Highlights
-
•
Poor adherence to therapy for T2DM is associated with lower reductions in HbA1c.
-
•
About one-third of patients initiating metformin discontinued within 12 months.
-
•
Fewer than 50% of all patients were adherent to metformin.
Keywords: Discontinuation, Adherence, Type 2 diabetes mellitus, Oral anti-hyperglycemic agents, Drug therapy, Metformin
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CPRD, Clinical Practice Research Datalink; MPR, medication possession ratio; PDC, proportion-of-days covered; UKPDS, United Kingdom Prospective Diabetes Study
Abstract
Aims
To describe discontinuation and adherence to metformin in the United Kingdom.
Methods
This was a retrospective analysis of data from the Clinical Practice Research Datalink database of type 2 diabetes patients aged ≥18 years with ≥1 metformin prescription in 2013. Metformin use was assessed in new and ongoing users, defined, respectively, as not having or having a prescription for metformin in the baseline period. Discontinuation was assessed in all patients and adherence in patients who did not discontinue metformin. Factors predictive of discontinuation and adherence were assessed.
Results
Discontinuation among new and ongoing users was 35.9% and 23.1%, respectively. Among the continuers of metformin treatment, the adherence rate was 40.5% and 44.3% among new and ongoing users, respectively. Among new users, baseline use of DDP-4 inhibitors (HR 1.276) and diabetes duration (HR 1.013) were associated with an increased risk of discontinuation, whereas increased age (HR 0.997), concomitant lipid-lowering therapy (HR 0.956), macrovascular disease (HR 0.952), and chronic kidney disease (HR 0.952) were associated with a decreased risk of discontinuation among ongoing users. Variables positively associated with adherence in both user groups were (HR values for all patients) age (1.021), smoking status (1.188), and baseline comorbidities: chronic kidney disease, depression, dementia, and chronic obstructive pulmonary disease (1.106, 1.192, 2.27, and 1.211, respectively), while obesity (0.936) and HbA1c 8.0–8.9% (0.862; reference <6.5%) were negatively associated with adherence.
Conclusions
About one-third of patients initiating metformin discontinued within 12 months and fewer than 50% of all patients are adherent to metformin.
Introduction
Among adults, aged ≥18 years, there was an estimated 451 million cases of diabetes in 2017 globally [1]. The highest age-adjusted prevalence was found in the North American and Caribbean regions (10.8%) while the lowest was found in the African region (4.2%) [1]. The prevalence of type 2 diabetes among adults in the United Kingdom is estimated at 5.6–5.8% [2], [3]. Unless contraindicated, metformin is the recommended first-line glucose-lowering pharmacotherapy for adults with type 2 diabetes [4]. This recommendation follows results of the United Kingdom Prospective Diabetes Study (UKPDS), which showed a 32% reduction in any diabetes-related endpoint, a 42% reduction in diabetes-related death, and a 36% reduction in all-cause mortality after treatment with metformin for a median of 10.7 years [5].
Metformin is the first anti-diabetic drug prescription for 80–90% of type 2 diabetes patients in primary care in the United Kingdom [6], [7], [8], [9]. Whether the patients prescribed metformin receive the therapeutic benefits achieved in the UKPDS clinical trial is dependent on their patterns of metformin use. In observational studies, poor adherence to pharmacotherapy for type 2 diabetes is associated with lower reductions in HbA1c and increased rates of hospitalization and mortality [10], [11].
Monitoring the rate of patients’ adherence to metformin is a continuing concern globally. The objective of this study was to report rates of metformin discontinuation and adherence, and to determine factors associated with these measures of metformin use in type 2 diabetes patients in the United Kingdom.
Materials and methods
Study design and data source
This was a retrospective cohort study of patients with type 2 diabetes identified in the Clinical Practice Research Datalink (CPRD) data set [12]. CPRD is a longitudinal, population-representative database, managed by the United Kingdom Department of Health, consisting of records for over 13 million patients entered by physicians in approximately 650 primary care practices. The study period was January 1, 2012 to December 31, 2014 (Fig. A.1). The date of the first metformin prescription in calendar year 2013 was designated as the index date, and the 12-month periods before and after the index date were defined as the baseline and follow-up periods, respectively. The study period (2012–2014) was determined a prior to the study analysis via a study protocol, thus eliminating the possibility of authors adjusting the study definitions to produce more favorable data.
Fig. A1.
Study design.
