Highlights
-
•
Sporadic cases of Burkholderia pseudomallei infections can happen in returning travel.
-
•
Burkholderia pseudomallei Infection leading to vascular aneurism is rarely observed.
-
•
Diagnosis can be enhanced by obtaining vascular tissue culture.
-
•
Early treatment including surgical intervention can be lifesaving.
Keywords: Melioidosis, Mycotic aneurysm, Burkholderia pseudomallei
Abstract
Melioidosis is endemic in Southeast Asia and Australia and cases outside those regions are often travel related. We present a case of melioidosis in a man, with no known risk factors who had an unusual presentation with an infected abdominal aortic aneurysm by Burkholderia pseudomallei in Saudi Arabia, a country with no previous reported cases of this infection. It occurred after traveling to Thailand and he was treated successfully with medical therapy and surgical intervention.
Introduction
Burkholderia pseudomallei is the cause of melioidosis. This case highlights the need for awareness of approaching recent travelers from endemic areas and to be aware of an unusual presentation.
Case
A 42-year-old previously well man, presented to our institution in Riyadh, Saudi Arabia, in 2014 with continuous fever for one month, associated with chills and rigors. He lost 8 kg during this period due to poor appetite. Two weeks prior to presentation he developed mid-back pain which radiated to both flanks, with lower abdominal pain radiating down to his groin. The patient had traveled to Thailand one month before his illness, where he visited rice fields and had contact with poultry and cattle. He denied any cough, joint pain, rash, headaches, or diarrhea and he did not consume alcohol or raw milk. On physical exam he appeared chronically ill, his temperature 38.4 °C, blood pressure was 140/85 mmHg, Pulse 102 beats per minute. respiratory rate 13 breaths per minute, the examination was noteworthy for a non-tender pulsatile mass in his epigastric area with auscultation revealing a loud bruit over it.
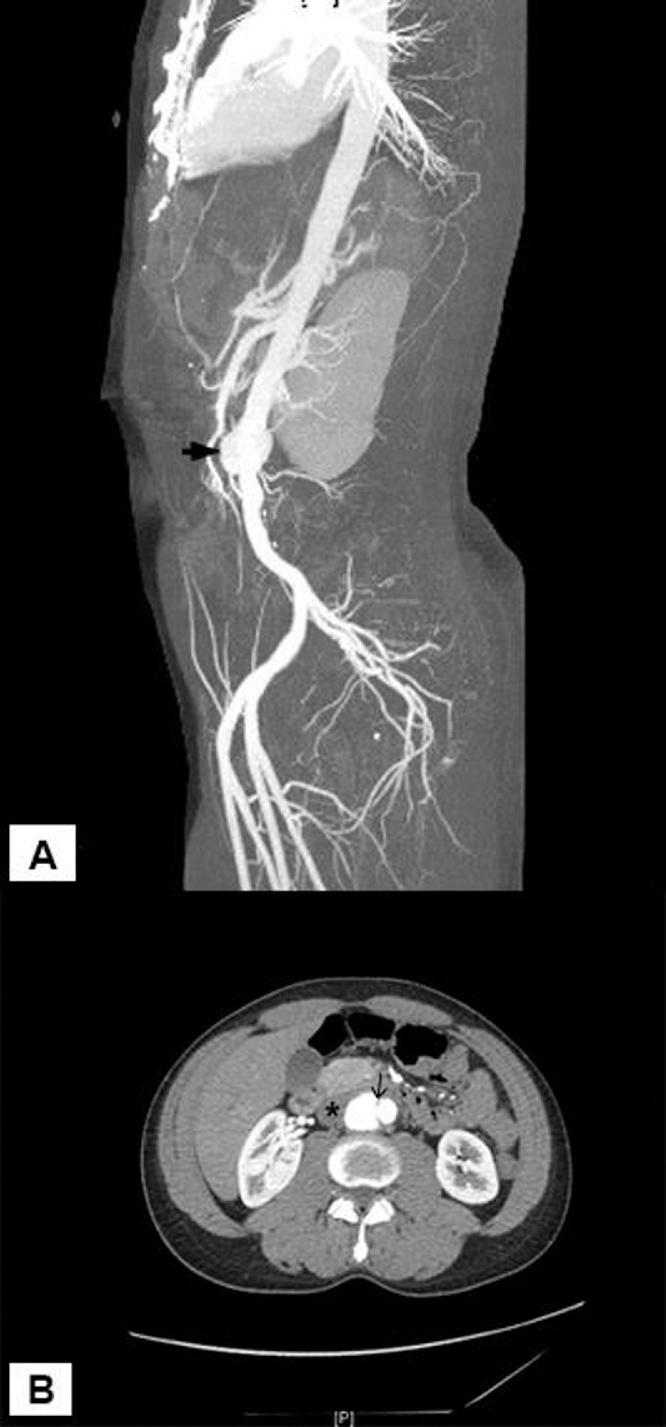
