Abstract
Background
Beta-hemolytic streptococci (BHS) are an uncommon cause of infective endocarditis (IE). The aim of this study was to describe the clinical features and outcomes of patients with BHS IE in a large multinational cohort and compare them with patients with viridans streptococcal IE.
Methods
The International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) is a large multinational database that recruited patients with IE prospectively using a standardized data set. Sixty-four sites in 28 countries reported patients prospectively using a standard case report form developed by ICE collaborators.
Results
Among 1336 definite cases of streptococcal IE, 823 were caused by VGS and 147 by BHS. Patients with BHS IE had a lower prevalence of native valve (P < .005) and congenital heart disease predisposition (P = .002), but higher prevalence of implantable cardiac device predisposition (P < .005). Clinically, they were more likely to present acutely (P < .005) and with fever (P = .024). BHS IE was more likely to be complicated by stroke and other systemic emboli (P < .005). The overall in-hospital mortality of BHS IE was significantly higher than that of VGS IE (P = .001). In univariate analysis, variables associated with in-hospital mortality for BHS IE were age (odds ratio [OR], 1.044; P = .004), prosthetic valve IE (OR, 3.029; P = .022), congestive heart failure (OR, 2.513; P = .034), and stroke (OR, 3.198; P = .009).
Conclusions
BHS IE is characterized by an acute presentation and higher rate of stroke, systemic emboli, and in-hospital mortality than VGS IE. Implantable cardiac devices as a predisposing factor were more often found in BHS IE compared with VGS IE.
Keywords: beta-hemolytic streptococci, cardiac devices, congestive heart failure, infective endocarditis, International Collaboration on Endocarditis
The microbiology of infective endocarditis (IE) has changed significantly between the pre- and post-antibiotic eras. In the pre-antibiotic era, streptococci were the most common causative organisms. More recently, staphylococci have become the most common agents [1]. Among the streptococci, the most common organisms belong to the α-haemolytic streptococci, the viridans group (VGS) [2, 3]. Beta-hemolytic streptococci (BHS) are recognized for their pathogenic role mainly in skin and soft tissue infections [4] and are an uncommon cause of infective endocarditis (IE) [5]. However, BHS IE seems to be an aggressive disease with a high 30-day mortality rate [5, 6]. Due to the uncommon nature of this disease in the antibiotic era, our understanding of BHS IE is still limited [6, 7].
The International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) is a large multinational database that recruited patients with IE prospectively using a standardized case report form. The purpose of ICE-PCS is to enhance our understanding of IE. In this report, we describe the characteristics and prognosis of patients with BHS IE and compare these with patients with IE caused by VGS. We also compare IE caused by group B streptococci (GBS) with VGS IE.
METHODS
Patient Population
Data were obtained from the International Collaboration on Endocarditis Prospective Cohort Study. The background and inclusion criteria of this international registry of IE patients have been reported previously [8, 9]. From June 2000 to September 2006, there were 4794 consecutive adult patients (≥18 years old) included in the registry with a diagnosis of definite IE according to the modified Duke criteria for endocarditis [10]. The ICE-PCS database is maintained at the Duke Clinical Research Institute, which is the data coordinating center for ICE studies. The ICE-PCS protocol was reviewed by institutional boards and ethics committees at all sites, per local standards.
Patient Selection
Patients were identified prospectively using site-specific procedures to ensure consecutive enrollment [9, 11]. Only patients with definite IE due to VGS or BHS were included in the current study. To preserve the assumption of independence of observations, only the first episode of IE recorded for an individual patient was used in the analysis.
Data Collection and Outcome
A standard case report form was used at all sites. Altogether, 275 variables were registered, including demographic and clinical data, outcome, and 1-year follow-up according to standard definitions [9, 12]. The primary outcome was in-hospital mortality.
Microbial Identification
Identification of bacterial species was performed according to Clinical and Laboratory Standards Institute (CLSI) protocols.
Statistical Analysis
Categorical variables are expressed as frequencies and percentages of the specified group. Continuous variables are presented as medians and 25th and 75th percentiles. Quantitative variables were compared using the Student t test. Qualitative variables were compared using the χ 2 or Fisher exact test, as appropriate. Univariate logistic regression was used to determine the clinical characteristics of patients with BHS IE associated with in-hospital mortality. A multivariate logistic regression model was used to determine the association between BHS etiology and in-hospital mortality in the total cohort of patients with streptococcal IE. All characteristics that differed between groups (significance level, P < .05) and those considered clinically relevant were included in the model and were successively excluded using a backward selection method with a P value threshold for exit of ≥.2. Unadjusted and adjusted associations are reported using odds ratios and 95% confidence intervals. Statistical significance was set at P < .05, and hypotheses were 2-sided. Statistical analyses were performed with SPSS Statistics 17.0.