Study population
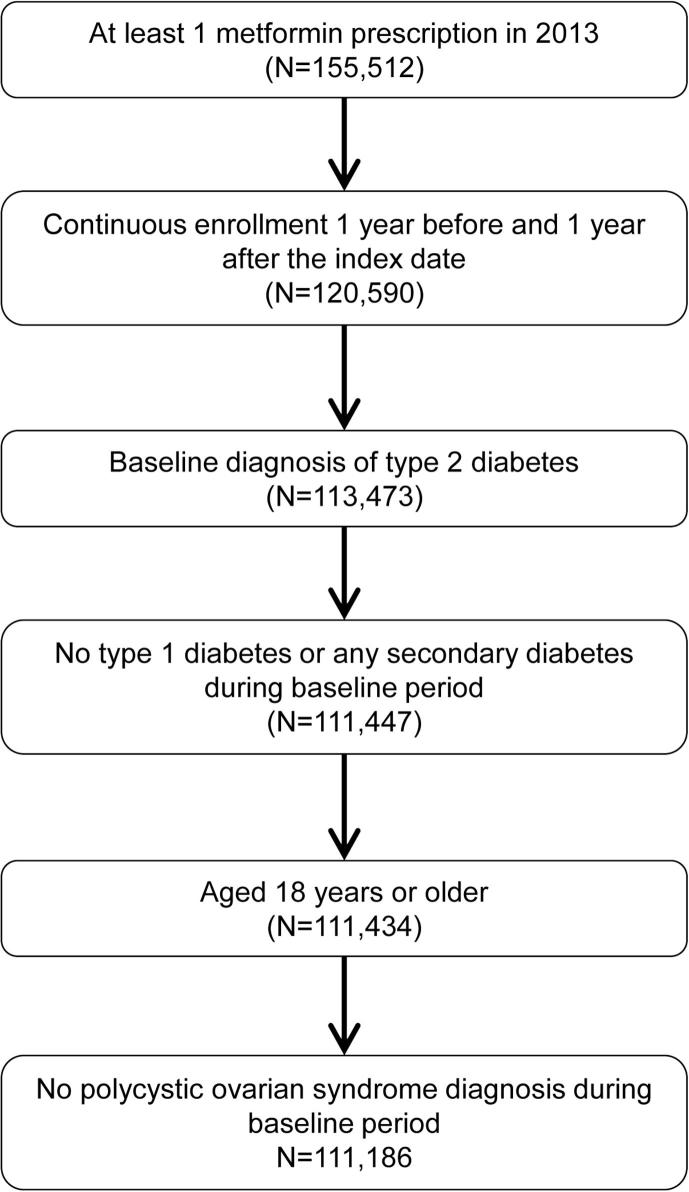
Patients were included in the analysis if they had Read/OXMIS code indicating a diagnosis of type 2 diabetes in the baseline period (Table A.1), were 18 years of age or older on the index date, were continuously enrolled during the baseline and follow-up periods, and had at least one metformin prescription during the 2013 calendar year. Patients were ineligible for inclusion if they had a diagnosis of type 1 diabetes mellitus, gestational diabetes or other forms of secondary diabetes or a diagnosis of polycystic ovarian syndrome during the baseline period.
Study variables
Patients’ demographic information was based on their member file for 2013. Clinical and laboratory variables were identified from Read codes recorded during the baseline period and included hypoglycemia, the Charlson comorbidity index (CCI), body mass index (BMI), HbA1c value, and common comorbidities of diabetes as listed by Suh et al. [13]. The study outcome variables were measures of metformin use during the follow-up period: rates of metformin discontinuation and adherence, expressed as proportions of patients. The total numbers of metformin prescriptions and metformin treatment days were determined. Discontinuation (yes/no) was defined as a metformin treatment gap of ≥90 days. Adherence was determined for patients who did not discontinue metformin. Adherence was defined as a proportion-of-days covered (PDC) value of ≥0.80, where PDC was calculated as the sum of days’ supply of metformin divided by the number of days between the first fill of a metformin prescription in the follow-up period and the end of the follow-up period.
