Abstract
Introduction
Tumourigenesis attributed to residual undifferentiated cells in a graft is considered to be a significant issue in cell therapy using human pluripotent stem cells. To ensure the safety of regenerative medicine derived from pluripotent stem cells, residual undifferentiated cells must be eliminated in the manufacturing process. We previously described the lectin probe rBC2LCN, which binds harmlessly and specifically to the cell surface of human pluripotent stem cells. We report here a technique using rBC2LCN to remove pluripotent cells from a heterogenous population to reduce the chance of teratoma formation.
Methods
We demonstrate a method for separating residual tumourigenic cells using rBC2LCN-bound magnetic beads. This technology is a novel use of their previous discovery that rBC2LCN is a lectin that selectively binds to pluripotent cells. We optimize and validate a method to remove hPSCs from a mixture with human fibroblasts using rBC2LCN-conjugated magnetic beads.
Results
Cells with the potential to form teratoma could be effectively eliminated from a heterogeneous cell population with biotin-labelled rBC2LCN and streptavidin-bound magnetic beads. The efficiency was measured by FACS, ddPCR, and animal transplantation, suggesting that magnetic cell separation using rBC2LCN is quite efficient for eliminating hPSCs from mixed cell populations.
Conclusions
The removal of residual tumourigenic cells based on rBC2LCN could be a practical option for laboratory use and industrialisation of regenerative medicine using human pluripotent stem cells.
Keywords: Pluripotent stem cell, rBC2LCN, Lectin, Cell separation
Highlights
-
•
A method using rBC2LCN-conjugated magnetic beads was developed to select hPSCs.
-
•
This method is applicable to eliminate hPSCs from a heterogeneous cell population.
-
•
The potential for teratoma formation was reduced by using this method.
1. Introduction
Human pluripotent stem cells (hPSCs), such as human embryonic stem cells (hESCs) [1] and human induced pluripotent stem cells (hiPSCs) [2], are expected to be applied to cell therapy, disease modelling, and drug development. It is possible to create hiPSCs from various types of somatic cells by the introduction of reprogramming factors, and various research projects using hiPSCs are ongoing.
Clinical trials using hESC-derived retinal pigment epithelium (RPE) and autologous hiPSC-derived RPE have been reported [[3], [4], [5]], and a clinical trial using allogeneic iPSC-derived RPE is underway. Another has been initiated for banked allogeneic hiPSCs to treat Parkinson's disease [6]. Further clinical trials have been planned to treat heart failure, spinal cord injury, Parkinson's disease, and aplastic anaemia, using cells created from hiPSCs [[7], [8], [9]]. Although hiPSC-based clinical and industrial applications are becoming realistic, it is still a major safety concern that residual hiPSCs in products for cell therapy could form tumours in transplanted sites [[10], [11], [12]]. The risk of teratoma formation by hPSCs has been reported in various animal studies [[13], [14], [15], [16], [17]]. It is reported that only 100 iPS cells are sufficient to produce a teratoma in a mouse model [13,18]. Therefore, the establishment of a method to detect and eliminate residual hPSCs from cell products without impairing the survival rate and functionality of the cells to be used for cell therapy, disease modelling, and drug development, is required.
Several strategies have been reported for selectively eliminating residual hPSCs from differentiated cells, including expressing a suicide gene into hPSCs [19], or using cytotoxic antibodies [[20], [21], [22]], chemical inhibitors [[23], [24], [25], [26]], and synthetic RNA or peptide [27,28]. Cell sorting methods using hPSC-specific antibodies [20,29,30] and lectins [31] have also been proposed. However, all of these methods have limitations with respect to specificity, throughput, and other issues, and therefore it is still necessary to develop alternative or additional technologies based on different mechanisms.
We have previously reported that a lectin designated recombinant N-terminal domain of BC2L-C lectin derived from Burkholderia cenocepacia (rBC2LCN) binds to various types of hiPSCs and hESCs, but not to differentiated somatic cells [32,33]. This lectin binds specifically to the Fuc1-2Gal1-3 motif that is highly expressed on hiPSCs [32,34]. In addition, podocalyxin, a type1 transmembrane protein, was identified as a predominant glycoprotein ligand of rBC2LCN [35]. As its main practical applications, fluorescence-labelled rBC2LCN allows live staining of hESCs/hiPSCs following its addition to the culture medium and is capable of separating live hPSCs by flow cytometry [33]. The staining is specific to undifferentiated cells and rapidly diminishes depending on their differentiation. Furthermore, based on the finding that rBC2LCN was internalised inside hPSCs after binding to the surface of these cells, recombinant lectin-toxin fusion proteins in which rBC2LCN was fused to several domains of Pseudomonas aeruginosa exotoxin A was developed for selective elimination of hPSCs [36,37].
In this study, we demonstrate an additional application of rBC2LCN, namely its potential in magnetic bead-based cell separation for reduction of tumourigenic hPSCs from differentiated cell populations. We evaluated cell separation efficiency by flow cytometry and digital PCR analyses. Effective elimination of hPSCs was also verified in a teratoma formation assay in a mouse model.
2. Materials and methods
2.1. Cell culture
The human ES cell line H9 hNanog-pGZ [1] was maintained in mTeSR1 (STEMCELL Technologies, Vancouver, BC, Canada) on a BD Matrigel growth factor reduced (GFR) matrix (BD Biosciences, San Jose, CA, USA) with zeocin, according to the WiCell feeder independent pluripotent stem cell protocols provided by the WiCell Research Institute (www.wicell.org). The human iPS cell line 201B7 [2] was maintained in mTeSR1 (STEMCELL Technologies) on the BD Matrigel hESC-qualified matrix (BD Biosciences), according to the manufacturer's instructions (STEMCELL Technologies). HDF (ATCC PCS-201-012) was maintained in 10% FBS containing DMEM (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). HDF cells were treated with 10 μg/ml of Mitomycin C (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) for 120 min to prevent proliferation. The experiments using hiPSCs and hESCs were approved by the National Institute of Advanced Industrial Science and Technology (AIST) (accreditation numbers and hi2016-099).
