Highlights
-
•
Human Bocavirus 1 (HBoV1) was commonly detected in a survey of circa 13,000 UK respiratory samples between 2015 and 2019.
-
•
Co-infection with other viruses was observed in approximately three quarters of samples.
-
•
However, mono-infection was also prevalent, and associated with clinically relevant disease.
-
•
Intensive care was required in 31% of HBoV1 mono-infections and ventilation in 17%.
-
•
Fatal multi-organ failure was observed in an apparently HBoV1 mono-infected and otherwise healthy child.
Keywords: Human Bocavirus, Respiratory virus, Viral co-infection, Viral mono-infection, Viral bronchiolitis, Viral pneumonia
Abstract
Background
Since its first isolation in 2005, Human Bocavirus (HBoV) has been repeatedly associated with acute respiratory tract infections, although its role in pathogenicity remains unclear due to high co-infection rates.
Objectives
To assess HBoV prevalence and associated disease in a cohort of respiratory patients in the East Midlands, UK between 2015 and 2019.
Study design
We initially investigated the undiagnosed burden of HBoV in a retrospective paediatric cohort sampled between 2015 and 2017 using an in-house PCR assay. HBoV was subsequently incorporated into the standard respiratory diagnostic pathway and we audited a calendar year of HBoV positive results between 2018 and 2019.
Results
Our retrospective PCR screening of previously routine diagnostic-negative samples from juvenile patients identified a 9% (n = 30) prevalence of HBoV type 1. These apparent HBoV1 mono-infections were frequently associated with respiratory tract symptoms, often severe requiring ventilation, oxygen and steroid intervention with 31% (n = 9) of individuals requiring intensive care. When HBoV screening was subsequently adopted into the routine respiratory diagnostic pathway, year-round infections were observed in both children and adults peaking in February. 185 of 9098 (2.03%) individuals were found to be HBoV positive with children aged 12–24 months the principally infected group. However, HBoV infection was also observed in patients aged over 60, predominantly as a mono-infection. 23% of the 185 unique patients were HBoV monoinfected and persistent low-level DNA positivity was observed in 15 individuals up to 6-months after initial presentation.
Conclusion
HBoV1 is a prevalent respiratory infection in the UK capable of causing serious monoinfections.
1. Introduction
Human Bocavirus 1 (HBoV1) was first identified in respiratory tract samples in 2005 and three further genotypes (HBoV2, 3, and 4) were subsequently detected in faecal specimens [[1], [2], [3]]. HBoV types 1–4 were determined to be members of the Bocaparvovirus genus in the Parvoviridae family, with sequence similarity to both bovine parvovirus and canine minute virus [4]. Despite HBoV1′s frequent detection in respiratory samples from subjects with acute respiratory tract infections (ARTIs), its role as a respiratory pathogen is not fully understood. High rates of co-infection (up to 70%–80% in some studies) with predominantly Respiratory Syncytial Virus, Rhinovirus, Parainfluenza, Adenovirus [5,6] and also frequent detection in asymptomatic subjects [7], have promoted a hypothesis of HBoV1 as a “passenger” virus [5,7,8]. In vitro culture models of the virus have been described [9,10] which could further elucidate pathogenicity.
Clinically, HBoV1 infections are typically characterized by mild self-limiting acute respiratory symptoms including cough, rhinitis, acute otitis media, and pharyngitis [7,11]. However, possibly due to the unknown pathogenicity and frequently asymptomatic or self-limiting nature of bocavirus infections, HBoV is often omitted from diagnostic investigation. Nevertheless, evidence to support HBoV1 as the aetiological agent in presentations of ARTI is growing, for instance by use of serological diagnosis using acute convalescent sera [12] and through correlation of HBoV1 viral loads with symptom severity in mono-infections [5,11,13]. Furthermore, the virus has been associated with respiratory symptoms in the absence of other viral, fungal, or bacterial agents, with a significant detection rate difference between cases and controls [5]. HBoV1 has been associated not only with self-limiting upper respiratory tract infections, but also lower respiratory tract symptoms including wheezing, bronchiolitis, respiratory distress, and pneumonia [5,8]. Indeed, there are several documented cases of bocavirus infection associated with severe lower respiratory illness requiring hospitalization, oxygen therapy, and even intensive care [[14], [15], [16], [17]]. Taken together, these findings suggest HBoV1 can function in isolation as a respiratory pathogen.