Statistical analysis
Rates of metformin discontinuation and adherence were expressed as proportions (percentages) of patients. These statistics were determined for the overall study cohort and stratified by new or ongoing metformin use, as defined by the occurrence or not of a metformin prescription in the baseline period. Baseline clinical and laboratory variables associated with metformin use patterns in the follow-up period were determined. Variables associated with metformin discontinuation were assessed by a Cox proportional hazards regression model with time-dependent covariates. A separate multivariate logistic regression analysis was conducted to assess variables associated with metformin adherence. Predictor variables were age on the index date, gender, smoking status, HbA1c % (<6.5, 6.5–6.9, 7.0–7.9, 8.0–8,9, ≥9.0), body mass index, number of medications in baseline period (5–10, >10), baseline use of prescription anti-diabetic drugs (DPP-4 inhibitors, sulfonylureas, glucagon-like peptide-1, thiazolidinediones, meglitinides, and alpha-glucose inhibitors), concomitant medication use (anti-hypertensive and lipid-lowering therapy), diabetes-related comorbidities (hypoglycemia, retinopathy, neuropathy, macrovascular disease, chronic kidney disease, depression, hypertension, obesity, hyperlipidemia, dementia, chronic obstructive pulmonary disease, and anxiety), and duration of type 2 diabetes. Hazard ratio (HR) and odds ratio (OR) values statistically significant at P < 0.01 and differing from the reference by >10% were considered meaningful.
Results
Patient characteristics
A total of 111,186 patients were included in the analysis (Fig. 1), of whom 11,227 (10.1%) were new metformin users and 99,959 (89.9%) were ongoing users. Their characteristics are shown in Table 1. The mean (SD) age for the entire study cohort was 66.37 (12.25) years, 58.6% were male, and the mean HbA1c value was 66 mmol/mol (8.2%). The duration of type 2 diabetes was mean (SD) 3.1 (4.7) years in the new user group and 8.2 (5.8) years in the ongoing group.
Fig. 1.
Patient selection.
Table 1.
Characteristics of type 2 diabetes patients.†
| All patients (N = 111,186) | New metformin users (N = 11,227) | Ongoing metformin users (N = 99,959) | |
|---|---|---|---|
| Age, mean (SD) years | 66.37 (12.25) | 62.90 (13.23) | 66.76 (12.07) |
| Male | 65,131 (58.6) | 6,461 (57.5) | 58,670 (58.7) |
| Smoker | 16,260 (14.6) | 1,921 (17.1) | 14,339 (14.3) |
| BMI, mean (SD) kg/m2 | 31.69 (6.48) | 32.38 (6.77) | 31.61 (6.44) |
| HbA1c mmol/mol, mean [% (SD)] | 66 [8.2 (3.1)] | 73 [8.8 (3.8)] | 66 [8.2 (3.0)] |
| <6.5% | 15,869 (14.3) | 898 (8.0) | 14,971 (15.0) |
| 6.5–6.9% | 18,352 (16.5) | 1,201 (10.7) | 17,151 (17.2) |
| 7.0–7.9% | 28,042 (25.2) | 1,482 (13.2) | 26,560 (26.6) |
| 8.0–8.9% | 11,697 (10.5) | 572 (5.1) | 11,125 (11.1) |
| ≥9.0% | 15,901 (14.3) | 1,169 (10.4) | 14,732 (14.7) |
| Missing | 21,325 (19.2) | 5,905 (52.6) | 15,420 (15.4) |
| Baseline medication use‡ | |||
| 5–10 medications | 54,358 (48.9) | 1,593 (14.2) | 52,765 (52.8) |
| >10 medications | 1,037 (0.9) | 15 (0.1) | 1,022 (1.0) |
| Baseline use of anti-diabetic drugs | |||
| Metformin | 99,959 (89.9) | – | 99,959 (100.0) |
| Sulfonylureas | 38,309 (34.5) | 1,149 (10.2) | 37,160 (37.2) |
| DPP-4 inhibitors | 15,095 (13.6) | 319 (2.8) | 14,776 (14.8) |
| Thiazolidinediones | 9,978 (9.0) | 153 (1.4) | 9,825 (9.8) |
| GLP-1 | 5,015 (4.5) | 89 (0.8) | 4,926 (4.9) |
| Other§ | 708 (0.6) | 14 (0.1) | 694 (0.7) |
| Concomitant medication use | |||
| Anti-hypertensive drugs | 84,399 (75.9) | 6445 (57.4) | 77,954 (78.0) |
| Lipid-lowering therapy | 87,932 (79.1) | 5661 (50.4) | 82,271 (82.3) |
| NSAIDs | 14,933 (13.4) | 1454 (13.0) | 13,479 (13.5) |
| CCI, mean (SD) | 2.48 (1.66) | 1.82 (1.55) | 2.56 (1.65) |
| Diabetes-related comorbidities¶ | |||
| Hypertension | 67,417 (60.6) | 5,574 (49.6) | 61,843 (61.9) |
| Retinopathy | 35,315 (31.8) | 1,486 (13.2) | 33,829 (33.8) |
| Depression | 34,358 (30.9) | 3,557 (31.7) | 30,801 (30.8) |
| Obesity | 28,391 (25.5) | 2,539 (22.6) | 25,852 (25.9) |
| Macrovascular disease | 26,733 (24.0) | 2,293 (20.4) | 24,440 (24.5) |
| Anxiety | 23,852 (21.5) | 2,588 (23.1) | 21,264 (21.3) |
| Chronic kidney disease | 20,967 (18.9) | 1,429 (12.7) | 19,538 (19.5) |
| Hyperlipidemia | 17,607 (15.8) | 1,442 (12.8) | 16,165 (16.2) |
| Malignant neoplasm | 11,444 (10.3) | 1,102 (9.8) | 10,342 (10.3) |
BMI, body mass index; CCI, Charlson comorbidity index; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; NSAIDs, nonsteroidal anti-inflammatory drugs.