2.2. Lectin labelling and magnetic cell separation
Recombinant BC2LCN lectin (rBC2LCN) (FUJIFILM Wako Pure Chemical Corporation) was labelled with a Biotin Labeling Kit-NH2 (Dojindo). Biotin-conjugated rBC2LCN (1–100 μg) or biotin-conjugated BSA were incubated with 50 μL of Dynabeads M−280 streptavidin (Thermo Fisher Scientific, Waltham, MA, USA) in 1 ml of MACS buffer [0.5% bovine serum albumin (BSA) and 2 mM EDTA in PBS] on a rotator for 30 min at room temperature. After incubation, the beads were rinsed twice with MACS buffer (rBC2LCN-magnetic bead and BSA-magnetic bead).
Cells (hESCs and hiPSCs) were dissociated with ESGRO Complete Accutase (Merck Millipore, Billerica, MA, USA) and mixed with HDF in a ratio of 1:1. HDF cells were pre-marked with a CellTrace Violet cell proliferation kit according to the manufacturer's protocol (Thermo Fisher Scientific) or with mitomycin C treated for proliferation inhibition, depending on the following analysis. A total of 2 × 106 mixed cells were incubated with 50 μL of the rBC2LCN-magnetic bead, BSA-magnetic bead or magnetic bead alone for 30 min at 4 °C in 1 ml of MACS buffer. The suspensions were placed in a DynaMag magnet (Thermo Fisher Scientific) for 2 min, and the supernatant with untouched cells was collected for flow cytometry, gene expression analysis and teratoma formation assay.
2.3. Flow cytometry
Flow cytometry was performed as described previously [33]. The cells were resuspended at approximately 1 × 106 cells/mL in MACS buffer and incubated with anti–TRA-1-60 antibodies (1:300 dilution; clone TRA-1-60, Merck Millipore) for 1 h at 4 °C. Normal mouse IgM (Merck Millipore) was used as an isotype control. The cells were rinsed with MACS buffer and then incubated with Alexa Fluor 488 goat anti-mouse IgM (1:300 dilution; Thermo Fisher Scientific). After further rinsing, cells were stained with propidium iodide (PI) (Thermo Fisher Scientific), and 20,000 cells were analysed using a Cell Sorter SH800Z (Sony Corporation, Tokyo, Japan). The data were analysed with FlowJo software (BD Biosciences).
2.4. Digital droplet polymerase chain reaction (ddPCR) analysis
Magnetically sorted or unsorted cells were stained with propidium iodide (PI), and PI-negative live cells were collected using a cell sorter SH800Z (Sony Corporation). Total RNA was extracted from frozen cell samples using ISOGEN (Nippon Gene Co., Ltd., Tokyo, Japan) and ddPCR was carried out using a QX200 droplet digital PCR system (Bio-Rad, Hercules, CA, USA), according to the manufacturer's instructions. Samples were analysed using 2x One-Step RT-ddPCR Supermix (Bio-Rad). The primers and TaqMan probe sequences, their concentrations, and thermal cycling conditions used in the ddPCR methods were according to a previous publication [38]. The probe and primer sequences are as follows: LIN28 probe, FAM-CGCATGGGGTTCGGCTTCCTGTCC-BHQ1, LIN28 forward primer, CACGGTGCGGGCATCTG, LIN28 reverse primer, CCTTCCATGTGCAGCTTACTC; NANOG probe, FAM-TGCTGAGGCCTTCTGCGTCACACC-BHQ1, NANOG forward primer, CTCAGCTACAAACAGGTGAAGAC, NANOG reverse primer, TCCCTGGTGGTAGGAAGAGTAAA. As the internal control, TaqMan GAPDH Control Reagent was used (Thermo Fisher Scientific). Each reaction was performed in duplicate. Total RNA quantities used for the reactions were 0.05, 5, and 50 ng for GAPDH, LIN28, NANOG, respectively. Fluorescence intensities of each droplet in samples were measured using a QX200 droplet reader (Bio-Rad). Positive and negative droplets were discriminated and counted by applying a threshold determined manually in QuantaSoft software. The same threshold was applied to all the wells for each gene on one PCR plate. We adopted the larger measurement result of accepted droplets among the duplicated wells. The number of adopted droplets was >10,000. The copy number concentration in the sample was calculated using the numbers of positive and accepted droplets. The concentration results in terms of target copies per microliter were provided by QuantaSoft software. The number of target copies per template RNA (ex, copies/50 ng of RNA in the case of NANOG) was calculated as concentration (copies/μl) × 20 (μl) × dilution factor of template RNA. The dilution factors were 1 for NANOG, 10 for LIN28 and 1000 for GAPDH, respectively. Absolute counts of NANOG or LIN28 were normalised to GAPDH. Experiments were performed in triplicate and repeated three times with similar results.