RESULTS
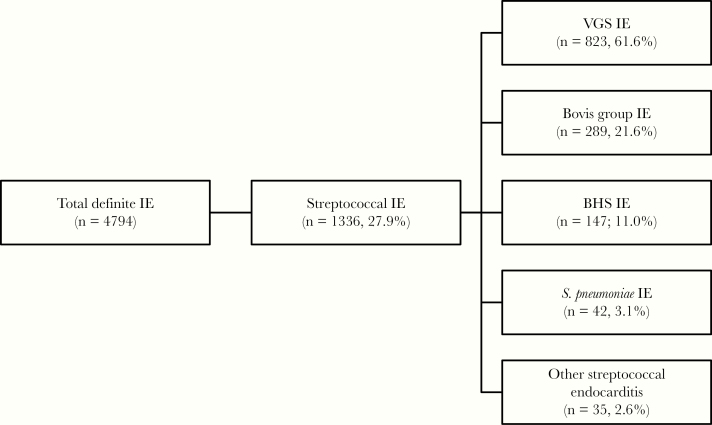
Among 4794 cases of definite IE in ICE-PCS, 1336 cases were caused by streptococci, 823 by VGS, and 147 by BHS (Figure 1). S. agalactiae (group B Streptococcus spp. [GBS]) was the most common pathogen among BHS (n = 95), followed by S. pyogenes (group A Streptococcus spp. [GAS]; n = 15). Overall, IE caused by BHS represented 3.1% of all IE episodes.
Figure 1.
Distribution of streptococcal IE episodes. Abbreviations: BHS, beta-hemolytic streptococci; IE, infective endocarditis; VGS, viridans streptococci.
The clinical characteristics of patients with IE caused by the various BHS are detailed in Table 1. Patients with GAS IE were younger, with a median age (interquartile range [IQR]) of 38.9 (25.6–71.8) years, compared with patients with GBS IE, where the median age (IQR) was 60.4 (47.9–73.5) years. The former group was also less likely to have diabetes (6.7 vs 20%) but more likely to report intravenous drug use (33.3 vs 3.2%). None of the patients with GAS IE developed stroke as a complication of IE, whereas 30.5% of the patients with GBS IE developed stroke.
Table 1.
Characteristics of Patients With IE Caused by BHS
| Variables | S. agalactiae (n = 95) | S. pyogenes (n = 15) | GCGG Streptococci (n = 37) |
|---|---|---|---|
| Demographics | |||
| Male sex | 56/95 (58.9) | 9/15 (60.0) | 28/37 (75.7) |
| Median age (IQR), y | 60.4 (47.9–73.5) | 38.9 (25.6–71.8) | 54.4 (41.7–65.7) |
| Underlying condition | |||
| Diabetes | 19/95 (20.0) | 1/15 (6.7) | 5/36 (13.9) |
| Cancer | 11/95 (11.6) | 1/15 (6.7) | 5/36 (13.9) |
| Hemodialysis | 0/55 (0) | 0/9 (0) | 1/21 (4.8) |
| HIV infection | 4/95 (4.2) | 1/15 (6.7) | 0/37 (0) |
| Predisposing conditions | |||
| IVDU | 3/95 (3.2) | 5/15 (33.3) | 0/35 (0) |
| Congenital heart disease | 3/92 (3.3) | 1/15 (6.7) | 7/35 (20.0) |
| Previous IE | 5/95 (5.3) | 1/15 (6.7) | 5/37 (13.5) |
| Native valve predisposition | 15/95 (15.8) | 2/15 (13.3) | 11/37 (29.7) |
| Endocavitary cardiac device | 7/94 (7.4) | 0/15 (0) | 6/37 (16.2) |
| Type of IE | |||
| NVIE | 76/94 (80.8) | 13/15 (86.7) | 25/37 (67.6) |
| PVIE | 16/94 (17.0) | 1/15 (6.7) | 9/37 (24.3) |
| Other | 2/94 (2.1) | 1/15 (6.7) | 3/37 (8.1) |
| PCN susceptibilitya | |||
| Susceptible | 84/91 (92.3) | 13/14 (92.9) | 32/35 (91.4) |
| Intermediate | 1/91 (1.1) | 0/14 (0) | 0/35 (0) |
| Resistant | 0/91 (0) | 1/14 (7.1) | 0/35 (0) |
| Clinical presentation | |||
| Community acquisition | 89/95 (93.7) | 15/15 (100) | 35/36 (97.2) |
| Admission delay <1 mo | 81/93 (87.1) | 13/14 (92.9) | 33/37 (89.2) |
| Fever | 91/92 (98.9) | 14/14 (100) | 35/37 (94.6) |
| Splenomegaly | 7/95 (7.4) | 2/15 (13.3) | 5/37 (13.5) |
| Osler’s nodes | 1/94 (1.1) | 1/15 (6.7) | 2/37 (5.4) |
| Roth spots | 2/94 (2.1) | 0/14 (0) | 1/37 (2.7) |
| Janeway lesions | 1/94 (1.1) | 2/14 (14.3) | 3/37 (8.1) |
| Conjunctival hemorrhages | 6/94 (6.4) | 1/14 (7.1) | 4/37 (10.8) |
| Splinter hemorrhages | 6/94 (6.4) | 1/14 (7.1) | 4/37 (10.8) |
| New murmur | 43/95 (45.3) | 7/15 (46.7) | 11/37 (29.7) |
| Worsening of old murmur | 9/91 (9.9) | 2/13 (15.4) | 6/36 (16.7) |
| Laboratory findings | |||
| Elevated rheumatoid factor | 4/91 (4.4) | 2/15 (13.3) | 3/36 (8.3) |
| Elevated CRP | 63/92 (68.5) | 13/15 (86.7) | 29/36 (80.5) |
| Elevated ESR | 58/92 (63.0) | 11/15 (73.3) | 20/36 (55.5) |
| Hematuria | 11/42 (26.2) | 3/7 (42.9) | 2/17 (11.8) |
| Echocardiographic findings | |||
| Presence of vegetations | 93/95 (97.9) | 13/15 (86.7) | 30/37 (81.1) |
| Vegetation on left side | 83/93 (89.2) | 10/13 (76.9) | 26/32 (81.2) |
| Moderate or severe regurgitation | 59/95 (62.1) | 11/15 (73.3) | 23/37 (62.2) |
| Paravalvular complication | 25/95 (26.3) | 5/15 (33.3) | 8/37 (21.6) |
| Complications | |||
| CHF | 31/95 (32.6) | 6/15 (40.0) | 13/36 (36.1) |
| CHF (NYHA Class III or IV) | 21/92 (22.8) | 4/13 (30.8) | 10/34 (29.4) |