Values are presented as N (%) unless otherwise indicated.
Excluding the index date.
Other anti-diabetic drugs were meglitinide, alpha-glucosidase, and sodium-glucose cotransporter inhibitors.
Only comorbidities present in ≥10% of the population were included.
Discontinuation and adherence rates
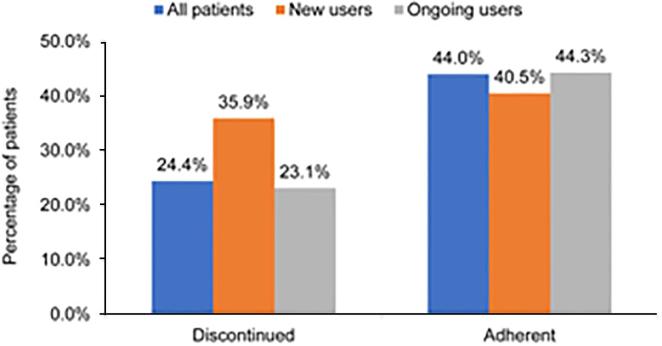
The numbers of patients who discontinued among new metformin and ongoing users were 4030 (35.9%) and 23,105 (23.1%), respectively (Fig. 2). Among new and ongoing metformin users who did not discontinue metformin (7197 and 76,854, respectively), 2914 (40.5%) and 34,082 (44.3%) patients were considered adherent (PDC ≥ 80%) (Fig. 2).
Fig. 2.
Patterns of metformin use. Discontinuation was assessed in the full study sample. Adherence was assessed in patients not discontinuing during the follow-up period. Sample sizes for analysis of discontinuation: all, 111,186; new, 11,227; and ongoing, 99,959. For adherence: all, 84,051; new, 7197; and ongoing, 76,854.
Regression results
In the new metformin user group, baseline use of DPP-4 inhibitors (HR 1.28) and duration of diabetes (HR 1.01) were associated with an increased risk of metformin discontinuation (Table 2). In the ongoing metformin user group, 4 variables were associated with a reduced risk (of <5%) of discontinuation (increased age, concomitant use of lipid-lowering therapy, macrovascular disease, and chronic kidney disease; Table 2).
Table 2.
Variables associated with discontinuation of metformin.
| New metformin users (N = 11,227) |
Ongoing metformin users (N = 99,959) |
|||
|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| Age | – | – | 0.997 | (0.996–0.999) |
| Baseline DPP-4 inhibitors | 1.276 | (1.069–1.524) | – | – |
| Concomitant lipid-lowering therapy | – | – | 0.956 | (0.927–0.987) |
| Macrovascular disease | – | – | 0.952 | (0.922–0.984) |
| Chronic kidney disease | – | – | 0.952 | (0.919–0.986) |
| Diabetes duration | 1.013 | (1.005–1.020) | – | – |
CI, confidence interval.
Statistically significant effect sizes at P < 0.01 are shown.
Obesity and an HbA1c value 8.0–8.9% were associated with a decreased odds of being adherent in both the new and ongoing metformin user groups, while the odds of adherence were increased by being a smoker and having at least one of several diabetes-related comorbidities: chronic kidney disease, depression, dementia, and chronic obstructive pulmonary disease (Table 3). Several other comorbidities were associated with a >10% increase in the odds of being adherent in the ongoing metformin user group: hypoglycemia, neuropathy, macrovascular disease, and anxiety.
Table 3.
