Abstract
Objectives
In areas of SARS-CoV-2 outbreak worldwide mean air pollutants concentrations vastly exceed the maximum limits. Chronic exposure to air pollutants have been associated with lung ACE-2 over-expression which is known to be the main receptor for SARS-CoV-2. The aim of this study was to analyse the relationship between air pollutants concentration (PM 2.5 and NO2) and COVID-19 outbreak, in terms of transmission, number of patients, severity of presentation and number of deaths.
Methods
COVID-19 cases, ICU admissions and mortality rate were correlated with severity of air pollution in the Italian regions.
Results
The highest number of COVID-19 cases were recorded in the most polluted regions with patients presenting with more severe forms of the disease requiring ICU admission. In these regions, mortality was two-fold higher than the other regions.
Conclusions
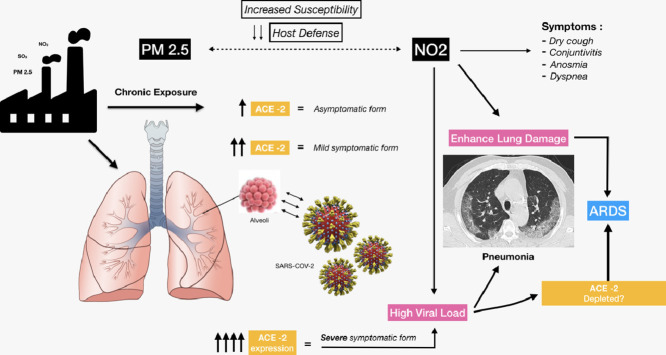
From the data available we propose a “double-hit hypothesis”: chronic exposure to PM 2.5 causes alveolar ACE-2 receptor overexpression. This may increase viral load in patients exposed to pollutants in turn depleting ACE-2 receptors and impairing host defences. High atmospheric NO2 may provide a second hit causing a severe form of SARS-CoV-2 in ACE-2 depleted lungs resulting in a worse outcome.
Graphical abstract
Background and work hypothesis
The potential correlation between air pollution and outbreaks of COVID-19 have been described in the recent literature.1., 2., 3., 4.
While SARS-CoV-2 diffusion has been documented in every country and a pandemic has been declared,5 there is still debate about the death rate and the severity of pneumonia encountered in specific countries, like Italy and China. In Italy the majority of severe cases and deaths occurred in the Po Valley, accounting for three quarters of the entire Italian caseload, pointing to a specific characteristic of the territory that may favour spread and severity of SARS-CoV-2 virus. The worst COVID-19 outbreaks have been reported in the Po valley, in the cities of Lodi, Cremona, Bergamo and Brescia which are known to be the four Italian cities with highest pollution levels6. Here we propose a hypothesis linking PM 2.5 and NO2 concentrations and the severity of SARS-CoV-2 which could have important implications in the prevention and management of the pandemic.
Methods
We collected population data provided by the Italian Civil Protection Agency and data concerning the emissions of air pollutants registered in every Italian region (air pollution data have been collected from Air-matters App and website – https://www.air-matter.com). Pearson correlation analysis was used to examine relationship between COVID-19 cases and PM 2.5 levels. Cut-off level for significance was set at P < 0.05. A two-way graph was created showing the simple association between PM 2.5 and number of ICU admission per cumulative days censored. Each Italian region population has been weighted for number of individuals over 65 years old. Patient data are official government data from Italian Civil Protection website (https://github.com/pcm-dpc/COVID-19). Population data were collected from the Italian Statistical Agency (https://dati.istat.it/Index.aspx?QueryId=42869). All analyses were performed using STATA 16 software.
Results
A clear association is apparent between PM 2.5 levels and COVID-19 outbreak distribution (see Table 1 and Table 2 ).
Table 1.