2.5. Teratoma formation assay
Eight-week-old immune-deficient NOD/ShiJic-scidJcl mice (CLEA Japan, Inc., Tokyo, Japan) were used for transplantation. NOD/ShiJic-scidJcl mice were anaesthetised using 2% isoflurane and an animal anesthetizer device (MK-AT210D, Muromachi Kikai Co., Ltd., Tokyo, Japan). The surgical area was disinfected with 70% ethanol. After cutting the centre of the scrotum, a testis was carefully pulled out and injected with 20 μL (~1.0 × 106 cells) of cell suspension with BD Matrigel hESC-qualified matrix (BD Biosciences). The same treatment was applied to the other testis. Cell-injected testes were returned to their original location, and the wound was sutured. The transplanted animals and tumour growth were observed routinely about once a week. They were sacrificed after the development of tumours larger than 2 cm in diameter or following an observation period of about 3 months. Tumours were fixed with 4% paraformaldehyde phosphate buffer Solution (FUJIFILM Wako Pure Chemical Corporation). Paraffin embedding, sectioning, and H&E staining of tumours were performed by UNITECH Co., Ltd (Chiba, Japan). Images were obtained using BZ-X710 microscope (Keyence, Osaka, Japan). This experiment was approved by the National Institute of Advanced Industrial Science and Technology (AIST) (accreditation number A2018-290).
2.6. Statistical analysis
A one-way ANOVA followed by Tukey's honest significant difference (HSD) test was used for analysis of ddPCR and teratoma size. Statistical significance was inferred where p < 0.05. The data were analysed with KaleidaGraph v.4.5.2 (Synergy Software, Reading, PA, USA).
3. Results and discussion
3.1. rBC2LCN-bound magnetic beads eliminated hESCs from a heterogeneous cell mixture
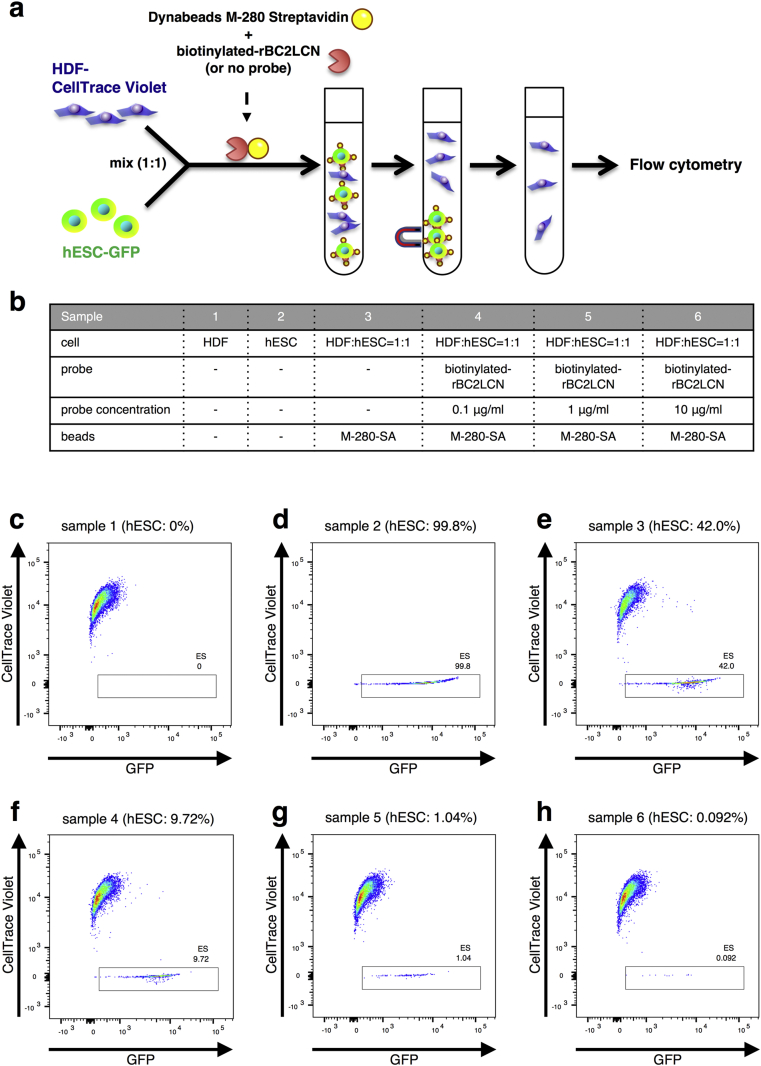
To facilitate the application of hPSC technology, it is important to detect and eliminate residual tumourigenic cells with high sensitivity. As a method to eliminate tumourigenic hPSCs from differentiated cell populations, we applied a magnetic bead-based cell separation system using rBC2LCN. We used hESCs (H9 hNanog-pGZ) [1] and normal human adult dermal fibroblasts (HDF) pre-labelled with CellTrace Violet. Biotin-labelled rBC2LCN was incubated with Dynabeads M−280 Streptavidin (Thermo Fisher Scientific). In this process, rBC2LCN was bound to magnetic beads. We first tested the capability of rBC2LCN-magnetic beads to separate undifferentiated hESCs from a mixed cell population containing hESCs (H9 hNanog-pGZ) expressing GFP and HDF pre-labelled with CellTrace Violet at a ratio of 1:1 (each 1 × 106 cells). The mixed cell populations were incubated with rBC2LCN-magnetic beads. The suspensions were treated with a magnet, and the supernatant containing unbound cells was collected for flow cytometry (Fig. 1). The flow of the experiment and details of each sample are shown in Fig. 1a and b, respectively. We tested three concentrations of rBC2LCN (0.1, 1, and 10 μg/ml) (Fig. 1b–h). Residual hESC levels evaluated by the percentage of GFP-positive and CellTrace Violet-negative cells after magnetic sorting were reduced up to 0.092% (Fig. 1h). The reduction in hESCs was correlated with increased rBC2LCN concentration (Fig. 1c–h). Magnetic beads alone as a negative control did not affect hESCs dislodged from the HDF/hESC mixture (Fig. 1e). These results suggest that rBC2LCN-bound magnetic beads efficiently eliminate live pluripotent stem cells from heterogeneous cell mixtures.
