metric.
p < .05.
p < .01.
Bonferroni corrected p < .00125.
3.4. The association of CT subtypes on cognitive performance controlling for gender and psychosis symptoms
We tested for the effect of CT subtypes on cognitive performance in SSDs and controlled for gender, positive and negative psychosis symptoms, and antipsychotic medication.
The analyses showed statistically significant effects for the regression models based on the predictors (CT subtypes, gender, psychosis symptoms, antipsychotics) on global cognitive performance, F(9, 62) = 2.95, p = .005, R2a = 0.19, visuospatial abilities, F(9, 65) = 2.67, p = .010, R2a = 0.16, learning, F(9, 65) = 2.65, p = .011, R2a = 0.16, memory, F(9, 65) = 3.75, p < .001, R2a = 0.25, and attention and working memory, F(9, 65) = 3.60, p = .001, R2a = 0.24, and processing speed, F(9, 65) = 3.57, p < .001, R2a = 0.24. Goodness of fit for the models (R2a) was assessed as small to moderate. No significant effects were found for the CT subtypes and executive functioning (p = .636) and verbal abilities (p = .122).
After correcting for multiple comparisons (Bonferroni adjustment 0.05/40 = p < .00125), the results indicate that the association between the predictors and cognitive impairment in SSDs is mainly driven by the CT subtype physical neglect (see Table 3). Increase in reported physical neglect predicted more impairment in attention/working memory abilities (p < .001; Table 3).
Table 3.
The effects of CTQ-SF subtypes on cognition by cognitive domain, controlling for antipsychotics, gender and psychosis symptoms.
| Global cognitive performance | Verbal abilities | Visuospatial abilities | Learning | Memory | Attention/working memory | Executive abilities | Processing speed | |
|---|---|---|---|---|---|---|---|---|
| Emotional abuse | 0.202 | 0.162 | 0.428 | −0.0389 | 0.340 | 0.0355 | 0.223 | 0.147 |
| (0.85) | (0.46) | (1.22) | (−0.16) | (1.27) | (0.14) | (0.49) | (0.50) | |
| Physical abuse | −0.223 | −0.772 | −0.132 | −0.239 | −0.502 | −0.286 | 0.615 | −0.216 |
| (−0.54) | (−1.26) | (−0.22) | (−0.56) | (−1.09) | (−0.67) | (0.77) | (−0.43) | |
| Sexual abuse | −0.0505 | 0.00466 | −0.0631 | −0.0577 | 0.231 | −0.0457 | −0.402 | −0.00674 |
| (−0.17) | (0.01) | (−0.15) | (−0.19) | (0.71) | (−0.15) | (−0.71) | (−0.02) | |
| Emotional neglect | 0.198 | 0.0567 | 0.184 | 0.437⁎ | 0.239 | 0.427⁎ | 0.0547 | 0.114 |
| (1.02) | (0.20) | (0.65) | (2.18) | (1.10) | (2.11) | (0.15) | (0.48) | |
| Physical neglect | −1.001⁎⁎ | −0.797 | −1.328⁎⁎ | −0.730⁎ | −0.994⁎⁎ | −1.082⁎⁎⁎ | −1.009 | −0.947⁎⁎ |
| (−3.31) | (−1.79) | (−3.15) | (−2.44) | (−3.07) | (−3.59) | (−1.74) | (−2.69) | |
| Gender | 0.731 | 2.986 | −0.426 | 1.734 | 0.145 | 0.749 | −1.031 | 1.644 |
| (0.45) | (1.26) | (−0.18) | (1.05) | (0.08) | (0.45) | (−0.33) | (0.84) | |
| PANSS positive | 0.109 | 0.287 | 0.123 | 0.0617 | −0.0399 | 0.0721 | 0.0610 | 0.145 |
| (0.78) | (1.38) | (0.59) | (0.42) | (−0.25) | (0.49) | (0.23) | (0.84) | |
| PANSS negative | −0.211 | −0.405 | −0.227 | −0.319⁎ | −0.410⁎⁎ | −0.130 | 0.0151 | −0.00123 |
| (−1.49) | (−1.94) | (−1.14) | (−2.26) | (−2.69) | (−0.91) | (0.06) | (−0.01) | |
| Antipsychotics DDD | −2.448⁎ | −0.500 | −3.435⁎ | −1.594 | −1.648 | −2.825⁎ | −2.438 | −5.129⁎⁎⁎ |