His CBC and chemistry profiles were normal, his erythrocyte sedimentation rate (ESR) was 99 mm/h (normal 01−17 mm/h), serologies for HIV, syphilis, brucellosis were all non-reactive and six thin and thick blood smears did not show any malaria parasites. Chest X-ray was normal and echocardiogram showed no vegetations. An abdominal CT with contrast showed a saccular aneurysm of the infrarenal abdominal aorta measuring 30 × 23 × 32 mm in antero-posterior, transverse and craniocaudal dimension respectively. It had a narrow neck and showed peripheral thrombosis, however, no leakage of contrast or dissection was seen (Fig. 1).
Fig. 1.
Maximum intensity projection angiogram (A) and axial CT angiogram (B) demonstrates infrarenal abdominal aortic pseudoaneurysm (arrowhead) arising with a narrow neck (arrow) and showing partial thrombosis (asterisk). There was no active contrast extravasation.
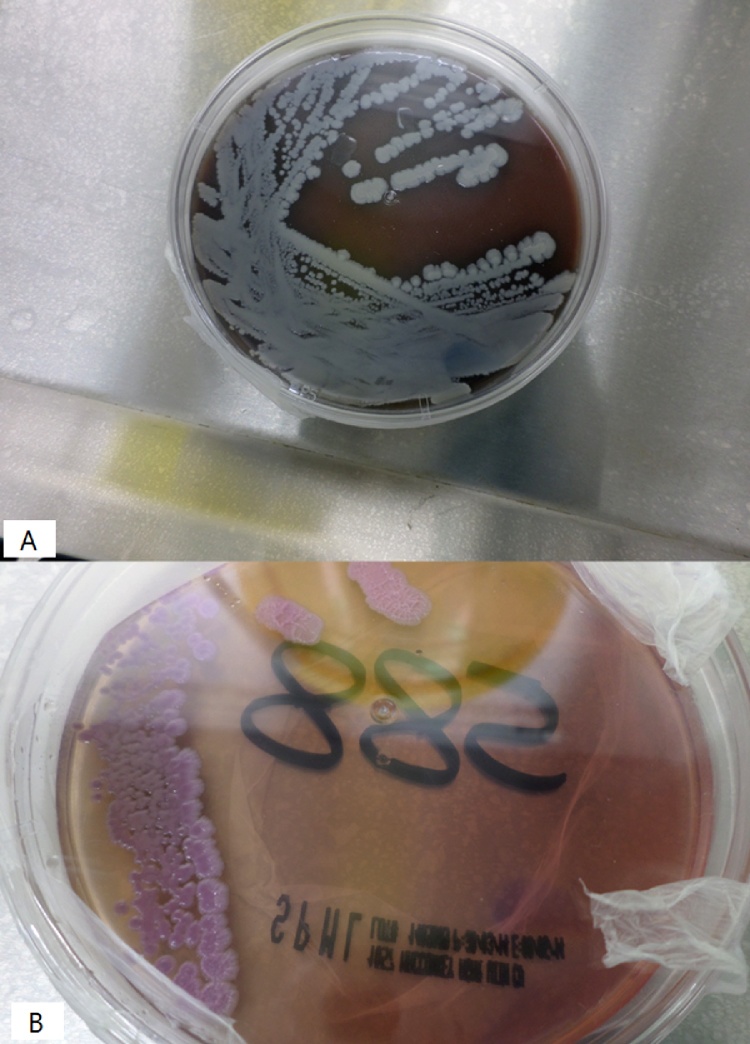
He was admitted to hospital with a diagnosis of infected abdominal aortic aneurysm (AAA) and started on imipenem 500 mg IV every 6 h and vancomycin 1gm IV every 12 h. Multiple sets of blood cultures were drawn and sent to microbiology laboratory. Two sets flagged positive after 2 days of incubation, Gram’s stain showed gram negative bacilli. 24 h later culture showed white to yellow small colonies which became wrinkled later on, growing on both blood and MacConkey agars (Fig. 2). The colonies were non-hemolytic on blood agar, the organism demonstrated bipolar staining and was oxidase positive and motile and it was identified as Burkholderia pseudomallei by Vitek 2 (bioMérieux) which gave it 98 % confidence. Ultimately a total of six sets of blood cultures grew the same organism.
Fig. 2.
burkholderia pseudomallei colonies on blood agar (A) and MacConkey agar(B).
He continued to have fever during his hospital stay, on day eight of admission, due to lack of medical response, he underwent endovascular aneurysm repair (EVAR). Tissue specimen was obtained from aorta and was sent for culture. This tissue culture grew the same organism with the same characteristics and same identification by Vitek 2. Susceptibility testing was set for this organism using E test on Mueller Hinton agar with inoculum equivalent to 0.5 MacFarland dilution as recommended by Clinical and Laboratory Standards Institute (CLSI), MICs were ciprofloxacin 0.75 μg/mL, imipenem 0.38 μg/mL, meropenem 1.5 μg/mL, doxycycline 0.75 μg/mL and colistin > 256 μg/mL. All work and manipulation