Fig. 1.
Magnetic cell separation using rBC2LCN eliminated hESCs from an hESC/HDF mixture. (a) Experimental design to remove hESCs using magnetic beads. HDF labelled with a CellTrace Violet, and hESC line H9 hNanog-pGZ (H9) were mixed in a ratio of 1 to 1. The cells incubated with Dynabeads M−280 Streptavidin (M-280-SA) and biotinylated-rBC2LCN or M-280-SA alone were separated by a magnet and then analysed by flow cytometry. (b) Details of sample preparation (c–h) Flow cytometry of cells selected negatively by magnetic beads. (c) HDF alone showed 0% of GFP-positive and CellTrace Violet-negative cells (sample 1). (d) hESC alone showed 99.8% GFP-positive and CellTrace Violet-negative cells (sample 2). (e) 42.0% of GFP-positive and CellTrace Violet-negative cells were detected in the prepared cells using M-280-SA alone (sample 3; control). Selection using (f) 0.1 μg/ml (sample 4), (g) 1 μg/ml (sample 5), or (h) 10 μg/ml (sample 6) of biotinylated-rBC2LCN reduced the ratio of GFP-positive and CellTrace Violet-negative cells in a rBC2LCN concentration (9.72, 1.04, and 0.092%, respectively)-dependent manner.
3.2. rBC2LCN-bound magnetic beads eliminated hiPSCs from a heterogeneous cell mixture
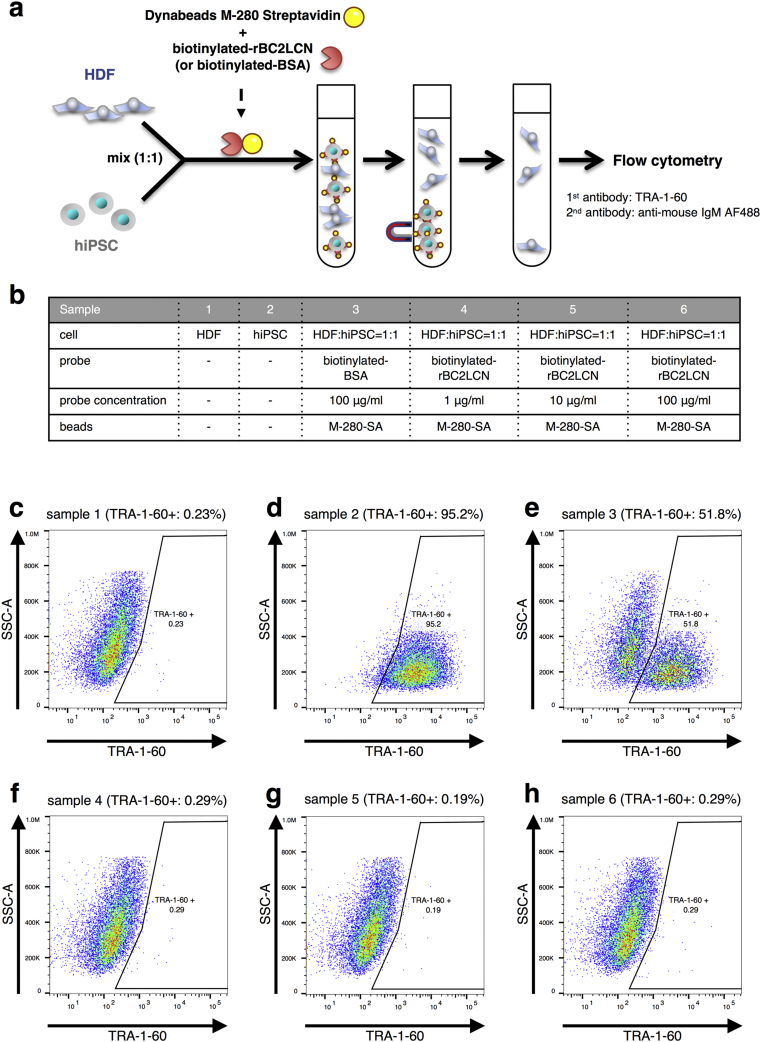
In order to test more realistic conditions for cell sorting, we next used Mitomycin C-treated HDF (MMC-HDF) and unlabelled hiPSCs (201B7) [2]. The hiPSC/MMC-HDF mixtures were incubated with rBC2LCN-magnetic beads. The subsequent analysis is the same as in Fig. 1, and the collected supernatant was used not only for flow cytometry (Fig. 2) but also for gene expression analysis by ddPCR (Fig. 3) and teratoma formation assay (Fig. 4). We detected hiPSCs as tumour rejection antigens-1–60 (TRA-1–60)-positive cells [39] (Fig. 2). The flow of the experiment and details of each sample are shown in Fig. 2a and b, respectively. The percentage of TRA-1-60-positive cells after magnetic sorting by rBC2LCN (0.19–0.29%) closely matched that of an MMC-HDF sample without iPSCs (0.23%) (Fig. 2c–h). We examined three concentrations of rBC2LCN (1, 10, and 100 μg/ml) (Fig. 2b), which were 10-fold higher than those used in Fig. 1, and no substantial difference in hiPSC removal efficiency was seen among them. The biotin-labelled BSA-magnetic beads as a negative control had no effect on eliminating hiPSCs from an MMC-HDF/hiPSC mixture (Fig. 2e). This result indicated that magnetic sorting using rBC2LCN efficiently functioned to eliminate hiPSCs from cell mixtures, and the separation efficiency was comparable to the limit of detection of flow cytometry with TRA-1-60. In subsequent experiments, we fixed the concentration of rBC2LCN to 10 μg/ml.