In order to better understand the epidemiology and disease burden (if any) imposed by HBoV infection, we retrospectively tested residual total nucleic acid (TNA) from 347 nasopharyngeal aspirates (NPAs) by pan-bocavirus degenerate PCR [18]. Samples were collected from January 2015 to April 2017 from individuals between 6 months and 5 years of age that were known to be negative for a panel of more established respiratory viruses. Having identified a significant number of bocavirus mono-infections associated with clinically severe disease, HBoV1 was included as a target in routine respiratory viral panel screening. HBoV1 detection was also reported throughout a calendar year (September 2018−August 2019) in upper and lower respiratory tract samples, again identifying significant numbers of patients infected with HBoV1, mainly children but also adults, encompassing both mono- and co-infections. Overall, our findings support the hypothesis that HBoV1 can be a significant human pathogen responsible for clinically relevant respiratory disease.
2. Methods
2.1. Samples
Nasopharyngeal aspirates (NPA) from children between 6 months and 5 years old were processed for routine diagnostic investigation at Nottingham University Hospitals Trust (NUHT) between January 2015 and April 2017 as previously described [19]. TNAs negative for Human Adenovirus, Influenza (A and B), Human Metapneumovirus, Parainfluenza viruses (1–4), Rhinovirus, Coronavirus and Human Respiratory Syncytial Virus (RSV; A and B) were selected for bocavirus screening. TNAs were pooled in groups of 10 for initial screening and if positive, residual individual samples were retrospectively tested.
A second study period (September 2018 to August 2019) investigated all respiratory samples (not limited by sample type or age) received for routine clinical diagnosis at NUHT. In this period, samples were routinely assessed with the AusDiagnostics Respiratory Viruses (16-well) target panel (REF 20602) for the High-Plex 24 system (REF 9150), including an HBoV target. Data were analysed using Microsoft Excel and Graphpad Prism software. Ethical approval for the use of residual material and association with anonymized patient information was provided under the Nottingham Health Science Biobank Research Tissue Bank, REC reference 15/NW/0685.
2.2. PCR assays
TNA extracts were screened by PCR using pan-Bocaparvovirus genus primers panBOV-F1 (TAATGCAYCARGAYTGGGTNGANCC) and panBOV-R1 (GTACAGTCRTAYTCRTTRAARCACCA) [20] to target the NS1 gene in 15 μL reactions comprising: 1.5 μL of QIAGEN 10× PCR buffer, 3 pmol of each primer, 6 nmol of dNTPs, 0.375U QIAGEN HotStarTaq DNA polymerase, 1 μL of TNA template and nuclease-free water. PCR was thermocycled as follows: 95 °C for 15 min, then 55 cycles of 95 °C for 20 s, 55 °C for 20 s, and 72 °C for 45 s. Amplification of a 1798bp NS1 fragment was also performed as above, except with 45 cycles and 72 °C for 120 s, using novel primers hBocaNS1f (TCTCAACCTGCTTTYACYTATGT) and hBocaNS1r (AGAATTGTCAGCRSTATGAGSAA). All positive pools and individual samples were confirmed by agarose gel electrophoresis and sequencing as previously described [19]. Phylogenetic analysis of sequences was performed using MEGA7 software (version 7.0.25) and all human reference sequences were downloaded from GenBank circa May 2017.
3. Results
347 residual TNAs from NPAs collected January 2015 to April 2017 from individuals between 6 months and 5 years of age (negative for routinely screened viral pathogens) were identified and retrospectively screened for the presence of Bocavirus by degenerate, pan-genus PCR in 35 pools. 19 pools were positive for Bocavirus and their component samples rescreened individually, identifying 30 (8.66%) TNAs positive for HBoV1, representing 29 unique patients. Sample HBoV1 positivity was confirmed by Sanger sequencing and BLAST analysis, revealing circa 99% conservation with Genbank HBoV1 reference strains in the conserved 298bp NS1/2 region targeted (data not shown).
To further confirm identity and investigate potential HBoV1 sequence variability and relatedness, a larger 1798 bp fragment of the NS1 gene was amplified from 20 of the 30 positive samples. The products were sequenced from the 5′ end only, yielding 1170 bp from each patient (covering bases 366–1535 of the prototypical reference isolate NC007455, [1] and aligned with 196 reference sequences of HBoV1–4 downloaded from Genbank in May 2017. Maximum likelihood phylogenetic reconstruction of this NS1 region confirmed strong bootstrap support for genotypic clustering of our positives with HBoV1 reference sequences (>0.99, data not shown). However, little support for any other phylogenetic inference was observed due to a high degree of genetic conservation observed in both study and reference HBoV1 sequences.