| (−2.34) | (−0.32) | (−2.22) | (−1.46) | (−1.39) | (−2.56) | (−1.21) | (−3.98) | |
| Constant | 54.76 | 57.28 | 57.26 | 52.06 | 58.20 | 53.28 | 53.25 | 51.71 |
| (15.74) | (11.17) | (11.72) | (15.04) | (15.52) | (15.26) | (7.98) | (12.68) | |
| N | 72 | 73 | 75 | 75 | 75 | 75 | 72 | 75 |
Note. t-statistics in parenthesis. Constant = The value of the dependent variable holding all predictors constant. PANSS = The Positive and Negative Syndrome Scale. CTQ-SF = Childhood trauma questionnaire short-form. DDD = the assumed average maintenance dose per day for a drug used for its main indication in adults. Unstandardized coefficients are reported due to the independent variables being measured in the same metric.
p < .05.
p < .01.
Bonferroni corrected p < .00125.
Lastly, we performed the analyses including education as a predictor for the cognitive domains. The analyses showed statistically significant effects for the regression models based on the predictors (CT subtypes, gender, psychosis symptoms, antipsychotics and education) on global cognitive performance, F(10, 61) = 4.59, p < .001, R2a = 0.33, verbal abilities, F(10, 62) = 3.42, p < .001, R2a = 0.25, visuospatial abilities, F(10, 64) = 5.05, p < .001, R2a = 0.35, learning, F(10, 64) = 4.34, p < .001, R2a = 0.31, memory, F(10, 64) = 5.85, p < .001, R2a = 0.40, attention and working memory, F(9, 65) = 3.60, p < .001, R2a = 0.24, and processing speed, F(9, 65) = 3.57, p < .001, R2a = 0.23. Goodness of fit for the models (R2a) was assessed as moderate to large. No significant effects were found for the CT subtypes and executive functioning (p = .722).
After correcting for multiple comparisons (Bonferroni adjustment 0.05/40 = p < .00125), the results indicate that the association between the predictors and cognitive impairment in SSDs is mainly driven by the CT subtype physical neglect (see Table 4). Increase in reported physical neglect predicted more impairment in attention/working memory abilities (p < .001; Table 4).
Table 4.
The effects of CTQ-SF subtypes on cognition by cognitive domain, controlling for antipsychotics, education, gender and psychosis symptoms.
| Global cognitive performance | Verbal abilities | Visuospatial abilities | Learning | Memory | Attention/working memory | Executive abilities | Processing speed | |
|---|---|---|---|---|---|---|---|---|
| Emotional abuse | 0.192 | 0.147 | 0.432 | −0.0360 | 0.344 | 0.0355 | 0.223 | 0.147 |
| (0.88) | (0.46) | (1.40) | (−0.16) | (1.42) | (0.14) | (0.49) | (0.50) | |
| Physical abuse | −0.310 | −0.909 | −0.312 | −0.351 | −0.631 | −0.286 | 0.615 | −0.216 |
| (−0.82) | (−1.64) | (−0.59) | (−0.91) | (−1.52) | (−0.67) | (0.77) | (−0.43) | |
| Sexual abuse | 0.158 | 0.323 | 0.301 | 0.171 | 0.493 | −0.0457 | −0.402 | −0.00674 |
| (0.57) | (0.80) | (0.79) | (0.61) | (1.65) | (−0.15) | (−0.71) | (−0.02) | |
| Emotional neglect | 0.124 | −0.0477 | 0.0592 | 0.359 | 0.149 | 0.427⁎ | 0.0547 | 0.114 |
| (0.70) | (−0.18) | (0.24) | (1.95) | (0.76) | (2.11) | (0.15) | (0.48) | |
| Physical neglect | −0.769⁎⁎ | −0.452 | −0.994⁎ | −0.520 | −0.754⁎ | −1.082⁎⁎⁎ | −1.009 | −0.947⁎⁎ |
| (−2.72) | (−1.10) | (−2.62) | (−1.87) | (−2.54) | (−3.59) | (−1.74) | (−2.69) | |