Fig. 2.
Magnetic cell separation using rBC2LCN eliminated hiPSCs from an hiPSC/MMC-HDF mixture. (a) Experimental design to remove hiPSCs using magnetic beads. HDF and hiPSCs were mixed in a ratio of 1 to 1. The cells incubated with Dynabeads M−280 Streptavidin (M-280-SA) and either biotinylated-rBC2LCN or biotinylated-BSA, were separated by a magnet, and then analysed by flow cytometry. (b) Details of sample preparation (c–h) Flow cytometry of cells selected negatively by magnetic beads. (c) HDF alone showed 0.23% of TRA-1-60-positive cells (sample 1). (d) hiPSC alone showed 95.2% TRA-1-60-positive cells (sample 2). (e) TRA-1-60-positive cells (51.8%) were detected in the prepared cells using 100 μg/ml of biotinylated-BSA (sample 5; control). Selection using (f) 1 μg/ml (sample 4), (g) 10 μg/ml (sample 5) or (h) 100 μg/ml (sample 6) of biotinylated-rBC2LCN reduced the ratio of TRA-1-60-positive cells to levels approximately equal to that of HDF alone (0.19–0.29% vs 0.23%, respectively).
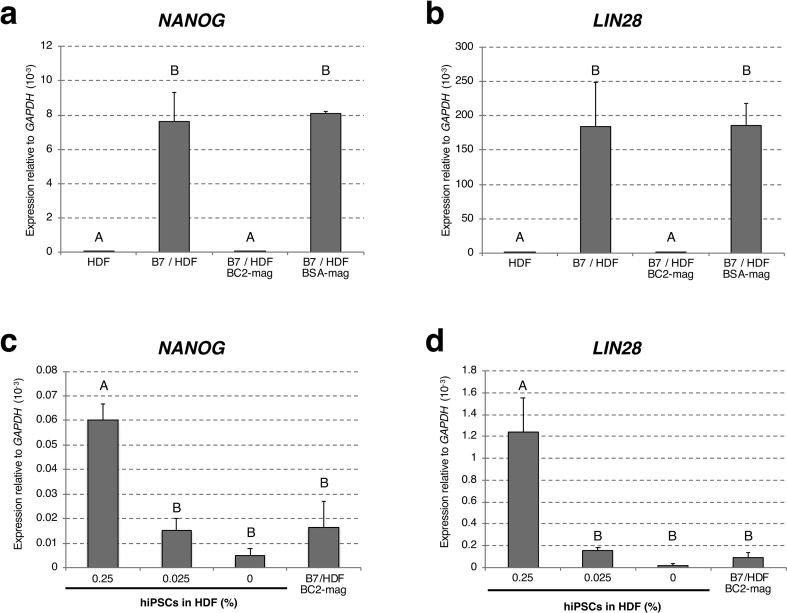
Fig. 3.
Detection of hiPSCs by droplet digital RT-PCR. Droplet digital PCR analysis to estimate residual hiPSC frequency after magnetic cell separation by rBC2LCN was performed by evaluating the expression of pluripotent stem cell marker genes, NANOG (a, c) and LIN28 (b, d). After mixing 1 × 106 of iPSCs and HDF at a ratio of 1: 1 respectively, negative-sorted cells by rBC2LCN-magnetic beads were analysed (a–d) Absolute counts were normalised to GAPDH (per 1000 copies of GAPDH). Results are presented as mean ± standard deviation of independent triplicate experiments (n = 3). Data were analysed by one-way ANOVA followed by Tukey's HSD test (a, b) The relative copy numbers of NANOG (a) and LIN28 (b) mRNAs in 50 ng of total RNA derived from magnetically sorted or unsorted cell mixtures. Significant differences are represented by different letters (Fig. 3a: p < 0.001; Fig. 3b: p < 0.005) (c, d) Relative copy number comparison between NANOG (c) and LIN28 (d) mRNAs in 50 ng of total RNA derived from magnetically sorted cell mixtures, and comparison samples in which HDF-derived total RNA was spiked with hiPSC-derived total RNA at a ratio of 0, 0.025%, or 0.25% by weight. There are significant differences between different letters (p < 0.001). B7: human iPS cell line 201B7. BC2-mag: rBC2LCN-magnetic beads. BSA-mag: BSA-magnetic beads.
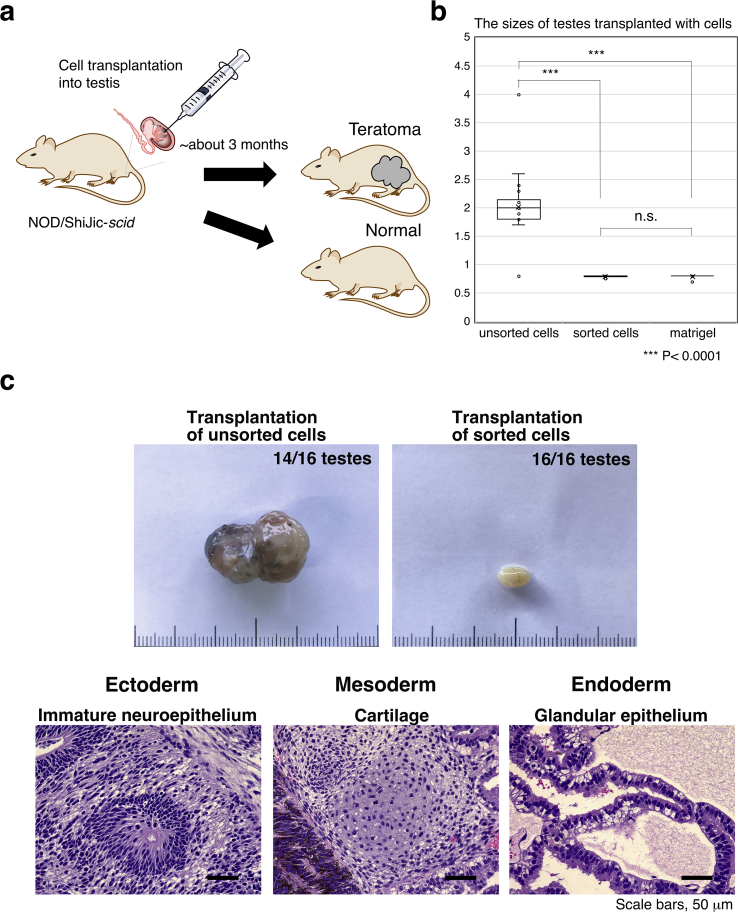
Fig. 4.
