Key Points
Question
What are the expected associations of intensive vs standard blood pressure control with residual life span in middle-aged and older adults who are at high cardiovascular risk but without diabetes mellitus?
Findings
In this second analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) randomized clinical trial, applying age-based methods and assuming consistent treatment responses over time, it was estimated that intensive blood pressure control improves projected survival by 6 months to 3 years, depending on the age at which this treatment strategy is started.
Meaning
These actuarial analyses from the SPRINT trial support the survival benefits of intensive blood pressure control, especially among middle-aged adults at risk.
This post hoc actuarial analysis of the SPRINT randomized clinical trial assesses the survival gains of intensive vs standard blood pressure control in adults 50 years or older who were at high cardiovascular risk and without diabetes.
Abstract
Importance
High blood pressure (BP) is a leading contributor to premature mortality worldwide. The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated a 27% reduction in all-cause death with intensive (vs standard) BP control. However, traditional reporting of survival benefits is not readily interpretable outside medical communities.
Objective
To estimate residual life span and potential survival gains with intensive compared with standard BP control in the SPRINT trial using validated nonparametric age-based methods.
Design, Setting, and Participants
This secondary analysis of data from an open-label randomized clinical trial included data from 102 enrolling clinical sites in the United States. Adults who were 50 years or older, were at high cardiovascular risk but without diabetes, and had a screening systolic BP between 130 and 180 mm Hg were enrolled between November 2010 and March 2013. Data analysis occurred from May 2019 to December 2019.
Interventions
A 1:1 randomization to intensive (target, <120 mm Hg) or standard (target, <140 mm Hg) systolic BP targets.
Main Outcomes and Measures
We calculated age-based estimates of projected survival (at a given age) using baseline age rather than time from randomization as the time axis. In each treatment arm at every year of age, residual life span was estimated using the area under the survival curve, up to a maximum of 95 years. Differences in areas under the survival curves reflect the estimated treatment benefits on projected survival.
Results
A total of 9361 adults were enrolled (mean [SD] age at randomization, 68 [9] years; 6029 [64.4%] were men; 5399 [57.7%] were non-Hispanic white individuals). Mean survival benefits with intensive vs standard BP control ranged from 6 months to up to 3 years. At age 50 years, the estimated residual survival was 37.3 years with intensive treatment and 34.4 years with standard treatment (difference, 2.9 years [95% CI, 0.9-5.0 years]; P = .008). At age 65 years, residual survival was 24.5 years with intensive treatment and 23.3 years with standard treatment (difference, 1.1 years [95% CI, 0.1-2.1 years]; P = .03). Absolute survival gains with intensive vs standard BP control decreased with age, but the relative benefits were consistent (4% to 9%).
Conclusions and Relevance
Intensive BP control improves projected survival by 6 months to 3 years among middle-aged and older adults at high cardiovascular risk but without diabetes mellitus. These post hoc actuarial analyses from SPRINT support the survival benefits of intensive BP control, especially among middle-aged adults at risk.
Trial Registration
ClinicalTrials.gov Identifier: NCT01206062
Introduction
Elevated blood pressure (BP) is a leading contributor to excess premature mortality worldwide,1,2 and plateauing trends in BP control have been implicated in recent stalled progress on reducing cardiovascular deaths in the United States.3,4 The Systolic Blood Pressure Intervention Trial (SPRINT; ClinicalTrials.gov Identifier: NCT01206062) demonstrated that intensive BP control (systolic BP target, <120 mm Hg) was superior to standard control (systolic BP target, <140 mm Hg), reducing cardiovascular events by 25% and all-cause mortality by 27% among middle-aged and older adults at high cardiovascular risk but without diabetes mellitus.5
Traditional reporting of the association of treatment responses with survival in clinical trials is challenging to interpret and communicate to patients, clinicians, and health systems. First, hazard ratios do not easily translate to forecasted future life-years lost. Second, while clinical trials are designed to assess therapeutic efficacy and safety over a finite time frame, preventive approaches, such as intensive BP control, may be implemented over longer periods. Clinical trials of long-term therapeutic exposure may be resource intensive and infeasible to conduct. Finally, summary statistics may not capture potential variation in expected survival benefits across ages.