Distribution of SARS-CoV-2 cases on March 31th 2020 (total number of cases, ICU admitted patients, deaths) according to each Italian region and the mean value of PM 2.5 registered in each region during February 2020, the month before the beginning of the outbreak in Italy. (Air pollution data have been collected from Air-matters app which include daily measurements from air quality measurement stations all over Italian territory. Data from P.A. Bolzano, Sardegna, Valle d'Aosta, Molise and Basilicata were unavailable at the time of writing).
| Italian regions | Mean PM 2.5 in February 2020 (µg/m3) | Total number of cases (n) | DEATHS (n) | Total number of hospitalized patients (n) | ICU admissions (n) |
|---|---|---|---|---|---|
| Lombardia | 35 | 43,208 | 7199 | 13,207 | 1324 |
| Emilia-Romagna | 30 | 14,074 | 1644 | 4118 | 353 |
| Piemonte | 39 | 9301 | 854 | 3626 | 452 |
| Veneto | 35 | 9155 | 477 | 2036 | 356 |
| Toscana | 12 | 4608 | 244 | 1413 | 293 |
| Marche | 5 | 3825 | 452 | 1115 | 169 |
| Liguria | 5 | 3416 | 428 | 1332 | 179 |
| Lazio | 12 | 3095 | 162 | 1300 | 173 |
| Campania | 12 | 2092 | 133 | 634 | 133 |
| Puglia | 11 | 1803 | 110 | 714 | 105 |
| P.A. Trento | 11 | 1746 | 164 | 434 | 80 |
| Sicilia | 7 | 1647 | 81 | 575 | 72 |
| Friuli Venezia Giulia | 23 | 1593 | 113 | 275 | 60 |
| Abruzzo | 8 | 1401 | 115 | 408 | 73 |
| P.A. Bolzano | n/a | 1371 | 76 | 311 | 62 |
| Umbria | 7 | 1078 | 37 | 219 | 43 |
| Sardegna | n/a | 722 | 31 | 141 | 28 |
| Calabria | 8 | 659 | 36 | 149 | 17 |
| Valle d'Aosta | n/a | 628 | 56 | 117 | 26 |
| Basilicata | n/a | 226 | 7 | 54 | 17 |
| Molise | n/a | 144 | 9 | 37 | 8 |
Table 2.
Correlations between mean PM 2.5 registered in February 2020 and COVID-19 cases in terms of total number, hospitalized patients, ICU admissions per cumulative days and deaths (data updated to 31st March 2020).
| Correlations | Pearson's coefficient (r-value) | Significance (p-value) |
|---|---|---|
| Mean PM 2.5 – Total number cases | 0.64 | 0.0074 |
| Mean PM 2.5 – ICU admissions per day | 0.65 | 0.0051 |
| Mean PM 2.5 – Deaths | 0.53 | 0.032 |
| Mean PM 2.5 – Hospitalized cases | 0.62 | 0.0089 |
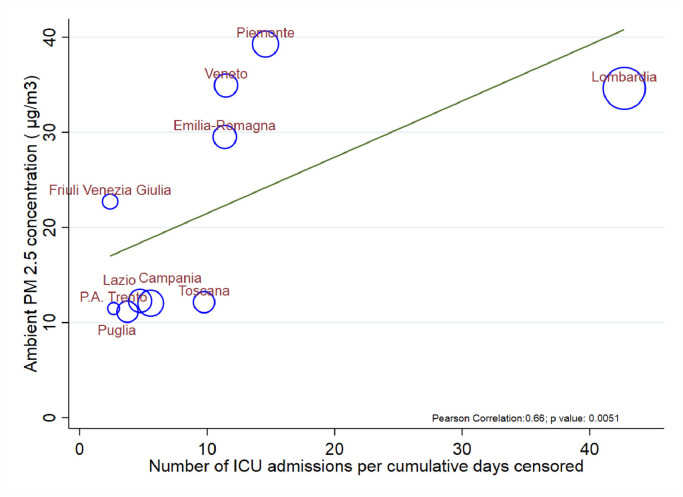
Our data show a significant relationship between the mean PM 2.5 concentration during February 2020, one month before the beginning of the outbreak, and the number of COVID-19 cases per region (updated to March 31st), confirming how more polluted areas are the ones where the contagion is more widespread. More significantly patients in polluted areas present with more severe forms of the disease requiring ICU. Mortality is two-fold higher than the other regions despite similar rates of ICU admission (crude death rate 14 vs 7%) (see Fig. 1 ).
