Abstract
Mucormycosis is a life-threatening infection caused by fungi in the Mucorales species. It most often affects immunocompromised hosts, including diabetics patients. It can affect a variety of organ systems with pulmonary manifestations being the second most common. In severe cases, significant bronchial necrosis and angioinvasion can be seen. Due to its rarity, such extensive cases are not often reported in literature. We present a case of a 32-year-old man who presented with severe bronchial necrosis, including bronchomediastinal fistula, due to Rhizopus. Despite prompt treatment, he developed massive hemoptysis from invasion of the pulmonary vasculature and died. We also provide a brief review of mucormycosis.
Keywords: Mucormycosis, Rhizopus, Bronchomediastinal fistula, Mediastinal emphysema, Massive hemoptysis
1. Introduction
Mucormycosis is a rare, life-threatening mycosis most often infecting immunocompromised hosts. Multiple organ systems can be involved, with pulmonary manifestations being the second most common [1]. We present a case of a diabetic, young man with pulmonary mucormycosis including bronchial necrosis causing a bronchomediastinal fistula and extensive mediastinal and subcutaneous emphysema.
2. Case description
A 32-year-old man with no previously known medical history presented to our institution, from a primary care office, for worsening dyspnea, cough and elevated glucose. Very limited information was obtained from the patient as he spoke a unique language from Guatemala called Tz'utujil, for which language interpretation was not available. In the emergency department he was diaphoretic, in the tripod position, and in moderate respiratory distress.
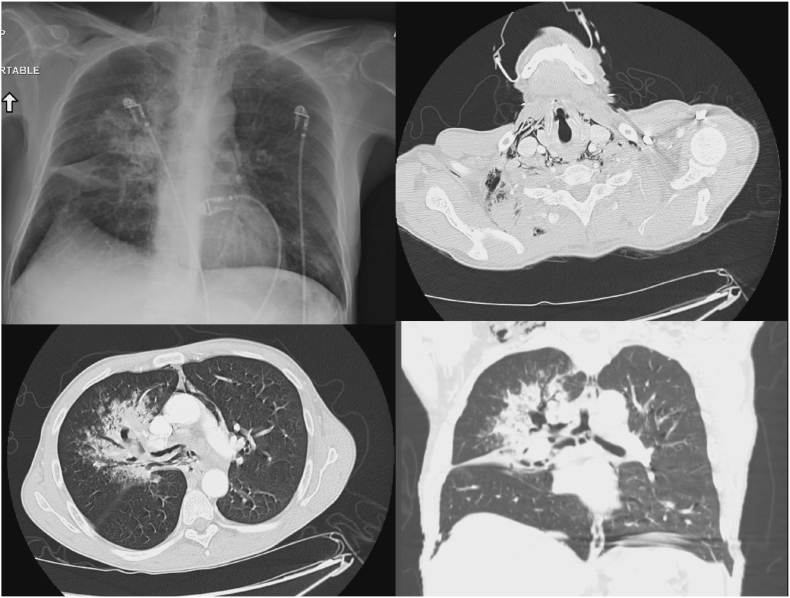
His condition was guarded initially on 10L oxymizer. Blood glucose was noted to be 415mg/dL. Chest x-ray was immediately performed and demonstrated subcutaneous air and pneumomediastinum. Chest CT was subsequently performed, revealing extensive right sided infiltration with probable erosion of the right mainstem bronchus, pulmonary emphysema in the right-sided airways, as well as right upper lobe (RUL) and right middle lobe (RML) infiltrates and centrilobular ground-glass nodules were also noted (Fig. 1). He was subsequently intubated due to increasing distress with a 35 french left sided, double lumen endobronchial tube (ET), performed under endoscopic guidance with a bronchoscope. Bronchoscopy revealed erythema at the carina along with copious thick, white secretions that extended into the left and right mainstem bronchus. Saline was used to wash and suction the secretions. Empiric antibiotic and anti-fungal treatment were started with Vancomycin, Piperacillin-Tazobactam, Doxycycline and Voriconazole. In addition, he was maintained on airborne precautions due to concern for tuberculosis. HIV testing, respiratory multiplex PCR, aspergillus antibodies, and blood cultures were obtained at this time. He was then transferred via helicopter to our tertiary institution for evaluation for interventional pulmonology.