SPRINT was terminated early for benefit after a median follow-up of 3.3 years.5 When assessing absolute treatment benefits on survival during these first 4 years of SPRINT, intensive BP control would be expected to prolong life span by 13 additional days compared with standard BP control.6 However, assuming consistent treatment responses over time, what would be the expected survival gains with long-term intensive BP control? Uniquely, SPRINT included a wide range of patients and enriched enrollment of patients 75 years or older, enabling estimation of projected survival at many points along the age spectrum. Using previously validated actuarial methods,7 we estimated residual life span and potential survival gains with intensive compared with standard BP control in the SPRINT trial.
Methods
The design8 and primary results5 of the SPRINT trial have been previously published. Between November 2010 and March 2013, SPRINT enrolled adults who were 50 years or older, were at high cardiovascular risk but without diabetes, and had a screening systolic BP between 130 and 180 mm Hg. High cardiovascular risk was defined as at least 1 of the following: clinical or subclinical cardiovascular disease (other than stroke), chronic kidney disease, a 10-year cardiovascular risk of an estimated 15% or more based on the Framingham risk score, or an age 75 years or older. All participants provided explicit written consent, and the study protocol was approved by the institutional review board at each participating site. The SPRINT primary outcome article data set was obtained for this analysis from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center after having received a waiver for secondary use by the institutional review board at Brigham and Women’s Hospital.
Participants were randomized to intensive (less than 120 mm Hg) or standard (less than 140 mm Hg) systolic BP targets. Background antihypertensive therapies were adjusted on a monthly basis for the duration of the trial and provided free of cost. Blood pressure was assessed as the mean of 3 measurements while the patient was seated, using an automated office BP machine.
Detailed description and external validation of these actuarial methods have been previously published.7 The end point of interest for this post hoc analysis was all-cause mortality. We additionally assessed the composite of death or the primary end point in SPRINT (myocardial infarction, other acute coronary syndromes, stroke, heart failure, or cardiovascular death). We calculated estimates of residual survival or survival free from the primary end point at a given age by using baseline age rather than the time from randomization as the time horizon and age at the time of an outcome event. Using Kaplan-Meier methods without other covariate inputs, we then calculated nonparametric, age-based estimates of survival or survival free from the primary end point for each treatment arm at every year of age. Projected survival or event-free survival was estimated using the area under the survival curve up to a maximum of 95 years.
Since baseline age and treatment arm were independent (as a function of randomization), differences in areas under the survival curves can be interpreted as the estimated treatment responses on residual survival (the projected number of years remaining for an individual patient) or event-free survival (the projected number of years alive, free from a primary end point). We iterated this process to produce estimated treatment responses for every age between 50 and 85 years. These treatment differences and accompanying 95% CIs were smoothed with a locally weighted scatterplot-smoothing procedure. To determine if treatment responses were stable over time, we tested for time-varying effects using a traditional time-from-randomization model.
All computations were performed using Stata, version 14.1 (StataCorp). P values less than .05 were considered significant.
Results
We included all participants randomized in SPRINT (N = 9361), with 4678 allocated to the intensive treatment arm and 4683 to the standard treatment arm. The mean (SD) baseline age was 67.9 (9.4) years; 6029 participants (64.4%) were men, and 5399 (57.7%) were non-Hispanic white individuals.
Over a median follow-up of 3.3 (interquartile range, 2.8-3.8) years, 365 deaths occurred, and 755 deaths or primary end points occurred. Estimated residual survival and event-free survival were numerically longer at every age in the intensive BP arm compared with the standard BP arm. We found no evidence of time-varying treatment responses of BP strategy for both end points of interest: death and the composite of death or a primary end point. As such, it was assumed that the treatment responses were stable for the duration of the trial.