Fig. 1.
Scatter plot showing the correlation of the mean concentration of PM 2.5 during February 2020 with the number of ICU admissions for severe COVID-19 infection per cumulative days (patient data updated at 31st March 2020). Regions which presented with the highest level of air pollution during the month of February 2020 are the ones presenting with more severe patients requiring ICU treatments). The size of the circles represents the proportion of population over 65 years of age.
One explanation for the geographical disparity in the number of cases, is that the high level of pollutants may favour the transmission of viral illnesses and increase their persistence in the community. A recent study indicates that the virus may remain viable in aerosol for some hours.7 The high pollutant levels and specific climate in the Po Valley may enhance aerosol stability of the virus, explaining the ongoing outbreaks and the associated high rate of contagion.
However, this does not explain the high fatality rate in the aforementioned areas. Atmospheric pollution may have a more fundamental role by increasing the susceptibility to the infection and mining pulmonary defence mechanisms favouring the establishment of more severe forms of the disease.
PM 2.5 local concentrations as the mainstem of viral susceptibility
PM 2.5 penetrates deep into the peripheral air spaces8 and may facilitate viral infection through the interaction with the lung renin-angiotensin system (RAS).
Pulmonary RAS consists of two axes participating in local inflammatory responses with opposing functions9: ACE/AngII/AT1R axis is involved in release of pro-inflammatory cytokines (such as IL-6 and TNF-alfa); ACE-2/Ang1-7/Mas axis culminates in Mas activation that represses STAT3 and ERK exerting an anti-inflammatory effect.
ACE-2 cleaves Ang II into the cardioprotective Ang 1–7, which acts through Mas receptors to counterbalance the detrimental effects of Ang II signalling. Therefore, ACE-2 protects against RAS-induced injuries through two processes: 1) degrading Ang I and Ang II to limit substrate availability in the adverse ACE/Ang II/AT1 receptor axis; 2) generating Ang 1–7 to increase substrate availability in the protective ACE-2/Ang 1–7/Mas receptor axis.9
ACE-2 knock-out mice are more prone to develop lung injury after exposure to PM 2.5 and pulmonary repair in this context is attenuated relative to controls. This suggests a crucial role for ACE-2 in lung protection from air pollutants.10
Chronic exposure to PM 2.5 in mice causes upregulation of pulmonary ACE expression and activity10 , 11 which may be a protective response to a chronic deleterious insult. Despite having normal lung structure and function, ACE-2 knockout mice exhibit very severe pathology of acute respiratory distress syndrome (ARDS) compared with wild-type control mice.10 , 12
The spike (S) protein of coronaviruses facilitates viral entry into target cells by engaging ACE-2 receptors.13 ACE-2 is expressed mainly at alveolar level, explaining the viral tropism for lower airways. Binding and entry of both SARS-CoV and SARS-CoV-2 into human cells is facilitated by the interaction between receptor-binding domain (RBD) of the S1 subunit on viral spike glycoproteins with the ectodomain of ACE- 2.14 SARS-CoV infection and challenge with recombinant SARS-Spike protein trigger a marked downregulation of ACE-2 expression in lungs and in cell culture causing more severe acute lung injury.15 Viral depletion of ACE-2 appears to be crucial in mediating lung injury.16 , 17
We postulate that patients chronically exposed to high levels of PM 2.5 overexpress ACE-2 facilitating viral penetration and subsequent depletion of ACE-2 leads to more severe forms of the disease.