Having identified the presence of previously undiagnosed HBoV1 infection in a significant proportion of NPA specimens investigated, clinical data in this pilot cohort were also retrospectively assessed (Table 1 ). All patients presented with a similar clinical picture of bronchiolitis variously including general respiratory distress, cough, coryza, and wheezing. 38% (11 of 29) of patients received oxygen, 38% nebulisers and 17% (5 of 29) were supported by mechanical ventilation. No respiratory or systemic bacterial co-infections were identified, but 38% of patients were administered antibiotics. Furthermore, 31% (9 of 29) were admitted to intensive care, with one patient dying of multi-organ failure and viral pneumonitis.
Table 1.
Clinical and laboratory data from HBoV1 positive patients, Nottinghamshire, United Kingdom, January 2015−March 2017.
| Sample number | Sample date | Age group | Hospital care | Ventilation | Nebuliser | Oxygen | Steroids | Bacterial co-infection | Antibiotics | Additional care and notes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jan 2015 | 2−3 years | Standard | Yes | Yes | Yes | Negative | Yes | Severe RSV infection >2 years prior | |
| 2 | Jan 2015 | 6−12 months | Intensive | Yes | Yes | Yes | Negative | Yes | IV salbutamol | |
| 3 | Jan 2015 | 6−12 months | Standard | unknown | ||||||
| 4 | Jan 2015 | 6−12 months | Standard | Yes | Not tested for | Atrovent given, premature | ||||
| 5 | Feb 2015 | 4−5 years | Standard | Negative | Yes | Prior gastrostomy | ||||
| 6 | Mar 2015 | 6−12 months | Intensive | Negative | Diarrhoea and vomiting, seizures, gastrostomy | |||||
| 7 | Mar 2015 | 6−12 months | Intensive | Yes | Negative | |||||
| 8 | Mar 2015 | 2−3 years | Standard | Yes | Yes | Negative | Yes | Died of multi-organ failure and viral pneumonitis | ||
| 9 | Mar 2015 | 6−12 months | Standard | Yes | Not tested for | |||||
| 10 | May 2015 | 13−24 months | Intensive | Yes | Not tested for | Yes | Adrenaline, Cerebral Palsy | |||
| 11 | Jun 2015 | ≤6 months | Standard | Not tested for | ||||||
| 12 | Oct 2015 | 13−24 months | Intensive | Yes | Yes | Negative | Yes | Salbutamol | ||
| 13 | Nov 2015 | 6−12 months | Standard | Yes | Not tested for | Yes | ||||
| 14 | Nov 2015 | 6−12 months | Intensive | Yes | Yes | Negative | Yes | Salbutamol | ||
| 15 | Nov 2015 | 6−12 months | Standard | Yes | Not tested for | |||||
| 16 | Dec 2015 | 13−24 months | Standard | Yes | Negative | Yes | ||||
| 17 | Dec 2015 | ≤6 months | Intensive | Yes | E. coli (urine) Negative (blood) | Yes | Other viral infections before and after | |||
| 18 | Jan 2016 | 13−24 months | Standard | Yes | Not tested for | |||||
| 19 | Jan 2016 | 6−12 months | Standard | Negative | Salbutamol | |||||
| 20 | Feb 2016 | 2−3 years | Standard | Yes | Not tested for | |||||
| 21 | Mar 2016 | ≤6 months | Standard | Yes | Yes | Negative | Yes | Premature at 25 weeks | ||
| 22 | Apr 2016 | 13−24 months | Standard | Yes | Yes | Not tested for | ||||
| 23 | Jul 2016 | 2−3 years | Standard | Yes | Not tested for | Salbutamol, Chemotherapy for Wilm's tumour | ||||
| 25 | Jul 2016 | 6−12 months | Intensive | Unknown | Subsequently Rhinovirus positive | |||||
| 24 | Aug 2016 | 13−24 months | Standard | Yes | Yes | Not tested for | ||||
| 26 | Dec 2016 | 13−24 months | Standard | Yes | Yes | Not tested for | ||||
| 27 | Feb 2017 | 6−12 months | Intensive | Unknown | Multiple underlying comorbidities, other viral infections before and after | |||||
| 28 | Feb 2017 | 6−12 months | Not admitted | Yes | Not tested for | |||||
| 29 | Mar 2017 | 6−12 months | Standard | Yes | Not tested for |
This retrospective in-house pilot study indicated a hidden HBoV1 burden of circa 9% in otherwise viral pathogen negative young children and encouraged the uptake of HBoV screening as part of routine diagnostic surveillance. Subsequently in August 2018 we began screening for HBoV in routine respiratory investigations using a commercial assay (AusDiagnostics) and undertook an audit of a calendar year of screening between 1st September 2018 and 31st August 2019. In this period 12,498 unique specimens were received for routine respiratory pathogen investigation, of which 208 (1.66%) were HBoV positive representing 185 of 9098 unique patients (2.03%). HBoV ranked seventh in viral prevalence behind Rhinovirus or Enterovirus (non-differentiated), Influenza A, RSV, Human Adenovirus, Parainfluenza Type 3 (PF3), Coronavirus and Human Metapneumovirus, but ahead of PF1, PF4, Parechovirus, PF2 and Influenza B (data not shown).