Fig. 1.
Top left: Initial chest x-ray on presentation showing right sided opacities and sub-cutaneous air in the neck. Top right: Axial cut of chest CT showing mediastinal air. Bottom right: Axial cut of CT demonstrating bronchomediastinal fistulas with necrotizing pneumonia. Bottom right: Coronal cut of the chest CT further demonstrating necrotizing areas in the right main bronchus and necrotizing pneumonia.
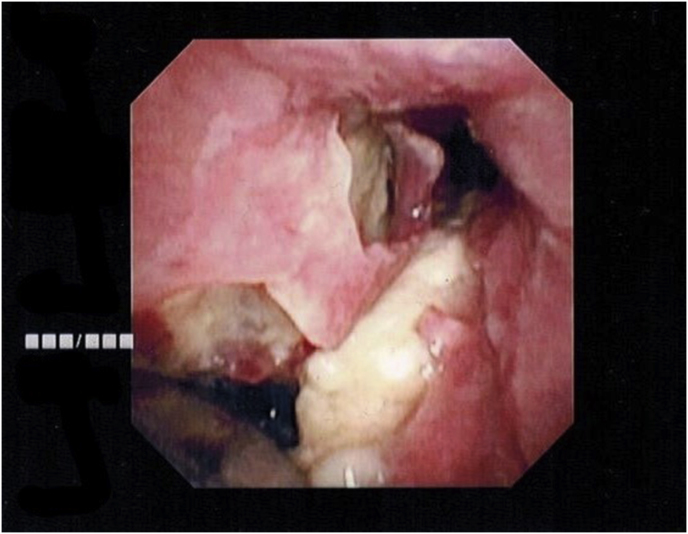
Cardiothoracic surgery evaluated the patient for bronchomediastinal fistula and recommended proceeding with further bronchoscopic intervention. Fiberoptic bronchoscopy was performed in the intensive care unit. Bronchoscopy revealed extensive submucosal sloughing and necrosis of the carina, right mainstem, right bronchus intermedius and RML lateral segment. In addition, there was an endobronchial flap with purulent material in the right mainstem bronchus. Broncheoalveolar lavage was obtained of the right lung as well as endobronchial biopsies of the right mainstem (Fig. 2). Continued broncoscopic management was recommended by cardiothoracic surgery at this time, with repeat debridement. The patient later became hypotensive requiring vasopressors, and broad-spectrum antibiotics and anti-fungal agents were continued.
Fig. 2.
Bronchoscopic view of the carina. Double lumen endobronchial tube seen inserted into the left mainstem bronchus. Significant necrotic tissue is seen with the bronchomediastinal fistula in the right mainstem bronchus.
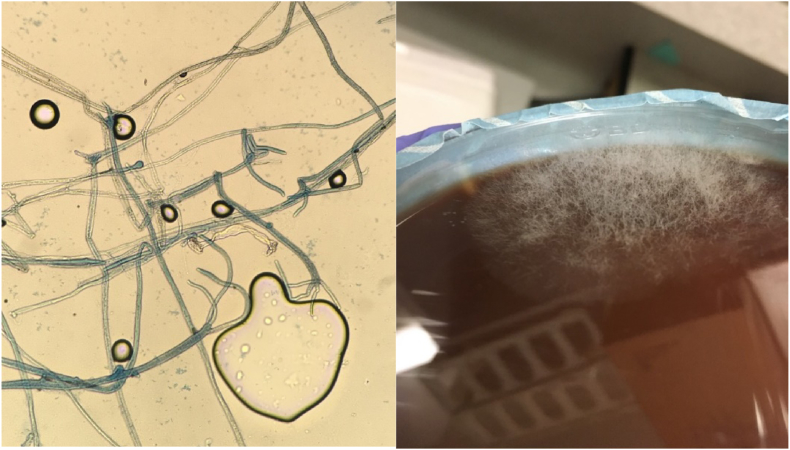
On hospital day 3, acid fast bacilli smear resulted as negative, bronchiolar alveolar lavage cultures grew rhizopus, candida (albicans or candida dubliniensis), and endobronchial biopsies revealed necrotic tissue with fungal hyphae suggestive of mucor species, as well as actinomyces (Fig. 3). His anti-fungal coverage was switched to liposomal Amphotericin B and micafungin. It was agreed that the etiology of patient's disease was invasive mucormycosis causing bronchial erosion and an MRI of the brain was ordered to look for fungal disease. Right sided mastoiditis was found with no other findings identified.
