Dear Editor-In-Chief,
Abbreviations/acronyms
| ACE2 | Angiotensin Converting Enzyme 2 |
| ADP | Adenosine 5’diphosphate |
| COVID-19 | Corona-Virus-Disease-2019 |
| DAPT | Dual Antiplatelet Therapy |
| DNA | Deoxyribonucleic Acid |
| DIC | Disseminated Intravascular Coagulation |
| ENT1 | Equilibrative Nucleoside Transporter 1 |
| IL | Interleukin |
| LMWH | Low molecular weight heparin |
| LPS | Lipopolysaccharides |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| NETs | Neutrophil Extracellular Traps |
| PLA | Platelets – Neutrophils aggregates |
| PCI | Percutaneous Coronary Intervention |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SIC | Sepsis-Induced Coagulopathy |
| TNF-α | Tumor Necrosis Factor alpha |
Recently, Yuki et al. published “COVID-19 pathophysiology: A review” [1]. We read with great interest this article. The authors mentioned that “coagulation dysfunction, thrombosis and pulmonary embolism have been observed in severe COVID-19 [1].” We would like to discuss a potential therapeutic strategy to prevent sepsis-induced coagulopathy (SIC) in coronavirus disease 2019 (COVID-19).
The outbreak of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread into a pandemic [1]. Mortality is high in intensive care unit (ICU), and associated with comorbidities such as diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, or cardiovascular disease [2].
COVID-19 is also associated with sepsis-induced coagulopathy (SIC) leading to disseminated intravascular coagulation (DIC) [3]. In COVID-19, elevation of D-dimer and fibrin/fibrinogen degradation products are the initial coagulopathy markers found [3]. COVID-19 patients also meet Virchow's triad criteria for thrombosis: i) endothelial injury, ii) hypercoagulability and iii) venous stasis. Two fibrinolysis markers, D-Dimer and fibrin degradation products, are the hallmarks of SIC-related mortality [3].
Low molecular weight heparin (LMWH) is a commonly used anticoagulant to prevent DIC and venous thromboembolism, but its efficiency in DIC remains controversial [3]. LMWH also exposes to the risk of heparin induced thrombocytopenia, an immune-mediated syndrome characterized by thrombocytopenia and a high risk for venous or arterial thrombosis [4].
Platelets modulate hemostasis and immune responses via interactions with immune cells, through secretion of immune-modulators and cell-cell interactions [5]. Platelets activate leukocytes through cell-cell interactions involving adhesion molecules such as P-selectin, a glycoprotein that, upon cell activation, is rapidly translocated from cytoplasmic α-granules to the cell surface [5]. Platelets-Leukocytes interactions are important in the pathogenesis of sepsis, and Platelets-Neutrophils Aggregates (PNA) and P-selectin secretion are altered in septic patients [5].
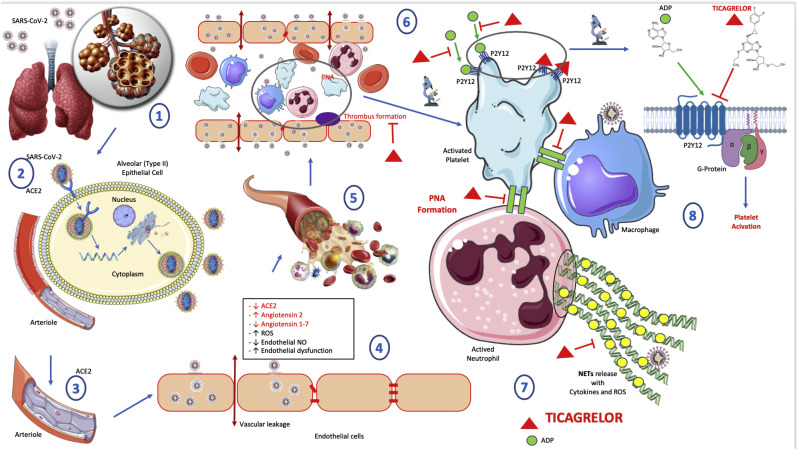
The platelet P2Y12, a G protein-coupled receptor that are expressed on platelet membranes for adenosine 5’diphosphate (ADP), plays a central role in platelet function, hemostasis, and thrombosis [5] (Fig. 1 ). The P2Y12 receptor is involved in platelet aggregation and is thus a biological target for the treatment of thromboembolisms and other clotting disorders [5] such as SIC and DIC.
Fig. 1.
Ticagrelor use to prevent sepsis-induced coagulopathy in COVID-19.
(1) SARS-CoV-2 entry into lungs through respiratory droplets.
(2) SARS-CoV-2 binds to ACE2 and enters into alveolar (type II) epithelial cell; SARS-CoV-2 replication into alveolar (type II) epithelial cell; Increased inflammation; Increased vascular remodelling; Endothelial dysfunction; Cardiopulmonary dysfunction.