Of the 208 positive specimens recorded, 165 (75.96%) were co-infected and 43 were from HBoV mono-infected patients (23.24%). Six mono- and 10 co-infected individuals were found to be HBoV positive during intensive care. Two of the 10 co-infected individuals had HBoV copy numbers 5- to 6-logs higher than the co-infecting virus.
116 (56%) of the 208 positive samples were throat swabs, 35% NPAs and 9% others including broncheoalveolar lavages, endotracheal aspirates and sputum. HBoV positivity rates by sample type were 73/1347 (5.42%) of NPAs, in contrast to only 116/10,249 (1.13%) of throat swabs and 19/902 (2.11%) of other samples received. Three individuals were sampled by both throat swab and NPA on the same or consecutive days. Two returned higher viral load values in the NPA (24- and 28-fold more viral target) whilst one indicated a 28-fold higher viral load in the throat swab compared to the NPA (data not shown).
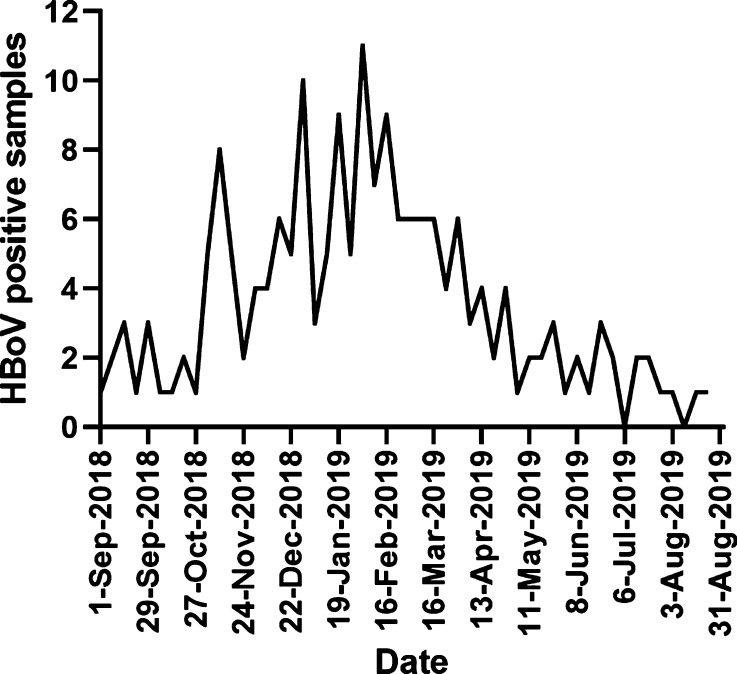
To determine if HBoV1 infections were associated with seasonality, weekly HBoV positivity rates were investigated (Fig. 1 ). Whilst HBoV positive samples were seen throughout the calendar year (commencing in week 36 of 2018 and finishing in week 35 of 2019) a peak of positivity was observed around the middle of February (week 7 of 2019, Fig. 1). No more than four positive samples per week were seen outside of weeks 45 to 18, suggesting a peak season from approximately the start of November 2018 until the end of April 2019.
Fig. 1.
Weekly HBoV PCR positive samples recorded at NUHT between week 36 of 2018 (commencing 1st September) and week 35 of 2019 (ending 31st August).