Variables associated with adherence to metformin (PDC ≥ 0.80).†
| All patients (N = 111,186) |
New metformin users (N = 7197) |
Ongoing metformin users (N = 76,854) |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Age | 1.021 | (1.020–1.023) | 1.018 | (1.013–1.023) | 1.022 | (1.020–1.023) |
| Male (Referent: Female) | 0.936 | (0.909–0.964) | – | 0.933 | (0.905–0.962) | |
| Smoker | 1.188 | (1.140–1.237) | 1.199 | (1.047–1.371) | 1.183 | (1.134–1.235) |
| HbA1c (Referent: HbA1c < 6.5) | ||||||
| 6.5–6.9 | 0.898 | (0.854–0.943) | – | 0.905 | (0.860–0.952) | |
| 7.0–7.9 | 0.888 | (0.848–0.930) | – | 0.894 | (0.853–0.937) | |
| 8.0–8.9 | 0.862 | (0.813–0.913) | 0.618 | (0.455–0.839) | 0.879 | (0.828–0.933) |
| BMI | 1.012 | (1.010–1.015) | – | 1.012 | (1.010–1.015) | |
| Baseline medications use (5–10 medications) | 1.073 | (1.035–1.112) | 1.256 | (1.074–1.468) | – | |
| Baseline use of anti-diabetic drugs | ||||||
| Sulfonylureas | 1.070 | (1.037–1.105) | – | 1.066 | (1.032–1.102) | |
| DPP-4 inhibitors | 1.091 | (1.046–1.138) | – | 1.085 | (1.041–1.132) | |
| Concomitant medication | ||||||
| Lipid-lowering therapy | 1.093 | (1.051–1.135) | – | 1.090 | (1.046–1.136) | |
| Diabetes-related comorbidities | ||||||
| Retinopathy | 0.946 | (0.915–0.978) | – | 0.934 | (0.903–0.966) | |
| Depression | 1.192 | (1.153–1.233) | 1.293 | (1.151–1.452) | 1.185 | (1.144–1.227) |
| Macrovascular disease | 1.185 | (1.144–1.228) | – | 1.192 | (1.149–1.236) | |
| Anxiety | 1.116 | (1.076–1.158) | – | 1.113 | (1.071–1.156) | |
| Chronic kidney disease | 1.106 | (1.064–1.150) | 1.340 | (1.143–1.571) | 1.094 | (1.051–1.138) |
| Hypoglycemia | 1.292 | (1.202–1.390) | – | 1.300 | (1.208–1.400) | |
| Neuropathy | 1.143 | (1.079–1.210) | – | 1.138 | (1.073–1.207) | |
| Obesity | 0.936 | (0.903–0.970) | 0.835 | (0.734–0.951) | 0.945 | (0.910–0.980) |
| Dementia | 2.270 | (2.016–2.555) | 2.115 | (1.379–3.244) | 2.282 | (2.017–2.582) |
| COPD | 1.211 | (1.140–1.286) | 1.502 | (1.219–1.851) | 1.190 | (1.117–1.267) |
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Adherence in patients who did not discontinue. Shown are statistically significant values at P < 0.01. Odds ratio values differing from unity by ±10% are in bold font.
Discussion
In this study, the rate of metformin discontinuation among new metformin users in the 12-month period after initiation was 35.9%, considerably higher than the 23.1% among ongoing users. Discontinuation was defined as a metformin treatment gap of ≥90 days, which does not differentiate a temporary from a permanent cessation of metformin, and the analysis presented here did not determine whether patients later resumed metformin use or switched to anti-hyperglycemic drugs in other classes. Other studies of the United Kingdom’s CPRD data set have used different definitions of discontinuation. A less stringent definition delivers a lower rate of discontinuation, while a more stringent definition delivers a higher rate of discontinuation. For example, in an analysis of patients with type 2 diabetes in the CPRD database for 2010–2017, discontinuation of an anti-diabetic drug was defined as a gap in prescriptions of ≥6 months and the rate of discontinuation in the 12 months after starting a first-line anti-diabetic drug (90% of which was metformin) was only 6% [14]. In an analysis of data from CPRD for years 2006–2011, discontinuation was defined as a 60-day gap in prescriptions, and the reported rate of discontinuation in the year after initiating metformin was 30%, higher than the rates observed among ongoing users in the current study [6].