(3) Pulmonary arteriole.
(4) SARS-CoV-2 binds to ACE2 and enters into endothelial cells; Decreased ACE2; Increased Angiotensin 2; Decreased Angiotensin 1-7; Increased ROS (Reactive Oxygen Species) ; Decreased endothelial NO (Nitric Oxide); Increased endothelial dysfunction; Vascular leakage.
(5) Arteriole and blood cells.
(6) Thrombus formation in dysfunctional and leaky arteriole; Platelet activation promotes thrombosis; PNA (Platelets-neutrophils aggregates) formation; Ticagrelor inhibits thrombus formation.
(7) Platelet activation by ADP binding P2Y12 receptor; PNA (Platelet-Neutrophil Aggregates) formation; NET release by neutrophil; NET contribute to triggering cytokine and ROS release by neutrophil and can alter endothelial barrier, and enhance leakage; Ticagrelor inhibits reversibly P2Y12 receptor and the whole cascade of events described below.
Ticagrelor is an orally administered platelet aggregation inhibitor with a cyclopentyl-triazolopyrimidine structure [5] (Fig. 1). It is a selective reversible P2Y12 receptor antagonist, which prevents P2Y12-mediated and ADP-mediated platelet activation and aggregation [5]. In addition, several studies indicate that ticagrelor may have pleiotropic effects in addition to its anti-platelet properties [6].
Here we aim at discussing the potential use of Ticagrelor in COVID-19, to reduce PNA, neutrophil extracellular traps (NETs) release, and vascular leakage.
Ticagrelor was approved for the prevention of severe cardiovascular events among patients with coronary ischemia or ischemic stroke [7].
Experimental models of sepsis and human clinical trials have also shown that platelet receptor P2Y12 inhibition reduced sepsis-induced mortality [[7], [8], [9], [10]]. In models of LPS-induced inflammation, Ticagrelor reduced cytokines IL-6, TNF-α, MCP-1 and IL-8 [10], and mortality [9]. In a model of intra-abdominal sepsis, Ticagrelor reduced sepsis-induced lung injury, neutrophils pulmonary infiltration, PNA formation, platelet activation and thrombocytopenia [8].
In the PLATelet inhibition and patient Outcomes (PLATO) study, Ticagrelor reduced mortality risk following pulmonary adverse events such as bacterial lung infection and sepsis in acute coronary syndrome patients [7]. According to neutrophils are a first-line defense against bacterial lung infection, a potential mechanism by which ticagrelor could influence host defense against bacterial lung infection is inhibition of cellular uptake of adenosine, a known regulator of neutrophil chemotaxis and phagocytosis [6,7].
In the human clinical trial “Targeting Platelet-Leukocyte Aggregates in Pneumonia with Ticagrelor (XANTHIPPE) study”, Ticagrelor reduced platelet-leucocyte interactions, and IL-6 levels within 24 h in patients with pneumonia [9]. It also limited NET release, thus improving lung function, and reducing vascular leakage and oxygen need [9]. The findings indicate a mechanistic link between platelets, leukocytes, and lung injury in settings of pneumonia and sepsis, and suggest possible therapeutic use of Ticagrelor to reduce complications. Ticagrelor also decreased D-Dimer levels in healthy volunteers [11].
One important point in the safe use of Ticagrelor. Pulmonary hemorrhage has been reported under dual antiplatelet therapy: Ticagrelor plus aspirin [12]. To avoid an increased bleeding risk, randomized controlled trial showed a net benefit of aspirin-free antiplatelet therapy [13]. This was demonstrated in the XANTHIPPE study, since Ticagrelor use was safe with no major bleeding reported [9].
COVID-19 is associated with coagulopathy through (Fig. 1): i) endothelial injury/dysfunction induced by the binding of SARS-CoV-2 to endothelial ACE2 receptor that leads to excess thrombin generation and fibrinolysis [14]; ii) platelet activation that induces hypercoagulability and membrane receptor expression enabling PNA between platelets and leukocytes [15]; iii) PNA formation that release pro-inflammatory cytokines, reactive oxygen species and NETs; iv) NETs released from activated neutrophils in response to infection such as SARS-CoV-2 can trap, and kill microorganisms [15]. They also activate platelets and coagulation, and contribute to thrombogenesis by forming a mesh with platelets and fibrin and accumulating coagulation factors such as Tissue Factor; v) Inflammation induced by NET-components (DNA, histone, and granule proteins including proteases such as elastase and pro-oxidant enzymes such as myeloperoxidase) also contribute to triggering cytokine release, oxidative stress and extracellular matrix proteolysis [15].
Cytokines and reactive oxygen species released by neutrophils can alter the endothelial barrier function, enhance leakage, and promote the development of tissue oedema and hypoxia [15] leading to acute respiratory distress and multiple organ dysfunction [14].
