This network meta-analysis identifies optimal antithrombotic regimens in terms of major bleeding and ischemic risk for patients with atrial fibrillation undergoing percutaneous coronary intervention by evaluating 5 randomized clinical trials.
Key Points
Question
What is the optimal antithrombotic regimen in terms of major bleeding and ischemic risk for patients with atrial fibrillation undergoing percutaneous coronary intervention?
Findings
This network meta-analysis of 5 randomized controlled trials found that the use of a combination of a non-vitamin K antagonist oral anticoagulant and a P2Y12 inhibitor (discontinuing the aspirin regimen a few days after percutaneous coronary intervention) reduced bleeding complications, including intracranial bleeding, whereas the combination of a vitamin K antagonist and dual antiplatelet therapy resulted in the highest rates of bleeding. The risk of ischemic events was comparable among the 4 tested regimens.
Meaning
The findings of this study may provide a rigorous and up-to-date evaluation of the safety and efficacy of available antithrombotic strategies to aid health care professionals in making informed treatment decisions.
Abstract
Importance
Antithrombotic treatment in patients with atrial fibrillation (AF) and percutaneous coronary intervention (PCI) presents a balancing act with regard to bleeding and ischemic risks.
Objectives
To evaluate the safety and efficacy of 4 antithrombotic regimens by conducting an up-to-date network meta-analysis and to identify the optimal treatment for patients with AF undergoing PCI.
Data Sources
Online computerized database (MEDLINE).
Study Selection
Five randomized studies were included (N = 11 542; WOEST, PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS, ENTRUST-AF PCI).
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in this network meta-analysis, in which bayesian random-effects models were applied. The data were analyzed from September 9 to 29, 2019.
Main Outcomes and Measures
The primary safety outcome was thrombolysis in myocardial infarction (TIMI) major bleeding and the primary efficacy outcome was trial-defined major adverse cardiovascular events (MACE).
Results
The total number of participants included in the study was 11 532. The mean age of the participants ranged from 70 to 72 years, 69% to 83% were male, 20% to 26% were female, and the participants were predominantly white (>90%). Compared with vitamin K antagonists (VKA) plus dual antiplatelet therapy (DAPT) (reference), the odds ratios (ORs) (95% credible intervals) for TIMI major bleeding were 0.57 (0.31-1.00) for VKA plus P2Y12 inhibitor, 0.69 (0.40-1.16) for non-VKA oral anticoagulant (NOAC) plus DAPT, and 0.52 (0.35-0.79) for NOAC plus P2Y12 inhibitor. For MACE, using VKA plus DAPT as reference, the ORs (95% credible intervals) were 0.97 (0.64-1.42) for VKA plus P2Y12 inhibitor, 0.95 (0.64-1.39) for NOAC plus DAPT, and 1.03 (0.77-1.38) for NOAC plus P2Y12 inhibitor.
Conclusions and Relevance
The findings of this study suggest that an antithrombotic regimen of VKA plus DAPT should generally be avoided, because regimens in which aspirin is discontinued may lead to lower bleeding risk and no difference in antithrombotic effectiveness. The use of a NOAC plus a P2Y12 inhibitor without aspirin may be the most favorable treatment option and the preferred antithrombotic regimen for most patients with AF undergoing PCI.
Introduction
Identifying an optimal antithrombotic regimen to prevent bleeding and ischemic events presents an unmet challenge to physicians treating patients with atrial fibrillation (AF) who require antiplatelet therapy for percutaneous coronary intervention (PCI) and/or acute coronary syndrome (ACS).1,2 Previous studies have compared various antithrombotic regimens.3,4,5,6,7 A 2019 network meta-analysis found that a regimen of non-vitamin K antagonist oral anticoagulants (NOACs) plus a P2Y12 inhibitor without aspirin was associated with lower rates of bleeding, including intracranial hemorrhage, compared with a regimen of vitamin K antagonists (VKA) plus dual antiplatelet therapy (DAPT).7 Since that publication, another randomized clinical trial (RCT) was completed, ENTRUST-AF PCI, which compared the safety of the use of edoxaban (trade names, Savaysa and Lixiana) plus a P2Y12 inhibitor with VKA plus DAPT in 1506 patients with AF who underwent PCI.8 Given the relative importance of this study, we have updated the previous literature search and network meta-analysis to provide readers with a current, state-of-the-art evidence base on antithrombotic regimens in this high-risk patient population.