This may explain the low incidence of severe pneumonia in children most of whom are asymptomatic. The limited exposure to PM 2.5 due to their young age may exempt them from pulmonary ACE-2 receptor overexpression. In China out of 72.314 infected patients <1% of the cases were children younger than 10 years old18 and developed milder disease.19 Similarly, chronic upregulation of ACE-2 in a PM 2.5 concentration-dependant manner could explain the high variability in clinical presentation ranging from asymptomatic patients to patients presenting with mild, moderate or severe form of the disease.20 , 21
Early in March 2020, the Italian village of Vo’ Euganeo near Padua, an area with relatively low atmospheric levels of pollution (yearly means PM 2.5 and NO2 respectively 19 and 14 µg/m3)22 experienced one of the first circumscribed outbreak of SARS-CoV-2 in Italy. Swabs were performed in the entire population of Vo'Euganeo, accounting for 3.275 inhabitants; SARS-CoV-2 had a prevalence of 2.6% in the overall population. Interestingly, it was found that 43% of patients positive to SARS-CoV-2 were completely asymptomatic,23 shedding insights in the incidence of asymptomatic infection and the possible role of asymptomatic carrier in spreading the disease.
Mean viral loads of severe cases of SARS-CoV2 have been reported to be 60 times higher than mild cases.24 In this case patients who had low exposure to PM 2.5 would have low pulmonary expression of ACE-2 with subsequent low viral load and mild symptoms.
Putative role of NO2 toxicity in the context of SARS-CoV-2 infection
Numerous sources of NO2 exist, making it one of the most common and widespread air pollutants25 with a geographical distribution overlapping that of PM 2.5.
Acute inhalation of high concentrations of NO2 occurring in conditions such as Silo filler's disease causes increased permeability pulmonary oedema.26 Long-term low- to moderate-level air pollutant exposure, including NO2, is associated with a greater risk of developing ARDS after severe trauma.27 Being a free radical, NO2 acts as a potent oxidant depleting anti-oxidant stores leading to impaired tissue defences, increased inflammatory response and cellular damage.
Two days after exposure to moderate levels of NO2 in an ice arena, exposed individuals experienced dry cough (97%), headache (45%), haemoptysis (35%) and dyspnoea (65%).28 The acute intoxication was secondary to the malfunctioning of ice resurfacer engine associated with poor ventilation. Interestingly, anosmia is frequently present also in the context of NO2 intoxication.29
Environmental concentrations of NO2 increase susceptibility to pneumonia by Klebsiella pneumoniae30 and Streptococcus pneumoniae31 in experimental murine models. The increased susceptibility seems to be linked to an impairment of pulmonary defence mechanisms, especially phagocytic activity at progressively higher concentrations32 , 33 and is associated with high mortality rates.34
Rose et al. reported that mice exposed to NO2 prior to cytomegalovirus infection required a viral load 100-fold lower than in control mice and re-infection from viral sources was more common.35 , 36 In this model, mice exposed to 5 ppm of NO2 were readily developing viral replication in lower respiratory tract soon after exposure evolving toward clinical form of acute lung injury as confirmed by histological samples showing pneumonitis. Inhalation of the same concentration of NO2 without viral exposure did not result in tissue damage. Re-inoculation of the virus after 30 days produced re-infection only in previously exposed mice.
These observations suggest a putative role of NO2 in worsening pulmonary damage in COVID-19 patients. Recent exposure to moderately high levels of nitric dioxide may have caused a worsening of the disease with exacerbation of the symptoms and of the respiratory distress. This would cause an overlap between the COVID-19 presentation and NO2-induced alveolar damage.
“Double-hit” hypothesis
The recent outbreak of COVID-19 is straining healthcare systems due to the high infectiousness and large number of patients with severe pneumonia requiring ICU treatment. Some geographical locations, such as the province of Hubei in China and Po Valley in Italy, have suffered the highest number of cases and deaths. This geographical distribution of the outbreaks shows a remarkable overlap with the local levels of air pollution.