| Stroke | 29/95 (30.5) | 0/15 (0) | 11/37 (29.7) |
| Other systemic embolism | 28/92 (30.4) | 7/15 (46.7) | 14/36 (38.9) |
| Persistent positive blood cultures | 2/94 (2.1) | 1/15 (6.7) | 1/36 (2.8) |
| Outcome | |||
| Surgery this episode | 52/95 (54.7) | 7/15 (46.7) | 14/37 (37.8) |
| In-hospital mortality | |||
| Overall | 19/95 (20.0) | 2/15 (13.3) | 6/37 (16.2) |
| NVIE | 11/76 (14.5) | 2/13 (15.4) | 5/25 (20.0) |
| PVIE | 8/16 (50.0) | 0/1 (0) | 1/9 (11.1) |
| 1-y mortality | 8/75 (10.7) | 2/9 (22.2) | 3/28 (10.7) |
| Relapse | 0/73 (0) | 0/9 (0) | 2/27 (7.4) |
Numbers represent n/N (%) unless otherwise specified.
Abbreviations: BHS, beta-hemolytic streptococci; CHF, congestive heart failure; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GCGG, group C and group G Streptococci; IE, infective endocarditis; IQR, interquartile range; IVDU, intravenous drug use; NVIE, native valve infective endocarditis; NYHA, New York Heart Association; PCN, penicillin; PVIE, prosthetic valve infective endocarditis.
aThe penicillin susceptibility for 6 GBS isolates out of 91 (6.6%) and 3 GCGG isolates out of 35 (8.6%) was missing.
Table 2 summarizes the characteristics of patients with VGS IE compared with those with BHS IE and GBS IE. Diabetes was the most common comorbidity observed in both the BHS and VGS IE groups (17.1% and 10.9%, respectively), followed by cancer (11.6% and 8.2%, respectively). Among the predisposing conditions, patients with BHS IE were less likely than those with VGS IE to have congenital heart disease (7.7 vs 19.3%; P = .002) and native valve predisposition (19.0 vs 46.4%; P < .005), whereas endocavitary cardiac devices were significantly more common in the BHS IE arm (8.9 vs 3.8; P = .03). Native valve predisposition includes rheumatic and other acquired valvular dysfunction and mitral valve prolapse with valvular regurgitation [13]. There was no difference between the 2 groups with regard to predilection for native or prosthetic valves, with native valve involvement being more common. BHS isolates were more likely to be fully susceptible to penicillin, with 11.7% of VGS isolates being intermediate and 2.0% being resistant to penicillin. One S. pyogenes isolate was found to be penicillin resistant. This is most likely a reporting error.
Table 2.
Comparison of BHS IE With VGS IE and GBS IE With VGS IE
| Variables | VGS IE (n = 823) | BHS IE (n = 147) | P a | GBS (n = 95) | P b |
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex | 585/823 (71.1) | 93/147 (63.3) | .06 | 56/95 (58.9) | .02 |
| Underlying condition | |||||
| Diabetes | 90/822 (10.9) | 25/146 (17.1) | .10 | 19/95 (20.0) | .01 |
| Cancer | 67/821 (8.2) | 17/146 (11.6) | .17 | 11/95 (11.6) | .26 |
| Hemodialysis | 11/526 (2.1) | 1/85 (1.2) | .79 | 0/55 (0) | .61 |
| HIV infection | 9/814 (1.1) | 5/147 (3.4) | .10 | 4/95 (4.2) | .04 |
| Predisposing conditions | |||||
| IVDU | 43/819 (5.2) | 8/145 (5.5) | .76 | 3/95 (3.2) | .62 |
| Congenital heart disease | 153/794 (19.3) | 11/142 (7.7) | .002 | 3/92 (3.3) | <.005 |
| Previous IE | 86/823 (10.4) | 11/147 (7.5) | .47 | 5/95 (5.3) | .15 |
| Native valve predisposition | 381/821 (46.4) | 28/147 (19.0) | <.005 | 15/95 (15.8) | <.005 |
| Endocavitary cardiac device | 31/812 (3.8) | 13/146 (8.9) | .03 | 7/94 (7.4) | .10 |
| Type of IE | .55 | .11 | |||
| NVIE | 641/792 (80.9) | 114/146 (78.1) | 76/94 (80.8) | ||
| PVIE | 130/792 (16.4) | 26/146 (17.8) | 16/94 (17.0) | ||
| Other | 21/792 (2.6) | 6/146 (4.1) | 2/94 (2.1) | ||
| PCN susceptibility | .001 | .003 | |||
| Susceptible | 646/793 (81.5) | 129/140 (92.1) | 84/91 (92.3) | ||
| Intermediate | 93/793 (11.7) | 1/140 (0.7) | 1/91 (1.1) | ||
| Resistant | 16/793 (2.0) | 1/140 (0.7) | 0/91 (0) | ||
| Clinical presentation | |||||
| Community acquisition | 758/799 (94.9) | 139/143 (97.2) | .23 | 89/95 (93.7) | .24 |