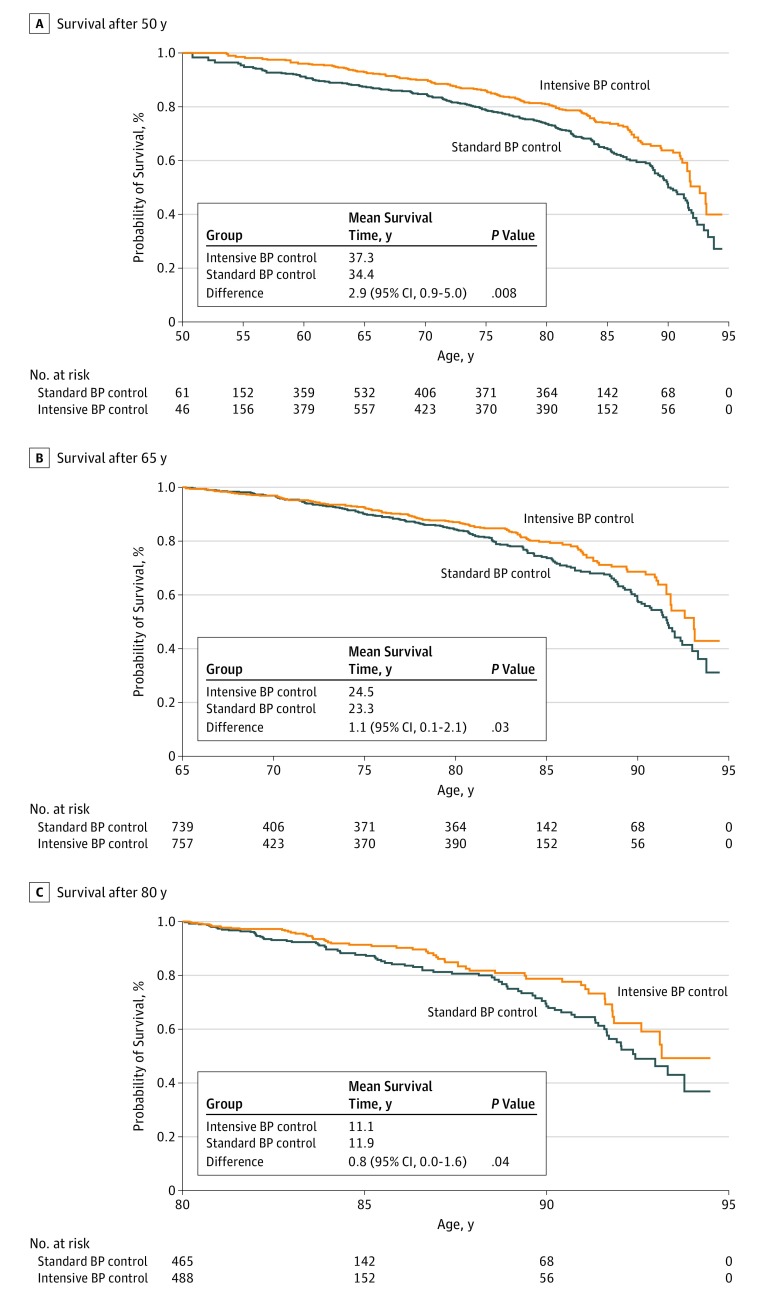
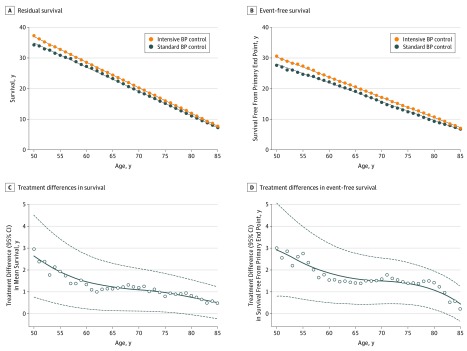
At age 50 years, the estimated residual survival was 37.3 years with intensive treatment and 34.4 years with standard treatment (difference, 2.9 years [95% CI, 0.9-5.0 years]; P = .008). At age 65 years, residual survival was 24.5 years with intensive treatment and 23.3 years with standard treatment (difference, 1.1 years [95% CI, 0.1-2.1 years]; P = .03). At age 80 years, residual survival was 11.9 years with intensive treatment and 11.1 years with standard treatment (difference, 0.8 years [95% CI, 0.0-1.6 years]; P = .04; Figure 1). Mean survival benefits with intensive vs standard BP control ranged from 0.5 years to 3 years. Mean event-free survival benefits similarly ranged from 0.2 years to 3 years (Figure 2). Based on a visual inspection of Figure 2, absolute projected survival gains with intensive vs standard BP control decreased with age. However, relative benefits on residual survival were relatively consistent across ages (4% to 9%) and more variable for event-free survival (without a clear pattern) across ages (3% to 15%).