| Gender | 0.664 | 3.010 | −0.523 | 1.673 | 0.0754 | 0.749 | −1.031 | 1.644 |
| (0.45) | (1.41) | (−0.25) | (1.11) | (0.05) | (0.45) | (−0.33) | (0.84) | |
| PANSS positive | 0.0337 | 0.168 | 0.000714 | −0.0151 | −0.128 | 0.0721 | 0.0610 | 0.145 |
| (0.26) | (0.89) | (0.00) | (−0.11) | (−0.89) | (0.49) | (0.23) | (0.84) | |
| PANSS negative | −0.149 | −0.317 | −0.144 | −0.267⁎ | −0.350⁎ | −0.130 | 0.0151 | −0.00123 |
| (−1.15) | (−1.67) | (−0.82) | (−2.07) | (−2.54) | (−0.91) | (0.06) | (−0.01) | |
| Antipsychotics DDD | −2.081⁎ | 0.0694 | −2.739⁎ | −1.156 | −1.147 | −2.825⁎ | −2.438 | −5.129⁎⁎⁎ |
| (−2.17) | (0.05) | (−2.00) | (−1.15) | (−1.07) | (−2.56) | (−1.21) | (−3.98) | |
| Education | 0.925⁎⁎⁎ | 1.445⁎⁎⁎ | 1.570⁎⁎⁎ | 0.989⁎⁎⁎ | 1.131⁎⁎⁎ | |||
| (3.72) | (3.99) | (4.42) | (3.81) | (4.08) | ||||
| Constant | 41.57 | 36.90 | 35.47 | 38.34 | 42.50 | 53.28 | 53.25 | 51.71 |
| (8.75) | (5.36) | (5.42) | (8.01) | (8.31) | (15.26) | (7.98) | (12.68) | |
| N | 72 | 73 | 75 | 75 | 75 | 75 | 72 | 75 |
Note. t-statistics in parenthesis. Constant = The value of the dependent variable holding all predictors constant. PANSS = The Positive and Negative Syndrome Scale. CTQ-SF = Childhood trauma questionnaire short-form. DDD = the assumed average maintenance dose per day for a drug used for its main indication in adults. Unstandardized coefficients are reported due to the independent variables being measured in the same metric. Years of education is included in the regression models only in domains that did not already have the correction in the cognitive test scoring norms.
p < .05.
p < .01.
Bonferroni corrected p < .00125.
4. Discussion
Reported levels of childhood physical neglect in our sample of SSDs predicted significant impairment in cognitive performance in attention/working memory abilities after adjusting for multiple comparisons, and controlling psychosis symptoms, antipsychotics, years of education, age and gender. In contrast, we found no significant differences in cognitive functioning between CT and no CT groups, nor between any other subtype of CT and the studied cognitive domains. Our findings regarding physical neglect indicate that CT subtypes might differentially influence cognitive abilities.
Half of our sample of patients with SSDs reported experiences of moderate to severe CT. Of those reporting CT, the majority had experienced up to three subtypes of CT. This is in line with previous studies on CT in SSDs (McGrath et al., 2017), and reports of co-occurrence of types of CT (Kessler et al., 2010). Our findings regarding associations between reports of CT and cognitive impairment, are in agreement with previous reports (Quide et al., 2016; Shannon et al., 2011). We did not find all types of CT to predict cognitive impairment in our sample of SSDs. This may in part explain inconsistency in previous research (Dauvermann and Donohoe, 2019): While some studies report associations between CT and cognitive impairment in SSDs (Aas et al., 2014), others, e.g. Ruby et al. (2017), did not find early trauma to predict cognitive impairment. Our findings indicate that CT subtype physical neglect may in part explain these discrepancies.