Teratoma formation assay. (a) Schematic illustration summarising the experimental design. Magnetically sorted cells were resuspended in Matrigel and transplanted by injection into a testis (20 μL volume with ≤1.0 × 106 cells). (b) Box plot analysis of the size of testes in mice transplanted with Matrigel alone (negative control; n = 8), unsorted (positive control; n = 16), or sorted (n = 16) cells. The number of days from transplantation to sampling of testes varied depending on teratoma growth. The sizes of testes transplanted with unsorted cell mixture were significantly larger than those transplanted with sorted cells or Matrigel alone. Data were analysed by one-way ANOVA followed by Tukey HSD test. ∗∗∗: p < 0.0001. (c) The appearance of the testes taken from NOD/ShiJic-scidJcl mice transplanted with unsorted (upper left) or sorted (upper right) cells. Representative H&E-stained sections of testes with teratomas transplanted with unsorted cell mixtures of iPSCs and MMC-HDF, showing the generation of all three germ layers. Teratoma sections contained immature neuroepithelium (ectoderm), cartilage (mesoderm), and glandular epithelium (endoderm). Scale bar: 50 μm.
3.3. Detection of hiPSCs by droplet digital RT-PCR after magnetic cell separation
We also performed ddPCR analysis, allowing evaluation of the frequency of hiPSCs remaining in the cell suspension after magnetic cell separation using rBC2LCN. For discrimination of dead cells, magnetically sorted or unsorted cells were stained with propidium iodide (PI). The population of PI-negative live cells was collected and analysed (Fig. 3a–d and see Supplementary Tables S1–3 online). Expression levels of NANOG (Fig. 3a) and LIN28 (Fig. 3b) relative to GAPDH were dramatically and significantly reduced in the sample sorted by rBC2LCN-magnetic beads, as compared to the sample sorted by BSA-magnetic beads, which was equivalent to the unsorted control sample (NANOG: p < 0.0001, LIN28: p = 0.0037). Furthermore, the levels of NANOG (Fig. 3c) and LIN28 (Fig. 3d) mRNAs in sorted cell mixtures were evaluated by comparing to the samples in which total RNA extracted from HDF (HDF RNA) was spiked with total RNA extracted from hiPSCs (hiPSCs RNA) at a ratio of 0, 0.025, or 0.25%. No statistically significant differences in expression levels of NANOG or LIN 28 were found among HDF RNA, HDF RNA mixed with 0.025% of hiPSCs RNA, or total RNA extracted from the cells sorted by rBC2LCN-magnetic beads. That is, the rBC2LCN magnetic beads removed iPSCs from a 1:1 mixture of iPSCs and HDF to a degree that was not significantly different from HDF alone in NANOG and LIN28 expressions. These results suggested that magnetic cell separation using rBC2LCN is quite efficient for eliminating hPSCs from mixed cell populations.
3.4. Teratomas were not produced by transplantation of negative-sorted cells by rBC2LCN-magnetic beads
The standard method to define pluripotent stem cells capable of generating tumoural structures containing tissues representing the three germ layers is to perform a teratoma formation assay [[40], [41], [42], [43], [44]]. To ensure effective removal of teratoma-forming cells, in vivo, we performed a teratoma formation assay in immunodeficient mice. We transplanted either cells magnetically sorted using rBC2LCN-magnetic beads or unsorted hiPSC/MMC-HDF mixtures into the testes of NOD/ShiJic-scidJcl mice and left them to engraft for 3 months (Fig. 4a). If any residual hiPSCs remained after sorting, these cells would form teratomas. In the animals transplanted with sorted cells or Matrigel alone, the rate of teratoma formation was 0% (n = 8 or four mice, n = 16 or eight testes for the injection of unsorted cells or Matrigel alone, respectively; Fig. 4b and c, and see Supplementary Table S4 online). In contrast, most testes transplanted with unsorted cell mixtures developed teratomas; 14 out of 16 transplanted testes formed large teratomas (≥1.7 cm in maximal diameter) (n = 8 mice; n = 16 testes) (Fig. 4b and c, and see Supplementary Table S4 online). Statistical analysis of maximal diameter of testes revealed significant size differences between unsorted and sorted cell transplantation (p < 0.0001, Fig. 4b and see Supplementary Table S5 online). Further microscopic observation by Haematoxylin and Eosin (H&E) staining showed these teratomas contained tissues derived from the three germ layers: ectoderm (immature neuroepithelium), mesoderm (cartilage), and endoderm (glandular epithelium) (Fig. 4c). In agreement with the results from flow cytometry (Fig. 2) and ddPCR (Fig. 3), magnetic-cell separation using rBC2LCN effectively removed hiPSCs and could exclude potential teratoma formation in NOD/ShiJic-scidJcl mice as no teratomas were observed in any of the tested animals (Fig. 4b and c).