In the latter analysis of CPRD data, a higher baseline HbA1c and younger age were associated with an increased likelihood of discontinuing oral anti-diabetic drug therapy in the period 6–18 months after initiation [6]. In the current study, however, demographic and clinical factors (except for duration of diabetes) were not associated with an increased risk of discontinuation in the new metformin user group. The explanation of the relationship between prior DPP-4 inhibitor use and an increased risk of discontinuation among new metformin users is unclear, but these patients comprised only 2.8% of new metformin users. Among ongoing metformin users, several factors, including increasing age, concomitant use of lipid-lower therapy, and certain comorbidities were associated with a decreased risk of discontinuing metformin, but the effect sizes were small (<5%).
The rate of metformin adherence over a 12-month period has been measured in several studies of electronic data sets in the United Kingdom [15], [16], [17]. All of these studies reported new use either of metformin or of oral anti-hyperglycemic agents (which consisted largely of metformin) and defined adherence as a PDC or medication possession ratio (MPR) value 0.80 (where the MPR was defined as the number of days of available medication divided by the number of days between the first and last prescription dates). In this study, rates of adherence were similar among new metformin and ongoing users who did not discontinue during follow-up (40.5% and 44.3%, respectively). This rate of adherence among new users is lower than rates determined in previous UK studies, however adherence rates have been found to vary with systematic reviews reporting adherence rates from 36 to 93% [18], [19]. Adherence rates of 81–82% were reported for the CPRD and GoDARTS cohorts in 2004–2014 for patients newly prescribed metformin who continued treatment for 1 year, 81.6% was reported for oral hypoglycemic agents (90.4% of which were metformin) for the CPRD data set in 2008–2016, and 60.1% was reported in a study of the IMS database of primary care practices for years 2009–2012 [15], [16], [17]. The reasons for the lower adherence rate in the present study is unclear but do not appear to be related to differences in the patient populations. Patient in these studies were, for instance, of similar age: average age 66 years in the present study, 65–66 years in other studies [15], [16]. It is possible that rates of adherence are affected by immediate release versus extended release forms of the drug. We did not collect information on the form of metformin used by patients included in the present study.
A number of different factors were associated with adherence in the current study. Obesity and an HbA1c value 8.08.9% were associated with a decrease in the odds of being adherent in both new and ongoing metformin user groups, while the odds of being adherent were increased by being a smoker and by the presence of several diabetes-related comorbidities. Age was also associated with adherence, although the effect size was small. Similarly, in the study of the UK IMS database, older age (≥65 years) was associated with adherence to oral anti-hyperglycemic therapy [15]. Adherence is largely driven by patients’ perceived susceptibility to disease and the belief that using medications will reduce their health threats [20]. It is possible that patients with several comorbidities had a higher perceived seriousness of type 2 diabetes or susceptibility to complications, and, therefore felt a stronger need to take medications in order to limit these health threats.
The present study is subject to limitations of a retrospective database analysis. PDC was used as a proxy for adherence which only show that a prescription was filled. It is unknown whether the patient took the prescription as prescribed. Thus, electronic prescription records are not necessarily reliable surrogates for medications taken. Furthermore, it is possible that rates of adherence were affected by immediate release versus extended release forms of the drug. We did not collect information on the form of metformin used by patients included in the present study. The analysis presented here did not determine whether patients who discontinued therapy later resumed their metformin use or switched to anti-hyperglycemic drugs in other classes. In the present study we required all patients to have continuous enrollment in the database for 12 months after the index date, thus, these patients might have different medication taking patterns than the general population. In addition, a 12-month follow-up period may not be sufficient to accurately describe a patient’s medication taking behavior. Finally, we were not able to adjust our analyses for other factors potentially associated with medication adherence and discontinuation, including race, socioeconomic status, lifestyle, health status (for example, changes in renal function), and family support.
Conclusions
In this study the rate of adherence to metformin was lower than reported in UK-based other studies: about one-third of patients initiating metformin discontinued within 12 months and fewer than 50% of all patients were adherent to metformin.
CRediT authorship contribution statement
Yuexin Tang: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Tracey Weiss: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Jinan Liu: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Swapnil Rajpathak: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Kamlesh Khunti: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Acknowledgments
Acknowledgements
The authors thank Anna Kaufman, MPH, in collaboration with ScribCo, for medical writing assistance.
The authors would also like to acknowledge Xin Chen for her programming and analytical assistance with this manuscript.
Kamlesh Khunti acknowledges support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and care – East Midlands (NIHR CLAHRC – EM) and the Leicester Biomedical Research Centre.
Funding
The study is funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Kenilworth, NJ, USA.
Conflicts of Interest
YT, TW, and SR are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and stockholders of Merck & Co., Inc., Kenilworth, NJ, USA.