Schalinski et al. (2016) suggested that some of the variance in cognitive impairment in SSDs could be explained by subtype of CT, as demonstrated by our findings that physical neglect is more closely associated with poorer cognitive performance. Our findings are in line with reports such as Li et al. (2017), whom in a sample of patients with SSDs found an association between physical neglect and impaired attention and memory, Traditionally, research has mainly focused on sexual and physical abuse (De Bellis et al., 2009). Although childhood neglect is frequently reported, the neurocognitive effects of neglect are understudied (De Bellis et al., 2009). As neglect entails an inability to meet basic emotional and physical needs, including nutrition and proper medical care during illness, and is related to other forms of abuse, the adverse neurocognitive consequences could be more extensive than for other types of abuse (Wells et al., 2019). Molina et al. (2018) found physical and emotional neglect to be negatively related to cognitive measures and report preliminary evidence for a role of early neglect in disrupted development of prefrontal cortex (PFC) connectivity and disturbed myelination regulation in SSDs. Early neglect at 3 years was found to predict hair cortisol concentration (HCC) in a transdiagnostic group (Schalinski et al., 2019b). HCC indicates cumulative cortisol levels associated with long term stress-reactions, indicating altered HPA-axis biology following inadequate care (Schalinski et al., 2019b). Thus, the absence of a reliable caregiver could be associated with negative impact on the developing brain (De Bellis et al., 2009) due to disrupting normative brain development during sensitive periods (Schalinski et al., 2019a), possibly affecting cognitive functioning in adulthood. Childhood neglect could thus be characterized as an impoverished parent-child relationship, which may in turn be a marker of an inherited cognitive vulnerability compounded by a gene-environment interaction, thus increasing psychopathology (Schalinski et al., 2019a). Maltreated and neglected children are also more likely to have parents who were themselves maltreated or traumatized, indicating intergenerational transmission involving maltreatment and neglect, deficient parenting skills, family stressors and genetic and epigenetic risk (Teicher and Samson, 2013).
When interpreting our findings, our limited sample size should be taken into account, as this boosts the risk of a Type II error. We did not use a control group in the present study, limiting knowledge on how levels of CT and cognitive performance compare to participants without SSDs. We were unable to control for cannabis use, socio-economic status or parental cognitive functioning, known to influence cognitive impairment in SSDs (Wells et al., 2019; Løberg et al., 2014). CT is measured retrospectively and by self-report, which might be associated to problems with validity and reliability. However, retrospective measurement of CT in SSDs is indicated to be valid and reliable (Fisher et al., 2011), albeit afflicted with common problems of retrospective self-reported methods of measuring CT (Baldwin et al., 2019).
Strengths of the study are the large clinical cognitive test-battery used, and the CTQ-SF is a well validated measure of CT, which allowed us to better differentiate between subtypes than much of the previous literature using other measures. Future research could benefit from a longitudinal design, with CT measured more close in time to the trauma and with additional measures to self-report.
Funding
The study was publicly funded by the Research Council of Norway (#213727) and the Western Norway Regional Health Authority (#911679, #911820) to EJ; by the Northern Norway Regional Health Authority (#PFP1300-16) to NM; as well as by the participating hospitals and universities. The study was not supported or funded by the pharmaceutical industry, nor did other funding agencies support data collection, interpretation, analyses or writing the report.