3.5. Cell sorting efficiency using rBC2LCN-magnetic beads
To evaluate an elimination efficiency of rBC2LCN magnetic beads, we predicted number of residual iPSCs in cell mixture used in teratoma formation assay from the ddPCR data. By calculating based on NANOG and LIN28 expression, the mean ± 2SD value of residual iPSCs in the transplanted cells in the teratoma assay was about 213 ± 615 and 65 ± 128 cells, respectively (Supplementary Fig. S1). These data indicated that approximately 99.8–100% of iPSCs were eliminated by rBC2LCN-magnetic beads. When using a mixture of differentiated cells and undifferentiated hPSCs in a ratio of 1:1, the separation efficiency of hPSCs by magnetic beads with SSEA4 or 57-C11 antibodies was less than 80% [30,45]. The separation efficiency was up to 99.5% using UEA-1 lectin [31]. Although the experimental conditions were not the same, simple comparisons should be avoided, but this technology showed superior performance compared to existing magnetic bead technology using antibodies and lectins. Our results indicate that even with this technique, trace amounts of hPSCs can remain in the cell products. When used as a quality control technology for cell therapy products, the removal efficiency of this technology is not sufficient for products with a high number of transplanted cells. Previous study reported that an antibody against SSEA-5 glycan expressed on hPSCs is a useful tool to removal of teratoma-forming cells. However, an anti-SSEA-5 antibody alone was insufficient to completely remove teratoma potential and complete removal was achieved only after combining SSEA-5 with two additional pluripotent surface markers (SSEA-5/CD9/CD90 or SSEA-5/CD50/CD200) [29]. Cell sorting by combination of rBC2LCN with other pluripotent surface markers may also improve separation efficiency. Proper combinations of various techniques, including rBC2LCN, to remove residual hPSCs in the manufacturing process are important to ensure the safety of cell therapy products.
4. Conclusions
We optimized and validated a method to remove hPSCs from a mixture with human fibroblasts using rBC2LCN-conjugated magnetic beads (Fig. 1, Fig. 2, Fig. 3). Our results suggested that magnetic-cell separation using rBC2LCN effectively removed teratoma-forming pluripotent stem cells from the cell mixture (Fig. 4). We propose that rBC2LCN is a suitable lectin marker for magnetic beads-based cell separation and that this application will contribute to the development of a practical and feasible method for removal of residual undifferentiated cells. As previously reported, the current magnetic activated cell sorting (MACS) technology is insufficient to meet the purification needs of cell therapy [46]. The relevance and validation of this technique for cellular transplantation will require more detailed experiments. In this study, we eliminated pluripotent stem cells from a fibroblast cell line acutely mixed together. For example, it will be more convincing to demonstrate that pluripotent cells can be separated from pluripotent stem cell-derived cell products or other cellular lineages that have been cultured together in the same conditions over time. It is crucial to properly combine the respective removal techniques according to the application scope. Further experiments are demanded to develop an optimised protocol for removal of residual undifferentiated cells.
Data availability statement
All data analysed during this study are included in this published article and its Supplementary Information files.
Ethics statement
This study was carried out in strict accordance with the National Institute of Advanced Industrial Science and Technology (AIST) guidelines for life science experiments (accreditation numbers hi2016–099 and A2018-290). Human embryonic stem cell experiment was approved by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT). Studies with mice were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments stipulated by the Science Council of Japan.
Author contributions statement
Y.H., Y.O., and Y.I. designed the research. Y.H., Y.O., Y.I., S.M., Y.N., Y.A., K.H., and M.S. carried out the experiments, Y.H., Y.O., and S.M. analysed the data. Y.H. and Y.O. wrote the paper. H.T. and J.H. supervised the research.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
Human ES cell line H9 hNanog-pGZ was obtained from the WiCell International Stem Cell (WISC) Bank. Human iPS cell line 201B7 (HPS0063) was provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18be0204429. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2020.03.017.
Contributor Information
Yoshikazu Haramoto, Email: y.haramoto@aist.go.jp.
Yasuko Onuma, Email: yasuko.onuma@aist.go.jp.
Shuuji Mawaribuchi, Email: mawaribuchi.s@aist.go.jp.
Yoshiro Nakajima, Email: ynakaji@koto.kpu-m.ac.jp.
Yasuhiko Aiki, Email: yasuhiko-aiki@aist.go.jp.
Kumiko Higuchi, Email: kumiko.higuchi@aist.go.jp.
Madoka Shimizu, Email: madoka-shimizu@aist.go.jp.
Hiroaki Tateno, Email: h-tateno@aist.go.jp.
Jun Hirabayashi, Email: jun-hirabayashi@aist.go.jp.