From this initial observation, we formulated our working hypothesis according to which a linkage exists between the air pollution levels and COVID-19 outbreak, in terms of transmission, number of patients, severity of presentation and number of deaths. Air pollutants, (such as PM 2.5 and NO2) plus SARS-CoV-2 give a “double-hit” to the lungs leading to acute lung injury by attenuating tissue remodelling and influencing local inflammatory response.
In Italy, the areas with the highest incidence of cases and deaths are the ones with levels of PM 2.5 and NO2 that are chronically high or with recent increases in the 2 months prior to the outbreak. Chronic exposure of the lungs to high levels of PM 2.5 causes upregulation of its protective mechanisms, such as ACE-2.
SARS-CoV-2 has shown a specific affinity for ACE-2 receptors and overexpression in patients subjected to chronic pollutants could represent a trojan horse for viral infection. Moreover, the SARS-CoV-2 binding to ACE-2 may induce deficient anti-inflammatory action leading to acute lung injury, attenuating local tissue repair. It is therefore likely that patients who present overexpression are the ones more readily infected and the ones with more severe presentations. Chronic lung exposure to NO2 may favour viral injury due to local damage induced by oxidative stress and due to local reduction of macrophage function and adaptive immune responses.
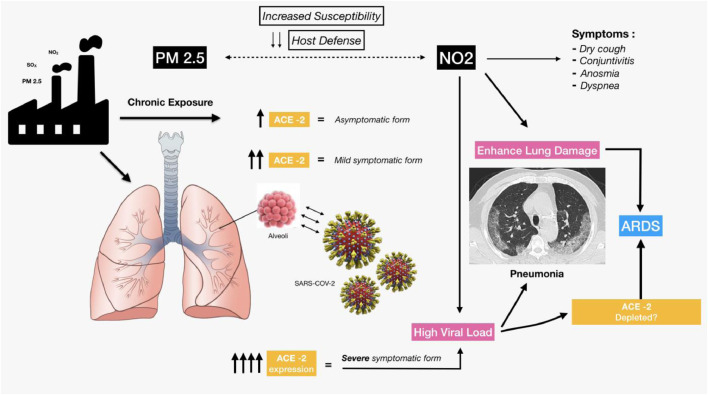
In addition, a putative role of NO2 in worsening pulmonary damage can be hypothesized in these patients. Many of the symptoms and signs of COVID-19 infection resemble that of moderate NO2 poisoning, including anosmia. It is possible that ACE-2 depletion following COVID-19 infection increases tissue vulnerability to NO2 toxicity that eventually contributes to the acute lung injury observed in patients with pneumonia-induced ARDS (Fig. 2 ).
Fig. 2.
Hypothesis of the SARS-CoV-2 infection mechanisms and severe lung disease induced by the combined effect of PM 2.5 and NO2. ACE-2 plays a bifunctional role as sort of “double-edged sword”: it turns off the RAS system and leads to beneficial effects but also mediates unique susceptibility to lung and CV disease in COVID-19 patients by serving as the SARS-CoV-2 receptor.
Undoubtedly, many other factors such as age, transmission patterns, population density and co-morbilities have an important impact on both the number and severity of COVID-19. A more detailed epidemiological analysis is necessary to provide a comprehensive understanding of the differences in severity of SARS-CoV-2 in different areas. Likewise, our observations are limited as detailed geographic measurements of pollutants, such as NO2 in the last few months have not yet been published. Nevertheless, we find the available evidence showing a direct link between atmospheric pollution and SARS-CoV-2 linked by ACE-2 receptor expression compelling. ACE-2 receptor expression can be obtained from swabs and if linked to alveolar expression from ex-vivo tissue samples may provide a method for testing this hypothesis.