CRediT authorship contribution statement
N. Mørkved:Writing - original draft, Writing - review & editing, Investigation, Visualization, Conceptualization.E. Johnsen:Data curation, Writing - original draft, Writing - review & editing, Conceptualization.R.A. Kroken:Data curation, Writing - original draft, Writing - review & editing, Conceptualization.R. Gjestad:Writing - original draft, Writing - review & editing, Formal analysis, Conceptualization.D. Winje:Writing - original draft, Writing - review & editing, Conceptualization.J. Thimm:Writing - original draft, Writing - review & editing, Conceptualization.F. Fathian:Data curation, Writing - original draft, Writing - review & editing, Conceptualization.M. Rettenbacher:Data curation, Writing - original draft, Writing - review & editing, Conceptualization.L.G. Anda:Data curation, Writing - original draft, Writing - review & editing, Conceptualization.E.M. Løberg:Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors would like to thank all participants and collaborators for participation in the project.
References
- Aas M., Dazzan P., Mondelli V., Melle I., Murray R.M., Pariante C.M. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:182. doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J.R., Reuben A., Newbury J.B., Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. Jama Psychiat. 2019;76:584–593. doi: 10.1001/jamapsychiatry.2019.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L. The Psychological Corporation, Harcourt Brace & Company; San Antonio, USA: 1998. Childhood Trauma Questionnaire. A Retrospective Self-report Manual. [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Harvey P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Bryden P.J., Roy E.A. A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn. 2005;58:258–268. doi: 10.1016/j.bandc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Carrion R.E., McLaughlin D., Auther A.M., Olsen R., Correll C.U., Cornblatt B.A. The impact of psychosis on the course of cognition: a prospective, nested case-control study in individuals at clinical high-risk for psychosis. Psychol. Med. 2015;45:3341–3354. doi: 10.1017/S0033291715001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K. Multi-health system; Toronto, Canada: 2002. Conners' Continuous Performance Test. [Google Scholar]
- Dauvermann M.R., Donohoe G. The role of childhood trauma in cognitive performance in schizophrenia and bipolar disorder - a systematic review. Schizophr Res Cogn. 2019;16:1–11. doi: 10.1016/j.scog.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Hooper S.R., Spratt E.G., Woolley D.P. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J. Int. Neuropsychol. Soc. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D.C., Kramer J.H., Kaplan E., Thompkins B.A.O. Psychological Corporation San Antonio; 1987. CVLT: California Verbal Learning Test-Adult Version: Manual. [Google Scholar]
- Delis D.C., Kaplan E., Kramer J.H. Pearson; 2001. Delis-Kaplan Executive Function System® (D-KEFS®). Examiner's Manual: Flexibility of Thinking, Concept Formation, Problem Solving, Planning, Creativity, Impluse Control, Inhibition. [Google Scholar]
- Dovran A., Winje D., Overland S.N., Breivik K., Arefjord K., Dalsbo A.S., Jentoft M.B., Hansen A.L., Waage L. Psychometric properties of the Norwegian version of the Childhood Trauma Questionnaire in high-risk groups. Scand. J. Psychol. 2013;54:286–291. doi: 10.1111/sjop.12052. [DOI] [PubMed] [Google Scholar]
- Erdodi L.A., Kirsch N.L., Sabelli A.G., Abeare C.A. The Grooved Pegboard Test as a validity indicator—a study on psychogenic interference as a confound in performance validity research. Psychol Inj Law. 2018;11:307–324. [Google Scholar]
- Fisher H., Craig T.K., Fearon P., Morgan K., Dazzan P., Lappin J., Hutchinson G., Doody G.A., Jones P.B., McGuffin P., Murray R.M., Leff J., Morgan C. Reliability and comparability of psychosis patients' retrospective reports of childhood abuse. Schizophr. Bull. 2011;37:546–553. doi: 10.1093/schbul/sbp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Randolph C., Carpenter C.J., Goldberg T.E., Weinberger D.R. The performance of patients with schizophrenia on the wechsler memory scale-revised. Clin. Neuropsychol. 1992;6:367–373. [Google Scholar]