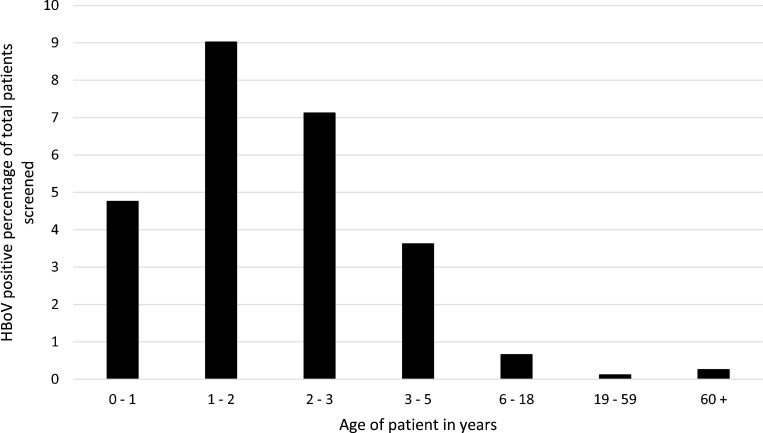
Age of HBoV-infected individuals was significantly skewed toward the young (p ≤ 0.0001, Chi-square test for trend), with 87% of unique HBoV-positive patients being aged 5 or under. A peak in positivity was seen in the 1−2-year-old age group, with 9% of these individuals screened presenting as HBoV positive (Fig. 2 ).
Fig. 2.
Proportion of HBoV positive patients in first positive sample observed between weeks 36 of 2018 to 35 of 2019 grouped by age.
Interestingly, of the 11 HBoV positive patients aged ≥60, seven were mono-infected and where co-infected, higher viral loads for HBoV were observed than for the co-infecting virus (data not shown).
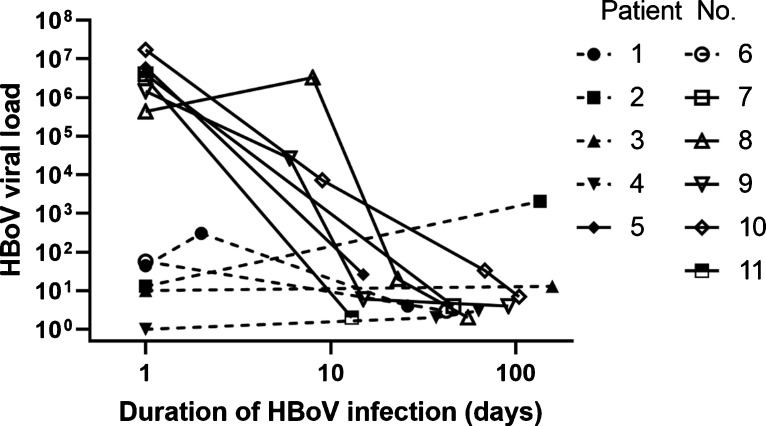
11 individuals were sampled on more than one occasion and >1 day apart, ranging from a 13 to a 157 day period (Fig. 3 ), all of whom were aged 5 years or under. Three individuals were sampled on four separate occasions (patients 8–10, Fig. 3), spanning 55, 91 and 104 days respectively: all of whom experienced a period of intensive care. Five of six patients presenting with higher initial viral load of >105 (data points connected by solid lines, Fig. 3), recorded their highest HBoV value on initial presentation, exhibiting a steady decline through the period of surveillance. Patient 8 initially presented with a viral load of 4.3 × 105, increasing by approximately one log to 3.3 × 106 (Fig. 3) one week later having been transferred to an intensive care unit after initial assessment and sampling. Co-infection was observed for at least one time point for all patients except one. However, in all six individuals presenting with HBoV values of ≥ 4 × 105, the HBoV viral load was higher than the co-infecting virus.
Fig. 3.
HBoV viral load determined by the AusDiagnostics assay in patients sampled more than once, more than one day apart in weeks 36 of 2018−35 of 2019.
4. Discussion
Failure to detect viral pathogens responsible for ARTIs can lead to inconclusive diagnosis, prolonged hospital stays and unnecessary antibiotic use, ultimately contributing to a burden on local and global health and economy. In clinical settings, most antibiotic prescriptions are erroneously prescribed for respiratory illnesses caused mainly by viruses [21]. Such overuse not only contributes to bacterial resistance, but can also affect commensal gastrointestinal microbiota required for healthy gut function and potentially cause unnecessary adverse effects in patients [21,22]. Expanding routine tested-for viral respiratory panels has the potential to reduce this public health burden.