Conclusions
A link between SARS-CoV-2 infection and air pollution is plausible and this may have a strong impact on the high rate of infection and mortality. If confirmed, our hypothesis has both immediate and long-term implications. In the short term it may help in identifying areas where serious cases are most likely to occur allowing timely allocation of limited and precious resources. It may also have prophylactic and treatment implications in the modulation of ACE-2 receptors before and after infection. In the medium and long-term our hypothesis should lead to an increased awareness that in specific environments defined by high density of population and high industrialization, together with certain climatic characteristics, influencing urban planning to bring about changes such as delocalization of factories, in order to avoid recreating the conditions associated with worse outcomes after viral infections. Weather forecasting and seasonal prediction systems may also contribute in fighting the virus spread.
Declaration of Competing Interest
The authors declare no conflict of interest and no financial support for this study.
Acknowledgements
We wish to thank all the people who assisted with this research, in particular: Rosalba Lembo (MSc, Anaesthesia and Intensive Care, San Raffaele Scientific Institute, Milan, Italy) for the graph and statistics.
References
- 1.Martelletti L., Martelletti P. Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Compr Clin Med. 2020;2:383–387. doi: 10.1007/s42399-020-00274-4. https://doi:10.1007/s42399-020-00274-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutheil F., Baker J.S., Navel V. COVID-19 as a factor influencing air pollution? Environ Pollut. 2020;263 doi: 10.1016/j.envpol.2020.114466. https://doi:10.1016/j.envpol.2020.114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. https://doi:10.1016/j.scitotenv.2020.138704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to COVID-19 infection zoning. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.045. https://doi:10.1016/j.jinf.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Coronavirus disease 2019 (COVID-19). Situation Report-51 (11th March 2020). 2020.
- 6.National air pollution data . 2018. Istituto Nazionale di Statistica (ISTAT)https://www.istat.it/it/archivio/236912 [Google Scholar]
- 7.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. https://doi:10.1056/NEJMc2004973 NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churg A., Brauer M. Human lung parenchyma retains PM2.5. Am J Respir Crit Care Med. 1997;155:2109–2111. doi: 10.1164/ajrccm.155.6.9196123. https://doi:10.1164/ajrccm.155.6.9196123 [DOI] [PubMed] [Google Scholar]
- 9.Parajuli N., Ramprasath T., Patel V.B. Targeting angiotensin-converting enzyme 2 as a new therapeutic target for cardiovascular diseases. Can J Physiol Pharmacol. 2014;92:558–565. doi: 10.1139/cjpp-2013-0488. https://doi:10.1139/cjpp-2013-0488 [DOI] [PubMed] [Google Scholar]
- 10.Lin C.-.I., Tsai C.-.H., Sun Y.-.L. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int J Biol Sci. 2018;14:253–265. doi: 10.7150/ijbs.23489. https://doi:10.7150/ijbs.23489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aztatzi-Aguilar O.G., Uribe-Ramírez M., Arias-Montaño J.A., Barbier O., De Vizcaya-Ruiz A. Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: angiotensin-II type-I receptor as a molecular target of particulate matter exposure. Part Fibre Toxicol. 2015;12:17. doi: 10.1186/s12989-015-0094-4. https://doi:10.1186/s12989-015-0094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. https://doi:10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou P., Yang X.-.L., Wang X.-.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. https://doi:10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047049. https://doi:10.1161/CIRCULATIONAHA.120.047049 CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 15.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. https://doi:10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. https://doi:10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Yang N., Tang J. Downregulation of angiotensin-converting enzyme 2 by the neuraminidase protein of influenza A (H1N1) virus. Virus Res. 2014;185:64–71. doi: 10.1016/j.virusres.2014.03.010. https://doi:10.1016/j.virusres.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020 doi: 10.1001/jama.2020.2648. https://doi:10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 19.Dong Y., Mo X., Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 https://doi:10.1542/peds.2020-0702 [Google Scholar]
- 20.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020 doi: 10.1001/jama.2020.2648. https://doi:10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. 2020.