- Heaton R.K. Psychological assessment resources, Inc; Odessa, FL: 1981. Wisconsin Card Sorting Test Manual. [Google Scholar]
- Johnsen E., Jorgensen H.A., Kroken R.A., Loberg E.M. Neurocognitive effectiveness of quetiapine, olanzapine, risperidone, and ziprasidone: a pragmatic, randomized trial. Eur Psychiatry. 2013;28:174–184. doi: 10.1016/j.eurpsy.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Keefe R.S. Schizophrenia is a cognitive illness: time for a change in focus. Jama Psychiat. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opfer L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Aguilar-Gaxiola S., Alhamzawi A.O., Alonso J., Angermeyer M., Benjet C., Bromet E., Chatterji S., de Girolamo G., Demyttenaere K., Fayyad J., Florescu S., Gal G., Gureje O., Haro J.M., Hu C.Y., Karam E.G., Kawakami N., Lee S., Lepine J.P., Ormel J., Posada-Villa J., Sagar R., Tsang A., Ustun T.B., Vassilev S., Viana M.C., Williams D.R. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Brit J Psychiat. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B., Lange F., Steinke A. The reliability of the Wisconsin Card Sorting Test in clinical practice. Assessment. 2019:1–16. doi: 10.1177/1073191119866257. 1073191119866257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R., Rennick P.M. Axon, Grosse Point; MI: 1979. Manual for the Repeatable Cognitive-Perceptual-Motor Battery. [Google Scholar]
- Li X.B., Bo Q.J., Zhang G.P., Zheng W., Wang Z.M., Li A.N., Tian Q., Liu J.T., Tang Y.L., Wang C.Y. Effect of childhood trauma on cognitive functions in a sample of Chinese patients with schizophrenia. Compr. Psychiatry. 2017;76:147–152. doi: 10.1016/j.comppsych.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Løberg E.M., Helle S., Nygård M., Berle J.O., Kroken R.A., Johnsen E. The Cannabis pathway to non-affective psychosis may reflect less neurobiological vulnerability. Front Psychiatry. 2014;5:159. doi: 10.3389/fpsyt.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J.J., McLaughlin K.A., Saha S., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., Bruffaerts R., de Girolamo G., de Jonge P., Esan O., Florescu S., Gureje O., Haro J.M., Hu C., Karam E.G., Kovess-Masfety V., Lee S., Lepine J.P., Lim C.C.W., Medina-Mora M.E., Mneimneh Z., Pennell B.E., Piazza M., Posada-Villa J., Sampson N., Viana M.C., Xavier M., Bromet E.J., Kendler K.S., Kessler R.C., Survey W.W.M.H. The association between childhood adversities and subsequent first onset of psychotic experiences: a cross-national analysis of 23 998 respondents from 17 countries. Psychol. Med. 2017;47:1230–1245. doi: 10.1017/S0033291716003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmetoglu M., Jakobsen T.G. Sage; UK: 2017. Applied Statistics Using Stata. A Guide for the Social Sciences. [Google Scholar]
- Miller E. Norland Software; Los Angeles: 1990. California Computerized Assessment Package. [Google Scholar]
- Molina V., Alvarez-Astorga A., Lubeiro A., Ortega D., Jimenez M., Del Valle P., Marques P., de Luis-Garcia R. Early neglect associated to prefrontal structural disconnectivity in schizophrenia. Schizophr. Res. 2018;192:487–488. doi: 10.1016/j.schres.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Mørkved N., Endsjø M., Winje D., Johnsen E., Dovran A., Arefjord K., Kroken R.A., Helle S., Anda-Ågotnes L.G., Rettenbacher M.A., Huber N., Løberg E.M. Childhood trauma in schizophrenia spectrum disorder as compared to other mental health disorders. Psychosis. 2017;9:48–56. [Google Scholar]