| Admission delay <1 mo | 423/780 (54.2) | 127/144 (88.2) | <.005 | 81/93 (87.1) | <.005 |
| Fever | 691/757 (91.3) | 140/143 (97.9) | .02 | 91/92 (98.9) | .01 |
| Splenomegaly | 108/791 (13.6) | 14/147 (9.5) | .07 | 7/95 (7.4) | .09 |
| Osler’s nodes | 22/788 (2.8) | 4/146 (2.7) | .16 | 1/94 (1.1) | .5 |
| Roth spots | 17/789 (2.1) | 3/145 (2.1) | .96 | 2/94 (2.1) | 1.0 |
| Janeway lesions | 27/788 (3.4) | 6/145 (4.1) | .12 | 1/94 (1.1) | .35 |
| Conjunctival hemorrhages | 33/789 (4.2) | 11/145 (7.6) | .20 | 6/94 (6.4) | .33 |
| Splinter hemorrhages | 70/789 (8.9) | 11/145 (7.6) | .88 | 6/94 (6.4) | .42 |
| New murmur | 306/817 (37.4) | 61/147 (41.5) | .52 | 43/95 (45.3) | .14 |
| Worsening of old murmur | 161/793 (20.3) | 17/140 (12.1) | .001 | 9/91 (9.9) | .02 |
| Laboratory findings | |||||
| Elevated rheumatoid factor | 45/781 (5.8) | 9/142 (6.3) | .10 | 4/91 (4.4) | .81 |
| Elevated CRP | 553/800 (69.1) | 105/143 (73.4) | .18 | 63/92 (68.5) | .90 |
| Elevated ESR | 514/805 (63.8) | 89/143 (62.2) | .58 | 58/92 (63.0) | .88 |
| Hematuria | 82/326 (25.1) | 16/66 (24.2) | .83 | 11/42 (26.2) | .88 |
| Echocardiographic findings | |||||
| Presence of vegetations | 705/821 (85.9) | 136/147 (92.5) | .08 | 93/95 (97.9) | .0003 |
| Vegetation on left side | 657/778 (84.4) | 119/138 (86.2) | .70 | 83/93 (89.2) | .22 |
| Moderate or severe regurgitation | 584/820 (71.2) | 93/147 (63.3) | .08 | 59/95 (62.1) | .07 |
| Paravalvular complication | 186/817 (22.8) | 38/147 (25.8) | .56 | 25/95 (26.3) | .44 |
| Complications | |||||
| CHF | 232/811 (28.6) | 50/146 (34.2) | .11 | 31/95 (32.6) | .41 |
| CHF (NYHA Class III or IV) | 139/767 (18.1) | 35/139 (25.2) | .05 | 21/92 (22.8) | .27 |
| Stroke | 118/812 (14.5) | 40/147 (27.2) | <.005 | 29/95 (30.5) | <.005 |
| Other systemic embolism | 147/811 (18.1) | 49/143 (34.3) | <.005 | 28/92 (30.4) | .005 |
| Persistent positive blood cultures | 20/806 (2.5) | 4/145 (2.8) | .82 | 2/94 (2.1) | 1 |
| Outcome | |||||
| Surgery this episode | 393/819 (48.0) | 73/147 (49.7) | .86 | 52/95 (54.7) | .21 |
| In-hospital mortality | |||||
| Overall | 68/823 (8.3) | 27/147 (18.4) | .001 | 19/95 (20.0) | <.005 |
| NVIE | 48/641 (7.5) | 18/114 (15.8) | .01 | 11/76 (14.5) | .04 |
| PVIE | 16/130 (12.3) | 9/26 (34.6) | .02 | 8/16 (50.0) | <.005 |
| 1-y mortality | 60/658 (9.1) | 13/112 (11.6) | .68 | 8/75 (10.7) | .66 |
| Relapse | 25/587 (4.3) | 2/109 (1.8) | .31 | 0/73 (0) | .10 |
Numbers represent n/N (%) unless otherwise specified.
Abbreviations: BHS, beta-hemolytic streptococci; CHF, congestive heart failure; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IE, infective endocarditis; IQR, interquartile range; IVDU, intravenous drug use; NVIE, native valve infective endocarditis; NYHA, New York Heart Association; PCN, penicillin; PVIE, prosthetic valve infective endocarditis; VGS, viridans group streptococci.
a P value between BHS IE and VGS IE.
b P value between BHS IE and GBS IE.
As far as clinical characteristics and outcome are concerned, the majority of patients with BHS IE were admitted within 1 month of symptom onset (88.2 vs 54.2%; P < .005), whereas patients with VGS IE had a more indolent disease where the diagnosis of IE was delayed for more than 1 month. Patients in the BHS IE arm more commonly developed New York Heart Association (NYHA) Class III or IV congestive heart failure (CHF; 25.2% vs 18.1%; P = .05), stroke (27.2% vs 14.5%; P < .005), and other systemic embolization (34.3% vs 18.1%; P < .005). In-hospital mortality was also significantly higher in patients with BHS IE (18.4% vs 8.3%; P = .001) in the overall cohort, as well as in the subgroups of native valve and prosthetic valve IE. There was no difference between BHS IE and VGS IE with regard to persistent bacteremia, requirement for surgery, 1-year mortality, and infection relapse.