of culture was done under class 2 biosafety cabinet in level 3 laboratory. Post-operatively the fever immediately subsided, vancomycin was stopped as soon as gram stain results from blood were obtained, repeated blood cultures post-operatively became negative, and he was continued on imipenem for total of six weeks. Then he was given another 6 weeks of doxycycline for eradication therapy. 6 months after completion of therapy on outpatient follow up he was alive and well.
Discussion
Burkholderia pseudomallei is an environmental gram-negative intracellular organism. Melioidosis, which is the disease caused by this organism, is the third cause of death from infectious diseases in northern Australia and Thailand [1]. Sporadic cases elsewhere are usually seen in immigrants or recent travelers [2]. B. pseudomallei is found naturally in soil and water and can be acquired through inhalation, percutaneous inoculation, or ingestion [1].
Melioidosis follows a seasonal pattern in which it occurs during heavy rains and floods [3]. In a study of over 204 cases with melioidosis, 84 % of patients were rice farmers. Other risk factors in the same study include diabetes mellitus, preexisting renal disease, alcoholism, thalassemia, and malignancy [4]. Our patient had no documented risk factors; however, visiting rice farms constitute a high risk of exposure.
Melioidosis can present with a myriad of clinical pictures ranging from simple local cutaneous infection to pneumonia and septic shock. Cardiovascular manifestations especially mycotic aneurysms are very rare. In a study following 540 patients over 20 years, only two patients were diagnosed with abdominal aortic aneurysm [5]. Elliot et al. suggested considering diagnosis of B. pseudomallei mycotic aneurysm in a febrile patient above the age of 40 with a recent visit to endemic area who presents with back pain, confirmed aneurysm, and no identifiable cause [6]. Ruling out tuberculosis and brucellosis which may mimic melioidosis and culturing the organism from blood and tissue, we were able to establish the diagnosis in our case. Thus far there has been limited cases of melioidosis in Saudi Arabia, all of which occurred in travelers coming from endemic areas [2,7]. This is the first case of mycotic aneurysm.