We tested this hypothesis by archiving and re-screening apparently viral-negative respiratory TNA extracts for HBoV, a relatively recently discovered, but widely reported viral pathogen, identifying 9% HBoV1 positivity of NPAs in patients aged 6 months to 5 years. Importantly, 10 (34%) patients with undiagnosed HBoV1 positive patients were prescribed empirical antibiotic therapy; eight in the absence of diagnosed bacterial co-infection whilst two were not investigated.
HBoV1 pathogenicity is not fully understood with HBoV frequently observed as a co-infection. Our initial in-house study supported a causal role of HBoV1 as a respiratory pathogen, with severe symptoms observed and intensive care required in the absence of co-infection with other typical viral or bacterial respiratory pathogens. Our subsequent one-year review of HBoV screening in routine diagnostic service confirmed previously observed high rates of co-infection with other viral pathogens.
High rates of coinfections and the presence of HBoV1 in asymptomatic individuals could be explained by long persistence periods and high prevalence of the virus. HBoV1 was found to persist in mucosa for more than four months following primary infections, which increases the chances of co-existence with other viral or bacterial pathogens [23,24] and the virus has been suggested to reactivate following a superinfection with another virus [4].We similarly observed persistence of HBoV in our cohort for up to 6 months. HBoV1′s high prevalence could also explain the high rate of co-infections. One study estimated HBoV1 infection rate to be as high as 59% in a cohort of children with respiratory illness and another reported that ∼90% of adults have HBoV-specific antibodies [23,25]. It is clear that detection of HBoV DNA can persist for many months, and therefore the presence of such DNA does not necessarily indicate a recent infection.
Our findings of frequent administration of oxygen, steroids and salbutamol in addition to ventilation and intensive care in HBoV1 mono-infection, supports previous reports of HBoV1 as a cause of serious lower respiratory tract infections and pneumonia [5,[13], [14], [15]].
HBoV1 has been reported to have very low genetic diversity worldwide [18]. Our sequencing and phylogenetic analysis of a region of NS1 (circa 22% of the genome) indeed confirmed these findings and would indicate whole genome sequencing is required for more robust investigation and interpretation of epidemiology. Sequencing of this NS1 region did strongly suggest all detected samples were of HBoV type 1, but the possibility of recombination cannot be ruled out without whole genome sequencing [18].
With respect to HBoV1 seasonality, our study showed higher prevalence during the late autumn and winter periods, consistent with prevalence reported worldwide [1,[26], [27], [28]]. However, few studies reported year-round detection and two studies reported peak detection during summer [[29], [30], [31], [32]]. The contradictory findings of these reports with many studies reporting winter and spring surges in HBoV1 infection rates could be explained by differences between strains isolated in different locations, but it could be also attributed to social or behavioural factors [33].
In summary, our study implicates HBoV1 as a currently prevalent respiratory pathogen in the UK capable of causing serious mono-infections. Increasing adoption of this relatively newly described virus into established diagnostic screening panels could be helpful in aiding clinical management of patients with respiratory tract disease and reducing unnecessary prescribing of antibacterial agents.
Funding sources
This study was funded internally by the University of Nottingham, UK, as part of a Master of Science degree project.
CRediT authorship contribution statement
Arwa A. Bagasi: Investigation, Writing - original draft, Writing - review & editing. Hannah C. Howson-Wells: Validation, Resources, Supervision, Writing - review & editing. Gemma Clark: Resources, Conceptualization, Writing - review & editing. Alexander W. Tarr: Supervision, Formal analysis, Writing - review & editing. Shiu Soo: Resources, Conceptualization, Writing - review & editing. William L. Irving: Resources, Conceptualization, Supervision, Writing - review & editing. C. Patrick McClure: Conceptualization, Methodology, Supervision, Formal analysis, Writing - original draft, Writing - review & editing.
Declarations of Competing Interest
None.