Methods
A full description of the methodology was previously published.7 In short, 2 of us (R.D.L. and R.E.H.) performed an updated literature review using the PubMed search engine and searched for the following: (1) RCTs with 2 or more comparator arms, (2) in patients with ACS and/or PCI, (3) a combination of anticoagulation and antiplatelet therapy, and (4) reported major bleeding and major adverse cardiovascular events (MACE) with a follow-up of 6 or more months. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Outcome Measures
The primary safety outcome was major bleeding according to the thrombolysis in myocardial infarction (TIMI) criteria.9 The primary efficacy outcome was trial-defined MACE, which was usually defined as a combination of either all-cause or cardiovascular mortality, myocardial infarction (MI), stroke, and stent thrombosis (eTable 1 in the Supplement). The RCTs, WOEST,3 AUGUSTUS,6 and ENTRUST-AF PCI8 reported the number of participants who had definite or probable stent thrombosis. The RCT, PIONEER AF-PCI,5 reported the number of participants who had stent thrombosis without specifying its category, and RE-DUAL PCI4 reported the number of participants who had definite stent thrombosis. Secondary efficacy outcomes were the individual components of this composite MACE outcome.
Data Collection Process
Two of us (H.H. and J.L.) independently extracted data on the study design, baseline characteristics, interventions, and outcomes.
Statistical Analysis
The data were analyzed from September 9 to 29, 2019. We fitted a bayesian random-effects network meta-analysis model to simultaneously compare multiple regimens. We estimated odds ratios (ORs) of the treatment effects of the 2 regimens and the associated 95% credible intervals (CrIs) using Markov chain Monte Carlo algorithms. To evaluate and rank regimens, we calculated rank probabilities (ie, probability of a regimen being the best, second-best, or worst for an outcome) and the Surface under the Cumulative Ranking (SUCRA). All analyses were conducted using the gemtc package (version 0.8-2) in R, version 3.6.1 (The R Foundation).
Results
Search Results
In an updated literature search, we found 38 unique studies that were published between April 19, 2019 (date of last search update) and September 14, 2019 (eTable 2 in the Supplement). Of them, 1 study (ENTRUST-AF PCI) was assessed in full-text and was found eligible.8 These data were combined with those of the 4 RCTs previously identified (WOEST, PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS).3,4,5,6
Study and Patient Characteristics
Baseline characteristics of patients in each RCT are provided in the Table. A total of 11 542 patients were included in the network meta-analysis. The mean age of the participants ranged from 70 to 72 years, 69% to 83% were male, 20% to 26% were women, and the participants were predominantly white (>90%). The prevalence of ACS ranged from 25% to 28% and, except for AUGUSTUS, all patients underwent PCI. Most patients were at high risk for thromboembolic and bleeding complications (>3% per annum). Risk of bias assessment, characteristics of the trial design, treatment regimens, and main results are provided in the Supplement (eTables 3-6 in the Supplement).
Table. Baseline Characteristics in Each Study.
| Characteristic | Treatment Regimen | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WOEST | PIONEER AF-PCI | RE-DUAL PCI | AUGUSTUS | ENTRUST-AF PCI | ||||||||||
| VKA + P2Y12 Inhibitor | VKA + DAPT | NOAC + P2Y12 Inhibitor | NOAC + DAPT | VKA + DAPT | NOAC (L) + P2Y12 Inhibitora | NOAC (H) + P2Y12 Inhibitora | VKA + DAPT | NOAC + DAPT | NOAC + P2Y12 Inhibitor | VKA + DAPT | VKA + P2Y12 Inhibitor | NOAC + P2Y12 Inhibitor | VKA + DAPT | |
| No. of participants (randomization) | 279 | 284 | 709 | 709 | 706 | 981 | 763 | 981 | 1153 | 1153 | 1154 | 1154 | 751 | 755 |
| Age, mean (SD), y | 70.3 (7.0) | 69.5 (8.0) | 70.4 (9.1) | 70.0 (9.1) | 69.9 (8.7) | 71.5 (8.9) | 68.6 (7.7) | 71.7 (8.9) | 70.7 (9.11) | 69.8 (9.31) | 70.5 (9.07) | 70.5 (9.13) | 69 (63-77)b | 70 (64-77)b |
