Highlights
-
•
Low sodium intake or sodium losses upregulate kidney expression of ACE2.
-
•
SARS-CoV-2 makes use of ACE2 to interact with target cells.
-
•
Low sodium status may increase the risk of kidney involvement during COVID-19.
Keywords: COVID-19, SARS-CoV-2, Sodium, Kidney, ACE2 receptor
Abstract
The angiotensin-converting enzyme 2 receptor (ACE2) is expressed in epithelial cells of many tissues including the kidney, and has been identified to interact with human pathogenic coronaviruses, including SARS-CoV-2. Although diffuse alveolar damage and acute respiratory failure are the main features of COVID-19 infection, two recent studies demonstrate that kidney impairment in hospitalized COVID-19 patients is common, and that kidney involvement is associated with high risk of in-hospital death. Interestingly, studies in rats have demonstrated that high dietary sodium intake results in down-regulation of the ACE2 expression in kidney tissue. We hypothesize that low sodium status makes kidney involvement during the course of COVID-19 infection more likely due to upregulation of membrane bound ACE2 in the kidneys. We propose that sodium intake and status should be monitored carefully during severe COVID-19 infections, and that low sodium intake be corrected early in its course, despite a potential conflict regarding common dietary recommendations to restrict dietary sodium intake in patients with hypertension, diabetes, and kidney disease.
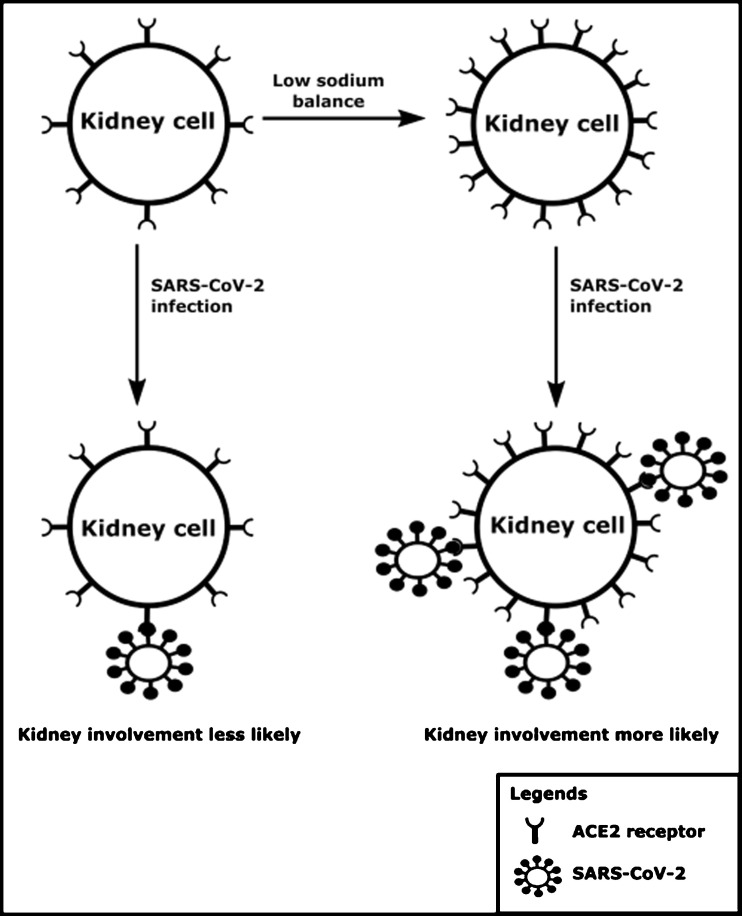
The angiotensin-converting enzyme 2 receptor (ACE2) is expressed in epithelial cells of many tissues including the kidney, and has been identified to interact with human pathogenic coronaviruses, including SARS-CoV-2 (Letko et al., 2020). Although diffuse alveolar damage and acute respiratory failure are the main features of COVID-19 infection, two recent studies demonstrate that kidney impairment in hospitalized COVID-19 patients is common, and that kidney involvement is associated with high risk of in-hospital death (Cheng et al., 2020; Li et al., 2020). If SARS-CoV-2 becomes systemic after the respiratory system has served as porte d’entrée, the kidney is a potential target for the virus, which is supported by results of RNA-sequencing studies, which found up to 100 times higher tissue ACE2 expression in the kidneys compared to the lungs (Fagerberg et al., 2014). Interestingly, studies in rats have demonstrated that high dietary sodium intake results in down-regulation of the ACE2 expression in kidneys (Cao et al., 2017; Berger et al., 2015). Indeed, Cao et al. demonstrated that a high sodium diet for 3 weeks more than halved the expression of ACE2 in rat kidney (Cao et al., 2017). Concordantly, Berger et al. demonstrated a nearly 5-fold higher ACE2 expression in spontaneously hypertensive rats fed a low sodium diet when compared to those on a high sodium diet (Berger et al., 2015). It should be noted that the high sodium group in the study by Cao et al. was exposed to a 20-times higher sodium intake than the low sodium group (Cao et al., 2017), while such an extreme difference in sodium intake is rarely seen between humans. Moreover, these experimental studies did not differentiate between cell membrane-bound ACE2 and soluble ACE2, which may be important in light of SARS-CoV-2 exposure to