Fig. 3.
Left: Biopsy specimen showing fungal hyphae consistent with mucormycosis. Right: Mucor growth on fungal medium.
The following day, the ventilator alarmed for high peak pressures. The ET appeared to be obstructed. An approximately 10cm long blood clot was retrieved from the ET, followed by large amounts of bright red blood. The patient was unable to be adequately ventilated or oxygenated and went into PEA arrest. Advanced cardiac life support (ACLS) protocol was immediately started. ET exchange was performed under glidescope visualization. Massive transfusion protocol was initiated simultaneously. Subsequently, crepitus was noted from mid-thigh to forehead, and bilateral needle decompression was performed with air and bright red blood return. ACLS protocol was continued for a total of 35 minutes, at which time the patient was pronounced dead.
3. Discussion
Mucormycosis (formerly zygomocosis) refers to infections caused by fungi in the order of Mucorales. The organisms are ubiquitous and most often affect immunocompromised hosts or those with diabetes mellitus. Rhizopus, Mucor, and Rhizomucor species account for >70% of cases, while Cunninghamella bertholethiae appears to be the most virulent [2]. Rhino-orbital-cerebral infection is the most common manifestation, followed by pulmonary mucormycosis and, less commonly, gastrointestinal, cutaneous and disseminated disease [1,4]. Mortality rates of pulmonary mucormycosis range from 50 to 80% [[1], [2], [3], [4]].
Common predisposing factors for pulmonary mucormycosis include uncontrolled diabetes, DKA, steroid use, chemotherapy, and use of other immunosuppressive agents [1,4]. In healthy individuals, oxidative and non-oxidative killing mechanisms of mononuclear and polynuclear phagocytes eliminate inhaled conidia [2]. In the case of diabetes mellitus, hyperglycemia and acidosis impair chemotaxis and the killing activity of phagocytic cells [2]. The hyphae of Mucor are known to be angioinvasive, causing hemorrhage, thrombosis, infarction, and tissue necrosis [3].
Presenting symptoms are non-specific, including dyspnea, cough, fever, and hemoptysis [5]. Constitutional symptoms such as weight loss and night sweats can be exhibited. A variety of radiographic patterns can be seen on chest films such as lobar consolidation, non-specific infiltrates, cavities, masses and nodules [6]. Halo and air-crescent signs are less frequently seen. Tracheobronchial mucormycosis is less common, accounting for 34% of pulmonary mucomycosis cases [7]. Endobronchial lesions can obstruct major airways or erode into pulmonary blood vessels causing massive hemoptysis, a manifestation more often in diabetic patients than those with malignancy [2,7]. Lobar bronchi are the most frequently involved location, with a predilection for upper lobes. Mainstem bronchi are the next most common, followed by the trachea. No predilection for the right or left side is observed [7]. Bronchoscopy reveals mucosal necrosis, hyperemic mucosa, mass-like lesions, and purulent exudates among other findings [7].
Diagnosis requires a high index of suspicion and depends largely on histopathologic demonstration of tissue invasion by the characteristic hyphae or isolation of Mucorales species in culture [2]. Tissue swabs, sputum, bronchioalveolar lavage samples, and blood cultures are often non-diagnostic [8]. Murcuales stains well with Grocott-Gomori methamine-silver stain [2] and appears as broad non-septated hyphae with branches at right angles [8]. Diagnosis can remain challenging, however, with the need for invasive procedures in potentially unstable patients. Bacteria, Candida albicans, and Aspergillus species are frequently isolated along with Mucorales species [7]. No cases in our literature search were found that isolated Mucorales species along with Actinomyces species as in this case.