Comparing the clinical characteristics of VGS IE and GBS IE (Table 2), we found out that patients who developed VGS IE were more likely to be males compared with patients with GBS IE (71.1% vs 58.9%; P = .02). For the underlying conditions, patients with GBS IE were more likely to have diabetes mellitus (DM; 20% vs 10.9%; P = .01) and HIV infection (4.2% vs 1.1%; P = .04). As for the predisposing conditions, patients with VGS IE were more likely to have congenital heart disease (19.3% vs 3.3%; P < .005) and native valve predisposition (46.4% vs 15.8%; P < .005). As for the clinical presentation, patients with GBS IE were more likely to present within 1 month of onset of symptoms (87.1% vs 54.2%; P < .005), and they were more likely to present with fever (98.9% vs 91.3%; P = .01). On the other hand, patients with VGS IE were more likely to have worsening of an old murmur on physical exam (20.3% vs 9.9%; P = .02). On echocardiography, patients with GBS IE were more likely to have vegetations (97.9% vs 85.9%; P < .005). The clinical course of patients with GBS IE is more likely to be complicated by stroke (30.5% vs 14.5%; P < .005) and other systemic embolisms (30.4% vs 18.1%; P = .005). Finally, in-hospital mortality was significantly higher for patients with GBS IE (20% vs 8.3%; P < .005). On univariate analysis, the variables associated with in-hospital mortality for BHS IE were age per 1-year increment (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.01–1.07; P = .004) and prosthetic valve IE (OR, 3.03; 95% CI, 1.17–7.84; P = .022). In addition, the complications associated with higher in-hospital mortality were CHF (OR, 2.51; 95% CI, 1.07–5.88; P = .03), especially NYHA Class III or IV CHF (OR, 4.14; 95% CI, 1.71–10.02; P = .002), and stroke (OR, 3.20; 95% CI, 1.34–7.62; P = .009) (Table 3).
Table 3.
Predictors of Mortality Among Patients With BHS IE
| Variable | OR (95% CI) | P |
|---|---|---|
| Age, per 1-y increment | 1.04 (1.01–1.07) | .004 |
| PVIE | 3.03 (1.17–7.84) | .02 |
| CHF | 2.51 (1.07–5.88) | .03 |
| CHF (NYHA Class III or IV) | 4.14 (1.71–10.02) | .002 |
| Stroke | 3.20 (1.34–7.62) | .009 |
| Etiology | ||
| S. agalactiae | 0.79 (0.14–4.47) | .79 |
| S. pyogenes | 1.29 (0.47–3.54) | .62 |
| Other BHS (reference) | 1 | – |
Abbreviations: BHS, beta-hemolytic streptococci; CHF, congestive heart failure; CI, confidence interval; IE, infective endocarditis; NYHA, New York Heart Association; OR, odds ratio; PVIE, prosthetic valve infective endocarditis.
Table 4 shows the analysis of factors associated with in-hospital mortality in the total cohort of streptococcal IE. In the univariate analysis, BHS as an etiology of IE was associated with a higher rate of in-hospital mortality (OR, 2.49; 95% CI, 1.53–4.05; P < .005). However, this association was not statistically significant in the multivariate analysis after controlling for confounders. Independent risk factors for mortality were age, HIV infection, presence of paravalvular complication, CHF, and stroke.
Table 4.
Independent Predictors of In-Hospital Mortality Among Patients with Streptococcal IE
| Variable | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age, per 1-y increment | 1.02 (1.01–1.04) | <.005 | 1.03 (1.01–1.04) | <.005 |
| Diabetes | 1.94 (1.24–3.02) | .004 | ||
| HIV infection | 5.13 (1.86–14.10) | .002 | 9.27 (2.90–29.62) | <.005 |
| Native valve predisposition | 0.58 (0.39–0.87) | .008 | ||
| PCN-susceptible streptococcus | 0.62 (0.42–0.93) | .02 | ||
| PVIE | 1.89 (1.25–2.87) | .003 | ||
| Paravalvular complication | 2.38 (1.64–3.46) | <.005 | 2.58 (1.72–3.89) | <.005 |
| CHF | 4.18 (2.89–6.04) | <.005 | 4.94 (3.36–7.27) | <.005 |
| CHF (NYHA Class III or IV) | 4.11 (2.83–5.98) | <.005 | ||
| Stroke | 4.03 (2.74–5.93) | <.005 | 4.81 (3.14–7.35) | <.005 |
| Etiology | ||||
| BHS IE | 2.49 (1.53–4.05) | <.005 | ||
| S. pneumonia | 1.85 (0.75–4.54) | .18 | ||
| Bovis group streptococci | 1.10 (0.68–1.76) | .70 | ||
| VGS (reference) | 1 | – |
Variables in the multivariate model: age per 1-year increment, diabetes, HIV infection, native valve predisposition, PVIE, paravalvular complication, CHF, stroke, and etiology. Final multivariate model: age per 1-year increment, HIV infection, PVIE, paravalvular complication, congestive heart failure, stroke (Hosmer-Lemeshow goodness of fit, 0.869; AUC, 0.8; Clustering coefficient [Cp], 9.950).