JL was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and owned stock in Merck & Co., Inc., Kenilworth, NJ, USA at the time of the study. JL is now an employee of Janssen.
KK reports personal fees from Amgen, AstraZeneca, Bayer, NAPP, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Berlin-Chemie AG/Menarini Group, Sanofi-Aventis, Servier, and Boehringer Ingelheim. KK reports grants from Pfizer, Boehringer Ingelheim, AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, and Servier.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100225.
Appendix A
Table A1.
Read/OXMIS codes.
| Read/OXMIS Code | Description |
|---|---|
| C109.12/C109.13 | Type 2 diabetes mellitus/Type II diabetes mellitus |
| C109011/C109012 | Type II diabetes mellitus with renal complications/Type 2 diabetes mellitus with renal complications |
| C109111/C109112 | Type II diabetes mellitus with ophthalmic complications/Type 2 diabetes mellitus with ophthalmic complications |
| C109211/C109212 | Type II diabetes mellitus with neurological complications/Type 2 diabetes mellitus with neurological complications |
| C109411/C109412 | Type II diabetes mellitus with ulcer/Type 2 diabetes mellitus with ulcer |
| C109511 | Type II diabetes mellitus with gangrene |
| C109611/C109612 | Type II diabetes mellitus with retinopathy/Type 2 diabetes mellitus with retinopathy |
| C109711/C109712 | Type II diabetes mellitus – poor control/Type 2 diabetes mellitus – poor control |
| C109911 | Type II diabetes mellitus without complication |
| C109A11 | Type II diabetes mellitus with mononeuropathy |
| C109B11 | Type II diabetes mellitus with polyneuropathy |
| C109C11/C109C12 | Type II diabetes mellitus with nephropathy/Type 2 diabetes mellitus with nephropathy |
| C109D11/C109D12 | Type II diabetes mellitus with hypoglycaemic coma/Type 2 diabetes mellitus with hypoglycaemic coma |
| C109E11/C109E12 | Type II diabetes mellitus with diabetic cataract/Type 2 diabetes mellitus with diabetic cataract |
| C109F11/C109F12 | Type II diabetes mellitus with peripheral angiopathy/Type 2 diabetes mellitus with peripheral angiopathy |
| C109G11/C109G12 | Type II diabetes mellitus with arthropathy/Type 2 diabetes mellitus with arthropathy |
| C109H11/C109H12 | Type II diabetes mellitus with neuropathic arthropathy/Type 2 diabetes mellitus with neuropathic arthropathy |
| C109J00/C109J12 | Insulin treated Type 2 diabetes mellitus/Insulin treated Type II diabetes mellitus |
| C109K00 | Hyperosmolar non-ketotic state in type 2 diabetes mellitus |
| C10D.00 | Diabetes mellitus autosomal dominant type 2 |
| C10D.11 | Maturity onset diabetes in youth type 2 |
| C10F.00/C10F.11 | Type 2 diabetes mellitus/Type II diabetes mellitus |
| C10F000/C10F011 | Type 2 diabetes mellitus with renal complications/Type II diabetes mellitus with renal complications |
| C10F100 | Type 2 diabetes mellitus with ophthalmic complications |
| C10F200 | Type 2 diabetes mellitus with neurological complications |
| C10F300/C10F311 | Type 2 diabetes mellitus with multiple complications/Type II diabetes mellitus with multiple complications |
| C10F400/C10F411 | Type 2 diabetes mellitus with ulcer/Type II diabetes mellitus with ulcer |
| C10F500/C10F511 | Type 2 diabetes mellitus with gangrene/Type II diabetes mellitus with gangrene |
| C10F600/C10F611 | Type 2 diabetes mellitus with retinopathy/Type II diabetes mellitus with retinopathy |
| C10F700/C10F711 | Type 2 diabetes mellitus – poor control/Type II diabetes mellitus – poor control |
| C10F900/C10F911 | Type 2 diabetes mellitus without complication/Type II diabetes mellitus without complication |
| C10FA00/C10FA11 | Type 2 diabetes mellitus with mononeuropathy/Type II diabetes mellitus with mononeuropathy |
| C10FB00/C10FB11 | Type 2 diabetes mellitus with polyneuropathy/Type II diabetes mellitus with polyneuropathy |
| C10FC00/C10FC11 | Type 2 diabetes mellitus with nephropathy/Type II diabetes mellitus with nephropathy |
| C10FD00 | Type 2 diabetes mellitus with hypoglycaemic coma |
| C10FE00/C10FE11 | Type 2 diabetes mellitus with diabetic cataract/Type II diabetes mellitus with diabetic cataract |
| C10FF00/C10FF11 | Type 2 diabetes mellitus with peripheral angiopathy/Type II diabetes mellitus with peripheral angiopathy |
| C10FG00 | Type 2 diabetes mellitus with arthropathy |
| C10FH00 | Type 2 diabetes mellitus with neuropathic arthropathy |
| C10FJ00/C10FJ11 | Insulin treated Type 2 diabetes mellitus/Insulin treated Type II diabetes mellitus |
| C10FK00 | Hyperosmolar non-ketotic state in type 2 diabetes mellitus |
Appendix B. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Holman N., Young B., Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32(9):1119–1120. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 3.McGovern A., Hinton W., Correa A., Munro N., Whyte M., de Lusignan S. Real-world evidence studies into treatment adherence, thresholds for intervention and disparities in treatment in people with type 2 diabetes in the UK. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline; 2015 December 2. Contract No.: NG28.