cells. Soluble ACE2 is formed through proteolytic cleavage of membrane bound ACE2 but probably represents only a small fraction of membrane bound ACE2 (Larouche-Lebel et al., 2019). It has been proposed that low sodium balance may lead to more severe COVID-19 (Post et al., 2020). Indeed, studies on electrolyte disturbances during COVID-19 have shown significantly lower sodium concentrations in patients with severe COVID-19 infection (Lippi et al., 2020). Here, we hypothesize that low sodium status also makes kidney involvement during the course of COVID-19 infection more likely due to upregulation of membrane-bound ACE2 in the kidneys. The hypothesized interaction between sodium balance, the ACE2 receptor, SARS-CoV-2, and the kidney is shown in Fig. 1 . We propose that sodium intake and status should be monitored carefully during severe COVID-19 infections and low sodium intake be treated early in its course, despite a potential conflict regarding common dietary recommendations to restrict dietary sodium intake in patients with hypertension, diabetes, and kidney disease.
Fig. 1.
A schematic overview of the hypothesized interaction between sodium balance, the ACE2 receptor expression, SARS-CoV-2 and the kidney.
Funding
None.
CRediT authorship contribution statement
Adrian Post: Conceptualization, Investigation, Visualization, Writing - original draft, Writing - review & editing. Robin P.F. Dullaart: Conceptualization, Investigation, Visualization, Writing - original draft, Writing - review & editing. Stephan J.L. Bakker: Conceptualization, Investigation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2020.198034.
Contributor Information
Adrian Post, Email: a.post01@umcg.nl.
Robin P.F. Dullaart, Email: dull.fam@12move.nl.
Stephan J.L. Bakker, Email: s.j.l.bakker@umcg.nl.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Berger R.C., Vassallo P.F., Crajoinas Rde O. Renal effects and underlying molecular mechanisms of long-term salt content diets in spontaneously hypertensive rats. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Della Penna S.L., Kouyoumdzian N.M. Immunohistochemical expression of intrarenal renin angiotensin system components in response to tempol in rats fed a high salt diet. World J. Nephrol. 2017;6(1):29–40. doi: 10.5527/wjn.v6.i1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Luo R., Wang K. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L., Hallstrom B.M., Oksvold P. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics. 2014;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche-Lebel E., Loughran K.A., Oyama M.A. Plasma and tissue angiotensin-converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J. Vet. Intern. Med. 2019;33(4):1571–1584. doi: 10.1111/jvim.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wu M., Guo J. medRxi. 2002. Caution on kidney dysfunctions of 2019-nCoV patients. (available http://medrxiv.org/content/early/2020/02/12/2020.02.08.20021212.abstract; (date of access: 16th May 2020)). [Google Scholar]
- Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann. Clin. Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A., Dullaart R.P.F., Bakker S.J.L. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur. J. Intern. Med. 2020;75:109. doi: 10.1016/j.ejim.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.