Abbreviations: AUC, area under the curve; BHS, beta-hemolytic streptococci; CHF, congestive heart failure; CI, confidence interval; IE, infective endocarditis; OR, odds ratio; NYHA, New York Heart Association; PCN, penicillin; PVIE, prosthetic valve infective endocarditis; VGS, viridans group streptococci.
DISCUSSION
In this prospective observational cohort study, we found that BHS IE was associated with a complicated clinical course and higher mortality compared with patients with VGS IE. These findings point toward the aggressive nature of the IE disease caused by BHS. This has also been demonstrated in previous studies [5, 6, 14–17].
In accordance with earlier reports [6, 14], the majority of patients with BHS IE in our cohort presented acutely, within 1 month of onset of symptoms. This was significantly different from VGS IE, where only approximately half of the patients presented acutely. Early-diagnosed IE is associated with poor prognosis and high mortality rate compared with late-diagnosed IE [18]. This association was observed in our cohort, where patients with BHS IE, who were more likely to be diagnosed early, had a higher in-hospital mortality rate (18.4%) compared with patients with VGS IE (8.3%; P = .001).
An important step in the initiation of infection is the ability of bacteria to bind host components, including fibrinogen, fibronectin, and platelets [19]. Many studies have highlighted the significance of bacterial binding to fibronectin and fibrinogen in the pathogenesis of IE [15, 20]. Oppegaard and colleagues [15] demonstrated that S. pyogenes strains causing IE had a higher representation of fibronectin and fibrinogen-binding genes compared with the isolates that cause skin and soft tissue infections (SSTIs) and respiratory tract infections (RTIs). Similarly, GBS is able to bind fibrinogen through its serine-rich surface glycoprotein Srr1 [19]. Among the different groups of BHS, GBS remains the most common cause of BHS IE [3, 6, 21, 22], which is the case in our cohort of patients. It is notorious for causing invasive neonatal disease; however, its incidence in the United States has declined since the introduction of intrapartum antibiotic prophylaxis. GBS causes a variety of invasive diseases, and adults aged 65 years or older are particularly susceptible [23]. Indeed, in our cohort, patients with GBS IE are older compared with patients with GAS IE or group C group G (GCGG) IE (Table 1). Among the BHS, the highest in-hospital mortality was observed among patients with GBS IE (20%), compared with patients with VGS IE (8.3%), with a P value < .005. However, GBS as the etiology of BHS IE was not a predictor of mortality (Table 3).
BHS IE tends to occur in the older population groups and is associated with chronic diseases such as alcoholism, DM, liver cirrhosis, and cancer [5, 6, 21, 24]. In our cohort, less than half of the patients (48/147) with BHS IE had underlying medical conditions. DM was the most common comorbidity among patients with VGS IE (10.9%) and BHS IE (17.1%), with no significant difference between the 2 groups. However, we found that DM is significantly more common among patients with GBS IE compared with patients with VGS IE (20 vs 10.9%; P = .01). Skoff et al. found that the prevalence of DM (44.4%) among adults with GBS disease is remarkably higher than that of the general US population (10.7%) [25]. On univariable analysis, DM was associated with in-hospital mortality for patients with streptococcal IE (OR, 1.94; 95% CI, 1.24–3.02; P = .004). This is in accordance with a previous report from the International Collaboration on Endocarditis [26], which showed that DM increases the risk of in-hospital mortality in patients with IE. In our subset of patients with BHS IE, DM did not pose an added risk for in-hospital mortality. In addition, HIV infection was found to be significantly more common in patients with GBS IE compared with patients with VGS IE (4.2 vs 1.1%; P = .04). HIV infection is rarely reported as a comorbidity among patients with GBS IE [5, 7, 14], although HIV infection was reported to be a predisposing condition for invasive GBS infection [24, 27]. This could be attributed to the small sample sizes reported in prior studies.
Among predisposing conditions, intravenous drug usage (IVDU) was seen in only 5.5% of the patients with BHS IE (8/145) and in 5.2% of the VGS IE cases (43/819). In a study by Lefort et al. [24], none of the patients with BHS IE reported drug use, and in other studies [6, 21], only 1 case among all BHS IE patients had IVDU as a predisposing factor. Overall, there were no differences between BHS and VGS IE cases in terms of predisposing IVDU, although IVDU was much more frequent in GAS IE (33.3%) than in GBS IE (3.2%). This is in accordance with the literature, where in BHS IE, GAS IE is the most common cause of endocarditis in IVDU [28].
The presence of an intracardiac device (ICD) was significantly higher in BHS IE patients (8.9%) compared with patients with VGS IE (3.8%; P = .03). None of the patients with GAS IE had an ICD. In addition, this difference becomes insignificant when comparing VGS IE and GBS IE (3.8 vs 7.4%; P = .1). In fact, the highest proportion of patients with an ICD was observed among patients with GCGG IE (16.2%). To our knowledge, this predisposing condition was never reported in previous studies [5, 6, 14, 21], except for 1 series comprised of 56 patients, where only 1 patient with BHS IE had a pacemaker [24]. This may be a reflection of an older population group [4, 5] with more comorbid medical conditions, including having endocavitary cardiac devices [21]. However, this was not associated with any increased risk of in-hospital mortality for patients with BHS IE on univariate analysis.