Therefore, when given at the early onset of COVID-19, Ticagrelor, through inhibition of platelet-neutrophil aggregates, NET release, and vascular leakage might prevent SIC, progression to DIC and associated morbi/mortality in COVID-19 patients. Ticagrelor could be a promised candidate to prevent SIC that it would be interesting to evaluate in a randomized clinical trial in COVID-19.
Author Contribution statement
LO: Conception, design, literature analysis, and manuscript redaction.
OM: Analysis, revising the manuscript for important intellectual content.
FP: Drafting the figure, and revising the manuscript for important intellectual content.
AJ: Analysis, redaction, and revising the manuscript for important intellectual content.
GM: Analysis, revising the manuscript for important intellectual content.
Each author revised the report and approved the submitted version of the manuscript.
Each author has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Permission Information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Ethics Approval
Not applicable.
Declaration of Competing Interest
All authors declare they have nothing to disclose, and no competing interests.
Acknowledgements
The authors wish to thank Ms. Hazel Chaouch for English language correction. The authors wish to thank Mr Loïc Fin, for his administrative contribution to the success of this study. The authors wish to thank the “CHU de Rennes – Corect” for their support for our work.
References
- 1.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raucci F., Mansour A.A., Casillo G.M., Saviano A., Caso F., Scarpa R., Mascolo N., Iqbal A.J., Maione F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun. Rev. 2020;102572 doi: 10.1016/j.autrev.2020.102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frazer C.A. Heparin-Induced Thrombocytopenia. J. Infus. Nurs. 2017;40:98–100. doi: 10.1097/NAN.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo M. P2Y 12 receptors: structure and function. J. Thromb. Haemost. 2015;13:S10–S16. doi: 10.1111/jth.12952. [DOI] [PubMed] [Google Scholar]
- 6.Alsharif K.F., Thomas M.R., Judge H.M., Khan H., Prince L.R., Sabroe I., Ridger V.C., Storey R.F. Ticagrelor potentiates adenosine-induced stimulation of neutrophil chemotaxis and phagocytosis. Vasc. Pharmacol. 2015;71:201–207. doi: 10.1016/j.vph.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storey R.F., James S.K., Siegbahn A., Varenhorst C., Held C., Ycas J., Husted S.E., Cannon C.P., Becker R.C., Steg P.G., Åsenblad N., Wallentin L. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25:517–525. doi: 10.3109/09537104.2013.842965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman M., Gustafsson D., Wang Y., Thorlacius H., Braun O.Ö. Ticagrelor reduces neutrophil recruitment and lung damage in abdominal sepsis. Platelets. 2014;25:257–263. doi: 10.3109/09537104.2013.809520. [DOI] [PubMed] [Google Scholar]
- 9.Sexton T.R., Zhang G., Macaulay T.E., Callahan L.A., Charnigo R., Vsevolozhskaya O.A., Li Z., Smyth S. Ticagrelor reduces Thromboinflammatory markers in patients with pneumonia. JACC Basic Transl. Sci. 2018;3:435–449. doi: 10.1016/j.jacbts.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas M.R., Outteridge S.N., Ajjan R.A., Phoenix F., Sangha G.K., Faulkner R.E., Ecob R., Judge H.M., Khan H., West L.E., Dockrell D.H., Sabroe I., Storey R.F. Platelet P2Y 12 inhibitors reduce systemic inflammation and its Prothrombotic effects in an experimental human model. Arterioscler. Thromb. Vasc. Biol. 2015;35:2562–2570. doi: 10.1161/ATVBAHA.115.306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traby L., Kollars M., Kaider A., Siller-Matula J.M., Wolkersdorfer M.F., Wolzt M., Kyrle P.A., Eichinger S. Differential effects of Ticagrelor with or without aspirin on platelet reactivity and coagulation activation: a randomized trial in healthy volunteers. Clin. Pharmacol. Ther. 2020;107:415–422. doi: 10.1002/cpt.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parasuraman S.K., Teo A.I., Millar C.G., Noman A. Pneumonitis and pulmonary haemorrhage after acute myocardial infarction. Clin. Med. Lond. Engl. 2015;15:591–593. doi: 10.7861/clinmedicine.15-6-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomaniak M., Chichareon P., Onuma Y., Deliargyris E.N., Takahashi K., Kogame N., Modolo R., Chang C.C., Rademaker-Havinga T., Storey R.F., Dangas G.D., Bhatt D.L., Angiolillo D.J., Hamm C., Valgimigli M., Windecker S., Steg P.G., Vranckx P., Serruys P.W., for the GLOBAL LEADERS Trial Investigators Benefit and Risks of Aspirin in Addition to Ticagrelor in Acute Coronary Syndromes: A Post Hoc Analysis of the Randomized GLOBAL LEADERS Trial. JAMA Cardiol. 2019;4:1092. doi: 10.1001/jamacardio.2019.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucoloto A.Z., Jenne C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019;6 doi: 10.3389/fcvm.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]