- 5.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England). 1998;352(9131):854-65. [PubMed]
- 6.Maguire A., Mitchell B.D., Ruzafa J.C. Antihyperglycaemic treatment patterns, observed glycaemic control and determinants of treatment change among patients with type 2 diabetes in the United Kingdom primary care: a retrospective cohort study. BMC endocrine disorders. 2014;14:73. doi: 10.1186/1472-6823-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma M., Nazareth I., Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1) doi: 10.1136/bmjopen-2015-010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta-Nemdharry P., Thomson A., Beynon J., Donegan K. Patterns of anti-diabetic medication use in patients with type 2 diabetes mellitus in England and Wales. Pharmacoepidemiol Drug Saf. 2017;26(2):127–135. doi: 10.1002/pds.4092. [DOI] [PubMed] [Google Scholar]
- 9.Desai U., Kirson N.Y., Kim J., Khunti K., King S., Trieschman E. Time to treatment intensification after monotherapy failure and its association with subsequent glycemic control among 93,515 patients with type 2 diabetes. Diabetes Care. 2018;41(10):2096–2104. doi: 10.2337/dc17-0662. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers L.R., Weedon M.N., Henley W.E., Hattersley A.T., Shields B.M. Cohort profile for the MASTERMIND study: using the Clinical Practice Research Datalink (CPRD) to investigate stratification of response to treatment in patients with type 2 diabetes. BMJ Open. 2017;7(10) doi: 10.1136/bmjopen-2017-017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khunti K., Seidu S., Kunutsor S., Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care. 2017;40(11):1588–1596. doi: 10.2337/dc16-1925. [DOI] [PubMed] [Google Scholar]
- 12.Herrett E., Gallagher A.M., Bhaskaran K., Forbes H., Mathur R., van Staa T. Data resource profile: clinical practice research datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh D.C., Choi I.S., Plauschinat C., Kwon J., Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the US population with type 2 diabetes, 1988–1994 to 1999–2004. J Diabetes Complications. 2010;24(6):382–391. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Dennis J.M., Henley W.E., McGovern A.P., Farmer A.J., Sattar N., Holman R.R. Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010–2017. Diabetes Obes Metab. 2019 doi: 10.1111/dom.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunceli K., Iglay K., Zhao C., Brodovicz K.G., Radican L. Factors associated with adherence to oral antihyperglycemic monotherapy in patients with type 2 diabetes mellitus in the United Kingdom. Diabetes Res Clin Pract. 2015;109(3):e27–e31. doi: 10.1016/j.diabres.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Farmer A.J., Rodgers L.R., Lonergan M., Shields B., Weedon M.N., Donnelly L. Adherence to oral glucose-lowering therapies and associations with 1-year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258–263. doi: 10.2337/dc15-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon J., McEwan P., Idris I., Evans M., Puelles J. Treatment choice, medication adherence and glycemic efficacy in people with type 2 diabetes: a UK clinical practice database study. BMJ Open Diabetes Res Care. 2018;6(1) doi: 10.1136/bmjdrc-2018-000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer J.A. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 19.Krass I., Schieback P., Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32(6):725–737. doi: 10.1111/dme.12651. [DOI] [PubMed] [Google Scholar]
- 20.Pinto S.L., Lively B.T., Siganga W., Holiday-Goodman M., Kamm G. Using the Health Belief Model to test factors affecting patient retention in diabetes-related pharmaceutical care services. Res Social Adm Pharm. 2006;2(1):38–58. doi: 10.1016/j.sapharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.