The majority of BHS endocarditis cases involved native valves (78.1%), whereas only 17.8% involved prosthetic valves. A similar distribution was observed in the VGS IE group. Similar rates for prosthetic valve BHS IE were reported by Baddour et al. and Lefort et al. (18% and 19.4%, respectively) [19, 21]. A higher rate for prosthetic valve IE of 41% was reported in a recent retrospective review [6] of 49 cases of BHS IE. In our study, univariate analysis revealed a 3-fold increased mortality for prosthetic valve IE (OR, 3.03; 95% CI, 1.17–7.84; P = .022). This is in accordance with a previous study by Sambola and colleagues [5] in which prosthetic valve IE was the only factor associated with increased mortality in patients with group B streptococcal IE (P < .001). However, a prosthesis was not associated with mortality in the study by Lefort et al. [24].
An important finding in our study is that BHS IE was associated with a higher rate of complications compared with VGS IE. This includes NYHA III or IV CHF, stroke, and other systemic embolizations. The clinical evolution of the latter is less frequently complicated by systemic embolization [21, 29]. In the literature, BHS IE is consistently associated with a high rate of extracardiac complications. Lefort and colleagues [24] reported a rate of 55%, including arterial emboli (23%), meningitis (11%), mycotic aneurysm (7%), and other focal events (34%). El Rafei et al. [6] reported a higher rate of extracardiac complications (80%), with 49% suffering from systemic embolization. The high rate of systemic emboli observed in patients with BHS IE is attributed to large vegetations with a high grade of friability [5, 6, 21, 30]. Typically, large vegetations have been associated with more indolent pathogens [6], S. aureus [7], or fungi [31]. Comparing GBS IE and VGS IE, the difference in stroke and other systemic emboli remained statistically significant, with stroke and other systemic emboli being more often seen with GBS IE (Table 2). Sambola et al. [5] reported 30 cases of GBS IE, among whom 28 cases underwent echocardiography. The 28 cases had vegetations on echocardiography. It is postulated that large vegetations potentially arise due to the lack of fibrinolysin production by GBS, leading to thrombi that are not broken down [5, 30]. In addition, Seo et al. [19] proposed that the binding of S. agalactiae Srr-1 to fibrinogen results in continued platelet recruitment, facilitating disease progression. Due to the risk of systemic embolization, some authors have advocated early surgery [21, 32].
In our study, there were similar rates of patients undergoing surgery in the BHS IE (50%) and in VGS IE (48%) groups, despite the former patients experiencing a higher number of complications. However, patients with GBS IE underwent surgery more often than patients with VGS IE. Yet, the difference was not statistically significant (54.7 vs 48%; P = .21). This increase in the number of patients with GBS IE undergoing surgery did not result in reduced mortality. The overall mortality of GBS IE was significantly higher than that of VGS IE (20 vs 8.3%; P < .005). The timing of surgery is an important determinant of the outcome of IE. We did not have the timing of surgery for our cohort of patients. The EASE trial [33] showed that early surgery reduced the risk of systemic embolization and death in large-vegetation IE. In 1 study [5], despite an increase in cardiac surgery frequency from 25% to 40% over time, mortality rates remained high (34% vs 45% in the earlier time period). The authors attributed this finding to the timing of surgery, which was performed relatively late in the course of the disease.
There are several limitations to this study. First, this is not a contemporary study. Patients were recruited during the period of 2000–2006; however, it is the largest published study to date, and we have no reason to believe that the features of BHS IE have changed over time. Features such as vegetation size, distinction between mechanical valves and bio-prosthetic valves, antimicrobial treatment regimens, and details regarding the role and timing of surgery were not available. Besides, we did not correct for multiple testing. Therefore, it is possible that some of the variables examined particularly in Table 2 could be statistically significant by pure chance alone. Finally, some S. anginosus strains can be beta hemolytic [34], and thus falsely grouped under BHS, over- or underestimating the differences found between VGS IE and BHS IE.
CONCLUSIONS
Our findings show that BHS IE is an aggressive disease characterized by an acute presentation. It is associated with more complications and increased incidence of in-hospital mortality compared with VGS IE. The excessive rate of systemic embolization and CHF suggests that early surgery may be important to prevent the progression of disease.
Notes
Financial support. This study was not funded.