B. pseudomallei is usually resistant to first and second generation of cephalosporins, colistin, macrolides, rifampicin, and aminoglycosides. However, carbapenems, third generation cephalosporins, trimethoprim-sulfamethoxazole and amoxicillin/calvulanate are effective [3]. There is no standardized regimen and duration of antimicrobials. In a meta-analysis comparing different methods of treating melioidosis which included 9 trials, ceftazidime containing intensive therapy followed by oral maintenance therapy (TMP-SMX, doxycycline, chloramphenicol) was associated with better survival [8]. Carbapenems have the lowest MIC against B. pseudomallei and shown to be as effective as ceftazidime [9].
In a case series of 6 patients with mycotic aneurysm due to melioidosis, case fatality was 17 % and relapse rate was 33 %. This fatality was linked with doxycycline maintenance therapy. [10]. Chetchotisakd et al. proved in a randomized trial that TMP-SMX monotherapy was non inferior to TMP-SMX plus doxycycline combination [11]. However, our case received doxycycline monotherapy without relapse.
In conclusion, this case is the first reported B. pseudomallei infected (mycotic) aneurysm in Saudi Arabia. Since a delayed diagnosis is associated with morbidity and mortality, physicians should be vigilant for endemic causes of mycotic aneurysm in returning travelers.
Author assignment
All authors were assigned equally and agreed to submitted changes.
Funding
None.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declarations of Competing Interest
Nothing to declare.
Acknowledgments
No acknowledgement.
Contributor Information
Mazin Barry, Email: mbarry@ksu.edu.sa.
Hebah Dada, Email: hdada@moh.gov.sa.
Mohammad Barry, Email: n3507612@kfshrc.edu.sa.
Abdulellah Almohaya, Email: amalmohaya@ksu.edu.sa.
Abdulwahab Aldrees, Email: abdaldrees@ksu.edu.sa.
References
- 1.Wiersinga W.J., Currie B.J., Peacock S.J. Medical progress: melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Alhatmi H., Alharbi A., Bosaeed M., Aldosary O., Aljohani S., Alalwan B. Melioidosis: case reports of confirmed Burkholderia pseudomallei in Saudi Arabia. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.01.310. [DOI] [PubMed] [Google Scholar]
- 3.Cheng A.C., Currie B.J. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suputtamongkol Y., Chaowagul W., Chetchotisakd P. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–413. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 5.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year darwin prospective study. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott J.H., Currie B.J. Diagnosis and treatment of mycotic aneurysm due to Burkholderia pseudomallei. Clin Infect Dis. 2005;41:572–573. doi: 10.1086/432126. [DOI] [PubMed] [Google Scholar]
- 7.Alwarthan S., Aldajani A., Al Zahrani I., Bukhari H. Melioidosis: can tropical infections present in nonendemic areas? A case report and review of the literature. Saudi J Med Med Sci. 2018;6:108. doi: 10.4103/sjmms.sjmms_118_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel M., Ti T. Interventions for treating melioidosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.cd001263. [DOI] [PubMed] [Google Scholar]
- 9.Smith M.D., Wuthiekanun V., Walsh A.L., White N.J. In-vitro activity of carbapenem antibiotics against /Mactam susceptible and resistant strains of Burkholderia pseudomallei. J Antimicrob Chemother. 1996;37 doi: 10.1093/jac/37.3.611. [DOI] [PubMed] [Google Scholar]
- 10.Low J.G.H., Quek A.M.L., Sin Y.K., Ang B.S.P. Mycotic aneurysm due to Burkholderia pseudomallei infection: case reports and literature review. Clin Infect Dis. 2005;40:193–198. doi: 10.1086/426590. [DOI] [PubMed] [Google Scholar]
- 11.Chetchotisakd P., Chierakul W., Chaowagul W. Trimethoprim-sulfamethoxazole versus trimethoprimsulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. 2014;383:807–814. doi: 10.1016/S0140-6736(13)61951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]