- 22.ARPA - Azienda regionale per la prevenzione e protezione ambientale del Veneto . Provincia di Padova; Relazione tecnica: 2018. Qualità dell'aria 2018. [Google Scholar]
- 23.Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. MedRxiv 2020:2020.04.17.20053157. 10.1101/2020.04.17.20053157. [DOI]
- 24.Liu Y., Yan L.-M., Wan L. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. https://doi:10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO guidelines for indoor air quality: selected pollutants. 2010. [PubMed]
- 26.Douglas W.W., Hepper N.G., Colby T V. Silo-filler's disease. Mayo Clin Proc. 1989;64:291–304. doi: 10.1016/s0025-6196(12)65249-5. https://doi:10.1016/s0025-6196(12)65249-5 [DOI] [PubMed] [Google Scholar]
- 27.Reilly J.P., Zhao Z., Shashaty M.G.S. Low to moderate air pollutant exposure and acute respiratory distress syndrome after severe trauma. Am J Respir Crit Care Med. 2019;199:62–70. doi: 10.1164/rccm.201803-0435OC. https://doi:10.1164/rccm.201803-0435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedberg K., Hedberg C.W., Iber C. An outbreak of nitrogen dioxide-induced respiratory illness among ice hockey players. JAMA. 1989;262:3014–3017. [PubMed] [Google Scholar]
- 29.Adams D.R., Ajmani G.S., Pun V.C. Nitrogen dioxide pollution exposure is associated with olfactory dysfunction in older U.S. adults. Int Forum Allergy Rhinol. 2016;6:1245–1252. doi: 10.1002/alr.21829. https://doi:10.1002/alr.21829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrlich R., Henry M.C. Chronic toxicity of nitrogen dioxide. Arch Environ Heal An Int J. 1968;17:860–865. doi: 10.1080/00039896.1968.10665342. https://doi:10.1080/00039896.1968.10665342 [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich R., Findlay J.C., Fenters J.D., Gardner D.E. Health effects of short-term inhalation of nitrogen dioxide and ozone mixtures. Environ Res. 1977;14:223–231. doi: 10.1016/0013-9351(77)90034-2. https://doi:10.1016/0013-9351(77)90034-2 [DOI] [PubMed] [Google Scholar]
- 32.Jakab G.J. Modulation of pulmonary defense mechanisms by acute exposures to nitrogen dioxide. Experientia Suppl. 1987;51:235–242. doi: 10.1007/978-3-0348-7491-5_40. https://doi:10.1007/978-3-0348-7491-5_40 [DOI] [PubMed] [Google Scholar]
- 33.Parker R.F., Davis J.K., Cassell G.H. Short-term exposure to nitrogen dioxide enhances susceptibility to murine respiratory mycoplasmosis and decreases intrapulmonary killing of Mycoplasma pulmonis. Am Rev Respir Dis. 1989;140:502–512. doi: 10.1164/ajrccm/140.2.502. https://doi:10.1164/ajrccm/140.2.502 [DOI] [PubMed] [Google Scholar]
- 34.Gardner D.E., Coffin D.L., Pinigin M.A., Sidorenko G.I. Role of time as a factor in the toxicity of chemical compounds in intermittent and continuous exposures. part I. effects of continuous exposure. J Toxicol Environ Health. 1977;3:811–820. doi: 10.1080/15287397709529615. https://doi:10.1080/15287397709529615 [DOI] [PubMed] [Google Scholar]
- 35.Rose R.M., Fuglestad J.M., Skornik W.A. The pathophysiology of enhanced susceptibility to murine cytomegalovirus respiratory infection during short-term exposure to 5 ppm nitrogen dioxide. Am Rev Respir Dis. 1988;137:912–917. doi: 10.1164/ajrccm/137.4.912. https://doi:10.1164/ajrccm/137.4.912 [DOI] [PubMed] [Google Scholar]
- 36.Rose R.M., Pinkston P., Skornik W.A. Altered susceptibility to viral respiratory infection during short-term exposure to nitrogen dioxide. Res Rep Health Eff Inst. 1989;24:1–24. [PubMed] [Google Scholar]