Figure 1. Probability of Residual Survival in the Systolic Blood Pressure (BP) Intervention Trial (SPRINT) by Age at Randomization.
Age-based Kaplan-Meier estimated curves are displayed for patients at age 50 years (A), 65 years (B), and 80 years (C) for survival between standard and intensive BP control arms. Residual life span was estimated using the area under the survival curve up to a maximum of 95 years.
Figure 2. Long-term Benefit of Intensive Blood Pressure (BP) Control on Life Expectancy in the Systolic Blood Pressure Intervention Trial (SPRINT).
Estimated mean survival times (A) and event-free survival times (B) in the intensive BP control and standard BP control arms of SPRINT are displayed. Treatment differences and 95% CIs are estimated for mean survival (C) and event-free survival (D) after application of a locally weighted scatterplot-smoothing procedure.
Discussion
We estimate intensive BP control improves projected survival and event-free survival by up to 3 years among middle-aged and older adults at high cardiovascular risk but without diabetes mellitus. These post hoc actuarial analyses from SPRINT project the survival benefits of intensive BP control, especially among middle-aged adults who are at risk.
Communicating prognosis and potential treatment benefits of a therapy or strategy has proved challenging in clinical practice.9 Provision of time frames of potential future adverse events may facilitate shared decision-making and patient and caregiver planning. Similarly, transmitting potential survival gains afforded by specific therapeutic approaches may more directly reinforce the rationale for treatment recommendations. Alternative-effect measures beyond traditional hazard ratios have been proposed to facilitate communication and interpretation of absolute (rather than relative) treatment responses. A difference in restricted mean survival times10,11 estimates the mean life expectancy gained (or lost) with an intervention over a certain period. However, restricted mean survival time only accounts for events that accrue over the duration of trial follow-up and does not project potential treatment responses over the lifetime of therapeutic application beyond the end of the study. For trials of long-term preventive interventions (such as BP lowering) in populations with low absolute annual risks,6 certain assumptions regarding continued treatment responses are needed to more robustly portray long-term treatment benefits. The SPRINT investigators previously presented a microsimulation model to estimate risk of death or clinical events that may occur beyond the trial duration; however, this model relied on 10 000 hypothetical patients with comparable clinical profiles as the SPRINT participants, with risk forecasted over the following decades based on estimates derived from American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator.12
To address the limitations of existing approaches, we developed an actuarial method that leverages individual patient–level trial data to estimate the lifetime benefit of a therapy. We previously validated these methods by applying them to a trial with known long-term follow-up.7 In the Studies of Left Ventricular Dysfunction Treatment (SOLVD) Treatment trial,7 which compared enalapril vs placebo over 3.5 years of follow-up, we anticipated a 12-year mean survival time of 5.7 (5.2-6.3) years in the enalapril arm. Actual long-term follow-up found a 12-year mean survival of 6.2 years in the enalapril arm, which fell within the expected confidence bounds.7 Since development, these age-based methods have been applied to trials of pharmacological therapies in heart failure,7 post–myocardial infarction left ventricular dysfunction,13 and diabetes mellitus.14
This age-based method also recognizes that the magnitude of treatment benefit may depend on the age of initiation, especially if therapies are intended for life-long use. Younger patients have longer projected survival times and longer anticipated exposure to BP lowering treatments over their lifetime. SPRINT was specifically designed to enrich enrollment of older adults, and events occurred across a broad range of ages, producing stable, age-specific estimates of residual survival. These data from SPRINT project a substantial absolute benefit of intensive BP control on survival, especially among middle-aged adults. Given concerning declines in US life expectancy for the last 3 years,3,4 these analyses help strengthen the public health message that population-wide BP control remains a critical strategy to improve life expectancy in the United States. Our analyses reaffirm the original SPRINT trial results and present them in alternative format that can be easily communicated to clinicians, patients, and the public at large.