Yuzuru Ito, Email: yuzu-itou@aist.go.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Thomson J a, Itskovitz-Eldor J., Shapiro S.S., Waknitz M a, Swiergiel J.J., Marshall V.S. Embryonic stem cell lines derived from human blastocysts [see comments] [published erratum appears in Science 1998 Dec 4. Science (80- ) 1998;282(5395):1827. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015 doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 4.Kamao H., Mandai M., Okamoto S., Sakai N., Suga A., Sugita S. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014 doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017 doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017 doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura M., Miyagawa S., Fukushima S., Saito A., Miki K., Ito E. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- 8.Okano H., Nakamura M., Yoshida K., Okada Y., Tsuji O., Nori S. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S., Takayama N., Hirata S., Seo H., Endo H., Ochi K. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014 doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Ben-David U., Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Canc. 2011 doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 11.Goldring C.E.P., Duffy P.A., Benvenisty N., Andrews P.W., Ben-David U., Eakins R. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013 doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentze H., Soong P.L., Wang S.T., Phillips B.W., Putti T.C., Dunn N.R. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009 doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kawai H., Yamashita T., Ohta Y., Deguchi K., Nagotani S., Zhang X. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cerebr Blood Flow Metabol. 2010 doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A.S., Tang C., Cao F., Xie X., Van Der Bogt K., Hwang A. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009 doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy N.S., Cleren C., Singh S.K., Yang L., Beal M.F., Goldman S.A. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006 doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T., Kawai H., Tian F., Ohta Y., Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011 doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- 18.Gropp M., Shilo V., Vainer G., Gov M., Gil Y., Khaner H. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PloS One. 2012 doi: 10.1371/journal.pone.0045532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuldiner M. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cell. 2003 doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 20.Ben-David U., Nudel N., Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013 doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- 21.Choo A.B., Tan H.L., Ang S.N., Fong W.J., Chin A., Lo J. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cell. 2008 doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 22.Tan H.L., Fong W.J., Lee E.H., Yap M., Choo A. mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cell. 2009 doi: 10.1002/stem.109. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David U., Gan Q.F., Golan-Lev T., Arora P., Yanuka O., Oren Y.S. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Lee M.O., Moon S.H., Jeong H.C., Yi J.Y., Lee T.H., Shim S.H. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards M., Phoon C.W., Goh G.T.W., Seng E.K., Guo X.M., Tan C.M.F. A new class of pluripotent stem cell cytotoxic small molecules. PloS One. 2014 doi: 10.1371/journal.pone.0085039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez-Martin A., Cufi S., Lopez-Bonet E., Corominas-Faja B., Oliveras-Ferraros C., Martin-Castillo B. Metformin limits the tumourigenicity of iPS cells without affecting their pluripotency. Sci Rep. 2012 doi: 10.1038/srep00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parr C.J.C., Katayama S., Miki K., Kuang Y., Yoshida Y., Morizane A. MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Sci Rep. 2016 doi: 10.1038/srep32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuang Y., Miki K., Parr C.J.C., Hayashi K., Takei I., Li J. Efficient, selective removal of human pluripotent stem cells via ecto-alkaline phosphatase-mediated aggregation of synthetic peptides. Cell Chem Biol. 2017 doi: 10.1016/j.chembiol.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Tang C., Lee A.S., Volkmer J.P., Sahoo D., Nag D., Mosley A.R. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011 doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim W.T., Lee H.M., Kim M.K., Choi H.S., Ryu C.J. In vivo evaluation of human embryonic stem cells isolated by 57-C11 Monoclonal antibody. Int J Stem Cells. 2016 doi: 10.15283/ijsc16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y.C., Nakagawa M., Garitaonandia I., Slavin I., Altun G., Lacharite R.M. Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011 doi: 10.1038/cr.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tateno H., Toyota M., Saito S., Onuma Y., Ito Y., Hiemori K. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011 doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onuma Y., Tateno H., Hirabayashi J., Ito Y., Asashima M. RBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Šulák O., Cioci G., Delia M., Lahmann M., Varrot A., Imberty A. A TNF-like trimeric lectin domain from Burkholderia cenocepacia with specificity for fucosylated human histo-blood group Antigens. Structure. 2010 doi: 10.1016/j.str.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Tateno H., Matsushima A., Hiemori K., Onuma Y., Ito Y., Hasehira K. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2LCN. Stem Cells Transl Med. 2013 doi: 10.5966/sctm.2012-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tateno H., Onuma Y., Ito Y., Minoshima F., Saito S., Shimizu M. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep. 2015 doi: 10.1016/j.stemcr.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tateno H., Saito S. Engineering of a potent recombinant lectin-toxin fusion protein to eliminate human pluripotent stem cells. Molecules. 2017 doi: 10.3390/molecules22071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroda T., Yasuda S., Matsuyama S., Tano K., Kusakawa S., Sawa Y. Highly sensitive droplet digital PCR method for detection of residual undifferentiated cells in cardiomyocytes derived from human pluripotent stem cells. Regen Ther. 2015 doi: 10.1016/j.reth.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews P.W., Banting G., Damjanov I., Arnaud D., Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984 doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 40.Brivanlou A.H., Gage F.H., Jaenisch R., Jessell T., Melton D., Rossant J. Setting standards for human embryonic stem cells. Science (80- ) 2003 doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- 41.Przyborski S.A. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cell. 2005 doi: 10.1634/stemcells.2005-0014. [DOI] [PubMed] [Google Scholar]
- 42.Gertow K., Przyborski S., Loring J.F., Auerbach J.M., Epifano O., Otonkoski T. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01b04s3. [DOI] [PubMed] [Google Scholar]
- 43.Prokhorova T.A., Harkness L.M., Frandsen U., Ditzel N., Schrøder H.D., Burns J.S. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cell Dev. 2009 doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 44.Wesselschmidt R.L. The teratoma assay: an in vivo assessment of pluripotency. Methods Mol Biol. 2011 doi: 10.1007/978-1-61779-201-4_17. [DOI] [PubMed] [Google Scholar]
- 45.Fong C.Y., Peh G.S., Gauthaman K., Bongso A. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) Stem Cell Rev Rep. 2009 doi: 10.1007/s12015-009-9054-4. [DOI] [PubMed] [Google Scholar]
- 46.Schriebl K., Lim S., Choo A., Tscheliessnig A., Jungbauer A. Stem cell separation: a bottleneck in stem cell therapy. Biotechnol J. 2010 doi: 10.1002/biot.200900115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this published article and its Supplementary Information files.