Potential conflicts of interest. Dr. Lamas received from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) a personal grant #E26/202.782/2015 regarding her research on infective endocarditis. The remaining authors have nothing to disclose. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McCartney AC. Changing trends in infective endocarditis. J Clin Pathol 1992; 45:945–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skinner S, Wudel B, Sanche SE.. Microbiology of infective endocarditis and microbiologic diagnosis. In: Chan KL, Embil J, eds. Endocarditis. Cham, Switzerland: Springer; 2016:49–65. [Google Scholar]
- 3. Kim SL, Gordon SM, Shrestha NK. Distribution of streptococcal groups causing infective endocarditis: a descriptive study. Diagn Microbiol Infect Dis 2018; 91:269–72. [DOI] [PubMed] [Google Scholar]
- 4. Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 2001; 33:556–61. [DOI] [PubMed] [Google Scholar]
- 5. Sambola A, Miro J, Tornos M, et al. Streptococcus agalactiae infective endocarditis: analysis of 30 cases and review of the literature, 1962–1998. Clin Infect Dis 2002; 34:1576–84. [DOI] [PubMed] [Google Scholar]
- 6. El Rafei A, DeSimone DC, DeSimone CV, et al. Beta-haemolytic streptococcal endocarditis: clinical presentation, management and outcomes. Infect Dis (Lond) 2016; 48:373–8. [DOI] [PubMed] [Google Scholar]
- 7. Ivanova-Georgieva R, Ruiz-Morales J, García-Cabrera E, et al. Left-sided infective endocarditis caused by Streptococcus agalactiae: rare and serious. Eur J Clin Microbiol Infect Dis 2018; 38(2):265–75. [DOI] [PubMed] [Google Scholar]
- 8. Murdoch DR, Corey GR, Hoen B, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabell CH, Abrutyn E. Progress toward a global understanding of infective endocarditis. Early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin North Am 2002; 16:255–72, vii. [DOI] [PubMed] [Google Scholar]
- 10. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 11. Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 2002; 162:90–4. [DOI] [PubMed] [Google Scholar]
- 12. Fowler VG Jr, Miro JM, Hoen B, et al. ; ICE Investigators Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–21. [DOI] [PubMed] [Google Scholar]
- 13. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990; 264:2919–22. [PubMed] [Google Scholar]
- 14. Georgieva RI, López MG, Ruiz-Morales J, et al. Streptococcus agalactiae left-sided infective endocarditis. Analysis of 27 cases from a multicentric cohort. J Infect 2010; 61:54–9. [DOI] [PubMed] [Google Scholar]
- 15. Oppegaard O, Mylvaganam H, Skrede S, et al. Clinical and molecular characteristics of infective β-hemolytic streptococcal endocarditis. Diagn Microbiol Infect Dis 2017; 89:135–42. [DOI] [PubMed] [Google Scholar]
- 16. Sendi P, Ericsson M, Olaison L. Infective endocarditis caused by group B Streptococcus: the role of aminoglycoside-combination. J Infect 2012; 64:127–9. [DOI] [PubMed] [Google Scholar]
- 17. Bläckberg A, Nilson B, Özenci V, et al. Infective endocarditis due to Streptococcus dysgalactiae: clinical presentation and microbiological features. Eur J Clin Microbiol Infect Dis 2018; 37:2261–72. [DOI] [PubMed] [Google Scholar]
- 18. N’Guyen Y, Duval X, Revest M, et al. ; AEPEI Study Group Time interval between infective endocarditis first symptoms and diagnosis: relationship to infective endocarditis characteristics, microorganisms and prognosis. Ann Med 2017; 49:117–25. [DOI] [PubMed] [Google Scholar]
- 19. Seo HS, Xiong YQ, Sullam PM. Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis. PLoS One 2013; 8:e64204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nomura R, Otsugu M, Naka S, et al. Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis. Infect Immun 2014; 82:5223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baddour LM. Infective endocarditis caused by β-hemolytic streptococci. The Infectious Diseases Society of America’s Emerging Infections Network. Clin Infect Dis 1998; 26:66–71. [DOI] [PubMed] [Google Scholar]
- 22. Kim SL, Gordon SM, Shrestha NK. Distribution of streptococcal groups causing infective endocarditis: a descriptive study. Diagn Microbiol Infect Dis 2018; 91:269–72. [DOI] [PubMed] [Google Scholar]
- 23. Raabe VN, Shane AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lefort A, Lortholary O, Casassus P, et al. ; Beta-Hemolytic Streptococci Infective Endocarditis Study Group Comparison between adult endocarditis due to beta-hemolytic streptococci (serogroups A, B, C, and G) and Streptococcus milleri: a multicenter study in France. Arch Intern Med 2002; 162:2450–6. [DOI] [PubMed] [Google Scholar]
- 25. Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 2009; 49:85–92. [DOI] [PubMed] [Google Scholar]
- 26. Kourany WM, Miro JM, Moreno A, et al. ; ICE MD Investigators Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International Collaboration on Endocarditis-Merged Database. Scand J Infect Dis 2006; 38:613–9. [DOI] [PubMed] [Google Scholar]
- 27. Farley MM, Harvey RC, Stull T, et al. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med 1993; 328:1807–11. [DOI] [PubMed] [Google Scholar]
- 28. Watanakunakorn C. Endocarditis due to beta-hemolytic streptococci. Chest 1992; 102:333–4. [DOI] [PubMed] [Google Scholar]
- 29. Sussman JI, Baron EJ, Tenenbaum MJ, et al. Viridans streptococcal endocarditis: clinical, microbiological, and echocardiographic correlations. J Infect Dis 1986; 154:597–603. [DOI] [PubMed] [Google Scholar]
- 30. Gallagher PG, Watanakunakorn C. Group B streptococcal endocarditis: report of seven cases and review of the literature, 1962–1985. Rev Infect Dis 1986; 8:175–88. [DOI] [PubMed] [Google Scholar]
- 31. Ellis M, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001; 32:50–62. [DOI] [PubMed] [Google Scholar]
- 32. Dolphin A, Cruickshank R. Penicillin therapy in acute bacterial endocarditis. Br Med J 1945; 1:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366:2466–73. [DOI] [PubMed] [Google Scholar]
- 34. Asam D, Mauerer S, Spellerberg B. Streptolysin S of Streptococcus anginosus exhibits broad-range hemolytic activity. Med Microbiol Immunol 2015; 204:227–37. [DOI] [PubMed] [Google Scholar]