- Mørkved N., Winje D., Dovran A., Arefjord K., Johnsen E., Kroken R.A., Anda-Ågotnes L.G., Thimm J.C., Sinkeviciute I., Rettenbacher M., Løberg E.M. Childhood trauma in schizophrenia spectrum disorders as compared to substance abuse disorders. Psychiatry Res. 2018;261:481–487. doi: 10.1016/j.psychres.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quide Y., O'Reilly N., Rowland J.E., Carr V.J., Elzinga B.M., Green M.J. Effects of childhood trauma on working memory in affective and non-affective psychotic disorders. Brain Imaging and Behav. 2016;11:722–735. doi: 10.1007/s11682-016-9548-z. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Reitan Neuropsychology Laboratory; 1986. Trail Making Test: Manual for Administration and Scoring. [Google Scholar]
- Ruby E., Rothman K., Corcoran C., Goetz R.R., Malaspina D. Influence of early trauma on features of schizophrenia. Early Interv Psychia. 2017;11:322–333. doi: 10.1111/eip.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski I., Teicher M.H., Nischk D., Hinderer E., Muller O., Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. doi: 10.1186/s12888-016-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski I., Breinlinger S., Hirt V., Teicher M.H., Odenwald M., Rockstroh B. Environmental adversities and psychotic symptoms: the impact of timing of trauma, abuse, and neglect. Schizophr. Res. 2019;205:4–9. doi: 10.1016/j.schres.2017.10.034. [DOI] [PubMed] [Google Scholar]
- Schalinski I., Teicher M.H., Rockstroh B. Early neglect is a key determinant of adult hair cortisol concentration and is associated with increased vulnerability to trauma in a transdiagnostic sample. Psychoneuroendocrinology. 2019;108:35–42. doi: 10.1016/j.psyneuen.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Shannon C., Douse K., McCusker C., Feeney L., Barrett S., Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr. Bull. 2011;37:531–537. doi: 10.1093/schbul/sbp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M.-S., Park S.-Y., Park S.-R., Seol S.-H., Kwon J.S. Clinical and empirical applications of the Rey–Osterrieth complex figure test. Nat. Protoc. 2006;1:892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- Shunk A.W., Davis A.S., Dean R.S. TEST REVIEW: Dean C. Delis, Edith Kaplan & Joel H. Kramer, Delis Kaplan Executive Function System (D-KEFS), The Psychological Corporation, San Antonio, TX, 2001. $415.00 (complete kit) Appl. Neuropsychol. 2006;13 275-27. [Google Scholar]
- Silva M.A. Development of the WAIS-III: a brief overview, history, and description. Grad J Couns Psychol. 2008;1:11. [Google Scholar]
- Spitzer R.L., Williams J.B.W., Gibbon M., First M.B. The structured clinical interview for Dsm-III-R (Scid). 1. History, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial-verbal reaction. J. Exp. Psychol. 1935;18:643–663. [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucok A., Kaya H., Ugurpala C., Cikrikcili U., Ergul C., Yokusoglu C., Bulbul O., Direk N. History of childhood physical trauma is related to cognitive decline in individuals with ultra-high risk for psychosis. Schizophr. Res. 2015;169:199–203. doi: 10.1016/j.schres.2015.08.038. [DOI] [PubMed] [Google Scholar]
- van Os J., Marsman A., van Dam D., Simons C.J. Evidence that the impact of childhood trauma on IQ is substantial in controls, moderate in siblings, and absent in patients with psychotic disorder. Schizophr. Bull. 2017;43:316–324. doi: 10.1093/schbul/sbw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varese F., Smeets F., Drukker M., Lieverse R., Lataster T., Viechtbauer W., Read J., van Os J., Bentall R.P. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr. Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological corporation; San Antonio, TX: 1997. Wechsler Memory Scale (WMS-III) [Google Scholar]
- Wells R., Jacomb I., Swaminathan V., Sundram S., Weinberg D., Bruggemann J., Cropley V., Lenroot R.K., Pereira A.M., Zalesky A., Bousman C., Pantelis C., Weickert C.S., Weickert T.W. The impact of childhood adversity on cognitive development in schizophrenia. Schizophr. Bull. 2019;46(1):140–153. doi: 10.1093/schbul/sbz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 1992. ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]
- Woods S.P., Delis D.C., Scott J.C., Kramer J.H., Holdnack J.A. The California Verbal Learning Test–second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]