Treatment requires prompt diagnosis and a multidisciplinary approach. Conventional amphotericin B is the anti-fungal agent of choice, though liposomal formulations of amphotericin B are now often used with their more favorable toxicity profile and comparable clinical outcomes [2]. There are no current guidelines on duration of treatment [9]. Due to tissue necrosis and thrombosis, penetration of antifungal agents is often poor, making surgical debridement a mainstay of therapy. Overall survival rates in medically managed patients were 35–46%, in comparison to surgically treated patients who demonstrated higher survival rates ranging 51–90%, respectively [4,[10], [11], [12]]. Removing as much infected tissues as possible while the infection is localized leads to improved outcomes [10]. Extensive surgery may be required, including lobectomy or pneumonectomy for more proximal disease. The benefit of resection lessens with dissemination of the disease [2].
4. Conclusion
Pulmonary mucormycosis is a well described phenomenon and has been seen causing necrosis of pulmonary parenchyma and bronchial structures. Necrosis causing such extensive bronchomediastinal fistula at presentation and progressing to invasion of vasculature has not previously seen in case reports to the best of our knowledge. Our case report demonstrates this unusual presentation.
Declaration of competing interest
No conflicts of interest.
References
- 1.Lin E., Moua T., Limper A.H. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45:443–448. doi: 10.1007/s15010-017-0991-6. [DOI] [PubMed] [Google Scholar]
- 2.Hamilos G., Samonis G., Kontoyiannis D.P. Pulmonary mucormycosis. Semin. Respir. Crit. Care Med. 2011;32:693–702. doi: 10.1055/s-0031-1295717. [DOI] [PubMed] [Google Scholar]
- 3.Heimes J., Zaheer S., Wallen J. Bronchial necrosis from mucormycosis: a case report and review. J. Pulm. Respir. Med. 2016;6:385. doi: 10.4172/2161-105X.1000385. [DOI] [Google Scholar]
- 4.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., Sein M., Sein T., Chiou C.C., Chu J.H. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 5.Ribes J.A., Vanover-Sams C.L., Baker D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000;13:236–301. doi: 10.1128/CMR.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams H.P., Rosado de Christenson M., Strollo D.C. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am. J. Roentgenol. 1997;168:1541–1548. doi: 10.2214/ajr.168.6.9168721. [DOI] [PubMed] [Google Scholar]
- 7.He R., Hu C., Tang Y., Yang H., Cao L., Niu R. Report of 12 cases with tracheobronchial mucormycosis and a review. Clin. Res. J. 2018;12:1651–1660. doi: 10.1111/crj.12724. [DOI] [PubMed] [Google Scholar]
- 8.Frater J.L., Hall G.S., Procop G.W. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab Med. 2001;125(3):375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 9.Cornely O.A., Cuenca-Estrella M., Meis J.F., Ullmann A.J. European society of clinical microbiology and infectious diseases (ESCMID) fungal infection study group (EFISG) and European confederation of medical mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin. Microbiol. Infect. 2014;20(Suppl 3):1–4. doi: 10.1111/1469-0691.12569. [DOI] [PubMed] [Google Scholar]
- 10.Lee F.Y., Mossad S.B., Adal K.A. Pulmonary mucormycosis: the last 30 years. Arch. Intern. Med. 1999;159:1301–1309. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 11.Tedder M., Spratt J.A., Anstadt M.P., Hegde S.S., Tedder S.D., Lowe J.E. Pulmonary mucormycosis: results of medical and surgical therapy. Ann. Thorac. Surg. 1994;57:1044–1050. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 12.Choi H., Lee H., Jeon K., Suh G.Y., Shin S., Kim H.K.…Kim H. Factors affecting surgical resection and treatment outcomes in patients with pulmonary mucormycosis. J. Thorac. Dis. 2019;11(3):892–900. doi: 10.21037/jtd.2019.01.75. [DOI] [PMC free article] [PubMed] [Google Scholar]