References
- 1.Allander T. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U S A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur J.L. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5(4):e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor A. A newly identified bocavirus species in human stool. J. Infect. Dis. 2009;199(2):196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jartti T. Human bocavirus-the first 5 years. Rev. Med. Virol. 2012;22(1):46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 5.Ghietto L.M. Comorbidity and high viral load linked to clinical presentation of respiratory human bocavirus infection. Arch. Virol. 2015;160(1):117–127. doi: 10.1007/s00705-014-2238-5. [DOI] [PubMed] [Google Scholar]
- 6.Ghietto L.M. High frequency of human bocavirus 1 DNA in infants and adults with lower acute respiratory infection. J. Med. Microbiol. 2012;61(Pt 4):548–551. doi: 10.1099/jmm.0.035600-0. [DOI] [PubMed] [Google Scholar]
- 7.Longtin J. Human bocavirus infections in hospitalized children and adults. Emerg. Infect. Dis. 2008;14(2):217–221. doi: 10.3201/eid1402.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q.B. Epidemic and molecular evolution of human bocavirus in hospitalized children with acute respiratory tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34(1):75–81. doi: 10.1007/s10096-014-2215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lüsebrink J. Detection of head-to-tail DNA sequences of human bocavirus in clinical samples. PLoS One. 2011;6(5):e19457. doi: 10.1371/journal.pone.0019457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkman R. Human bocavirus can be cultured in differentiated human airway epithelial cells. J. Virol. 2009;83(15):7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allander T. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 2007;44(7):904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Söderlund-Venermo M. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg. Infect. Dis. 2009;15(9):1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect. Dis. 2014;14:424. doi: 10.1186/1471-2334-14-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moesker F.M. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin. Microbiol. Infect. 2015;21(10):964. doi: 10.1016/j.cmi.2015.06.014. e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Körner R.W. Severe human bocavirus infection, Germany. Emerg. Infect. Dis. 2011;17(12):2303–2305. doi: 10.3201/eid1712.110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastien N. Human bocavirus infection, Canada. Emerg. Infect. Dis. 2006;12(5):848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskola V., Xu M., Soderlund-Venermo M. Severe lower respiratory tract infection caused by human bocavirus 1 in an infant. Pediatr. Infect. Dis. J. 2017;36(11):1107–1108. doi: 10.1097/INF.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor A. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010;201(11):1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagasi A.A. Trichodysplasia spinulosa polyomavirus in respiratory tract of immunocompromised child. Emerg. Infect. Dis. 2018;24(9):1744–1746. doi: 10.3201/eid2409.180829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor A. Identification and characterization of a new bocavirus species in gorillas. PLoS One. 2010;5(7):e11948. doi: 10.1371/journal.pone.0011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llor C., Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5(6):229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulliford M.C. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. doi: 10.1136/bmjopen-2014-006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin E.T. Frequent and prolonged shedding of bocavirus in young children attending daycare. J. Infect. Dis. 2010;201(11):1625–1632. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blessing K. Prolonged detection of human bocavirus DNA in nasopharyngeal aspirates of children with respiratory tract disease. Pediatr. Infect. Dis. J. 2009;28(11):1018–1019. doi: 10.1097/INF.0b013e3181a854ae. [DOI] [PubMed] [Google Scholar]
- 25.Lindner J. Humoral immune response against human bocavirus VP2 virus-like particles. Viral Immunol. 2008;21(4):443–449. doi: 10.1089/vim.2008.0045. [DOI] [PubMed] [Google Scholar]
- 26.Christensen A. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J. Clin. Virol. 2010;49(3):158–162. doi: 10.1016/j.jcv.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canducci F. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 2008;80(4):716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fry A.M. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J. Infect. Dis. 2007;195(7):1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung J.Y. Bocavirus infection in hospitalized children, South Korea. Emerg. Infect. Dis. 2006;12(8):1254–1256. doi: 10.3201/eid1208.060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meriluoto M. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg. Infect. Dis. 2012;18(2):264–271. doi: 10.3201/eid1802.111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W. [Detection and analysis of bocavirus in hospitalized children with respiratory infection] Zhongguo Dang Dai Er Ke Za Zhi. 2016;18(1):39–43. doi: 10.7499/j.issn.1008-8830.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn J.G. Human bocavirus isolated from children with acute respiratory tract infections in Korea, 2010-2011. J. Med. Virol. 2014;86(12):2011–2018. doi: 10.1002/jmv.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin. Microbiol. Infect. 2012;18(10):946–954. doi: 10.1111/j.1469-0691.2012.03968.x. [DOI] [PubMed] [Google Scholar]