Limitations
The primary limitation of our approach is that it assumes survival risks are independent of the duration of the treatment implementation. However, nonadherence, interval adverse events, or long-term variation in achieved systolic BP levels may attenuate treatment benefits over time. While these analyses were not able to directly estimate nonadherence (based on validated adherence scales that have been shown to be associated with the effectiveness of BP lowering in SPRINT),15 our models assumed that SPRINT participants adhered to treatments as observed during the trial duration, and time-varying analyses did not detect variation in treatment responses during this observation period. We acknowledge that the estimates of survival gains may be overly optimistic, because adherence to BP lowering in real-world settings is expected to be lower than observed during the trial (in which medications were provided free of charge). Discontinuation of antihypertensive treatment with a resultant rise in BP level would be expected to attenuate the observed benefits on survival and event-free survival. It is reassuring that simulations of decreased adherence after 5 years of intensive BP control still suggest durable improvements in long-term cardiovascular events and health status.12 Regardless, these data reinforce efforts to promote long-term adherence to this preventive strategy in clinical practice.
Other limitations include restricted generalizability beyond the scope of SPRINT trial eligibility criteria. We also did not account for individual patient–level factors in forecasting expected survival, as these methods were designed to generate population-level estimates. Despite these limitations, this novel, age-based method facilitates estimation of survival benefits in clinical trials when a patient’s life span (and potential therapeutic exposure) exceeds the trial duration.
Conclusions
We applied age-based methods to the SPRINT trial to estimate total survival and event-free survival gains with intensive BP lowering. Assuming consistent beneficial responses with long-term application, intensive BP control improves life expectancy and event-free survival by up to 3 years, depending on the age at which this treatment strategy is started. These data from the SPRINT trial reinforce that BP control and adherence to BP-lowering treatments, especially when started earlier in life, may meaningfully prolong life span free from cardiovascular disease.
References
- 1.Forouzanfar MH, Liu P, Roth GA, et al. global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165-182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 2.Marczak L, Williams J, Loeffler M; for the Institute for Health Metrics and Evaluation . Global deaths attributable to high systolic blood pressure, 1990-2016. JAMA. 2018;319(21):2163. doi: 10.1001/jama.2018.5119 [DOI] [PubMed] [Google Scholar]
- 3.Shah NS, Lloyd-Jones DM, O’Flaherty M, et al. Trends in cardiometabolic mortality in the United States, 1999-2017. JAMA. 2019;322(8):780-782. doi: 10.1001/jama.2019.9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. doi: 10.1001/jama.2019.16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stensrud MJ, Aalen JM, Aalen OO, Valberg M. Limitations of hazard ratios in clinical trials. Eur Heart J. 2019;40(17):1378-1383. doi: 10.1093/eurheartj/ehy770 [DOI] [PubMed] [Google Scholar]
- 7.Claggett B, Packer M, McMurray JJ, et al. ; PARADIGM-HF Investigators . Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289-2290. doi: 10.1056/NEJMc1509753 [DOI] [PubMed] [Google Scholar]
- 8.Ambrosius WT, Sink KM, Foy CG, et al. ; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532-546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paladino J, Lakin JR, Sanders JJ. Communication strategies for sharing prognostic information with patients: beyond survival statistics. JAMA. 2019;322(14):1345-1346. doi: 10.1001/jama.2019.11533 [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179-1180. doi: 10.1001/jamacardio.2017.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380-2385. doi: 10.1200/JCO.2014.55.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bress AP, Bellows BK, King JB, et al. ; SPRINT Research Group . Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377(8):745-755. doi: 10.1056/NEJMsa1616035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stienen S, Ferreira JP, Vincent J, et al. Estimated long-term survival with eplerenone. J Am Coll Cardiol. 2019;73(18):2357-2359. doi: 10.1016/j.jacc.2019.02.043 [DOI] [PubMed] [Google Scholar]
- 14.Claggett B, Lachin JM, Hantel S, et al. Long-term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease. Circulation. 2018;138(15):1599-1601. doi: 10.1161/CIRCULATIONAHA.118.033810 [DOI] [PubMed] [Google Scholar]
- 15.Haley WE, Gilbert ON, Riley RF, et al. ; SPRINT Study Research Group . The association between self-reported medication adherence scores and systolic blood pressure control: a SPRINT baseline data study. J Am Soc Hypertens. 2016;10(11):857-864.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]