| Male, % | 76.7 | 82.4 | 74.5 | 75.5 | 73.4 | 74.2 | 69.3 | 76.5 | 69.0 | 72.9 | 70.6 | 71.6 | 74.2 | 74.6 |
| BMI, mean (SD) | 27.5 (4.3) | 27.9 (4.2) | 28.6 (25.7-32.4)b | 28.4 (25.6-32.1)b | 29.0 (25.8-32.8)b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Diabetes, % | 24.3 | 25.4 | 28.8 | 28.1 | 31.3 | 36.9 | 34.1 | 37.9 | 37.1 | 35.9 | 35.9 | 36.6 | 34.5 | 34.2 |
| History, % | ||||||||||||||
| Myocardial infarction | 34.4 | 35.2 | 19.7 | 25.4 | 22.2 | 24.2 | 25.4 | 27.3 | NA | NA | NA | NA | 25.0 | 23.4 |
| PCI | 30.8 | 35.6 | NA | NA | NA | 33.2 | 31.3 | 35.4 | NA | NA | NA | NA | 26.5 | 25.8 |
| Coronary artery bypass graft | 20.1 | 26.1 | NA | NA | NA | 9.9 | 10.4 | 11.3 | NA | NA | NA | NA | 6.1 | 6.5 |
| Gastrointestinal bleeding | 5.0 | 4.9 | 1.0 | 1.3 | 0.7 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CHA2DS2-VASc score, %c | ||||||||||||||
| ≤2 | NA | NA | 26.7 | 23.7 | 20.8 | 23.4 | 32.4 | 19.7 | 20.8 | 21.5 | 20.8 | 19.1 | NA | NA |
| ≥3 | NA | NA | 73.3 | 76.3 | 79.2 | 76.6 | 67.6 | 80.3 | 79.2 | 78.5 | 79.2 | 80.9 | NA | NA |
| HAS-BLED score, %d | ||||||||||||||
| ≤2 | NA | NA | 27.6 | 32.0 | 29.5 | 33.2 | 40.5 | 29.4 | 50.5 | 51.7 | 50.9 | 49.6 | 31.8 | 27.0 |
| ≥3 | NA | NA | 72.3 | 68.0 | 70.5 | 66.8 | 59.5 | 70.6 | 49.5 | 48.3 | 49.1 | 50.4 | 62.2 | 66.6 |
| Arterial access, % | ||||||||||||||
| Radial | 26.5 | 25.0 | NA | NA | NA | 63.0 | 65.8 | 62.3 | NA | NA | NA | NA | 77.5 | 80.5 |
| Femoral | 73.1 | 73.2 | NA | NA | NA | 36.6 | 33.0 | 36.8 | NA | NA | NA | NA | 22.4 | 19.3 |
| Stent type, % | ||||||||||||||
| None | 1.8 | 1.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA | NA | NA | NA | NA |
| Bare metal | 31.9 | 30.3 | 32.6 | 31.2 | 31.8 | 15.2 | 16.1 | 13.6 | NA | NA | NA | NA | NA | NA |
| Drug eluting | 64.9 | 64.4 | 65.4 | 66.8 | 66.5 | 82.1 | 81.5 | 84.6 | NA | NA | NA | NA | NA | NA |
| Bare metal and drug eluting | 1.1 | 3.9 | 2.0 | 2.0 | 1.7 | 1.9 | 1.3 | 1.2 | NA | NA | NA | NA | NA | NA |
| Other | NA | NA | NA | NA | NA | 0.8 | 1.0 | 0.5 | NA | NA | NA | NA | NA | NA |
| Creatinine clearance, mL/min/1.73 m2, mean (SD) | NA | NA | 78.3 (31.3) | 77.5 (31.8) | 80.7 (30.0) | 76.3 (28.9) | 83.7 (31.0) | 75.4 (29.1) | 78.5 (31.5) | 79.4 (31.7) | 78.7 (30.2) | 80.0 (36.8) | 71.8 (53.7-91.1)b | 71.7 (54.0-90.9)b |
| Type of index event, % | ||||||||||||||
| NSTEMI | NA | NA | 18.5 | 18.3 | 17.8 | 20.7 | 23.5 | 21.0 | NA | NA | NA | NA | 21.7 | 20.8 |
| STEMI | NA | NA | 12.3 | 13.8 | 10.7 | 14.7 | 14.9 | 14.6 | NA | NA | NA | NA | 17.7 | 17.5 |
| Unstable angina | NA | NA | 20.7 | 21.1 | 23.7 | 19.9 | 16.5 | 16.9 | NA | NA | NA | NA | 14.9 | 16.3 |
| Type of AF, % | ||||||||||||||
| Persistent | NA | NA | 20.7 | 20.6 | 21.1 | 17.7 | 17.3 | 18.2 | NA | NA | NA | NA | 18.6 | 19.3 |
| Permanent | NA | NA | 37.4 | 33.6 | 34.5 | 32.6 | 32.8 | 32.4 | NA | NA | NA | NA | 27.8 | 33.1 |
| Paroxysmal | NA | NA | 42.8 | 46.1 | 44.4 | 49.6 | 49.8 | 49.4 | NA | NA | NA | NA | 53.5 | 47.4 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index (calculated as the weight in kilograms divided by height in meters); CHA2DS2-VASc score, congestive heart failure, hypertension, age ≥75 years, diabetes, stroke [double weight], vascular disease, age 65 to 74 years, and female sex; DAPT, dual antiplatelet therapy; HAS-BLED, hypertension, abnormal renal and liver function, stroke–bleeding, labile international normalized ratio, elderly, drugs or alcohol; NA, not available; NOAC, non-VKA oral anticoagulant; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction; VKA, vitamin K antagonist.
SI conversion factor: To convert creatinine clearance to milliliter/second/meter squared, multiply by 0.0167.
(L) and (H) indicate low- and high-dose schemes of NOAC used in the RE-DUAL PCI trial.
Median (IQR).
CHA2DS2-VASc scores reflect the risk of stroke, with values ranging from 0 to 9 and with higher scores indicating greater risk.
HAS-BLED scores reflect the risk of major bleeding, with values ranging from 0 to 9 and with higher scores indicating greater risk.
Structure of Network Meta-analysis
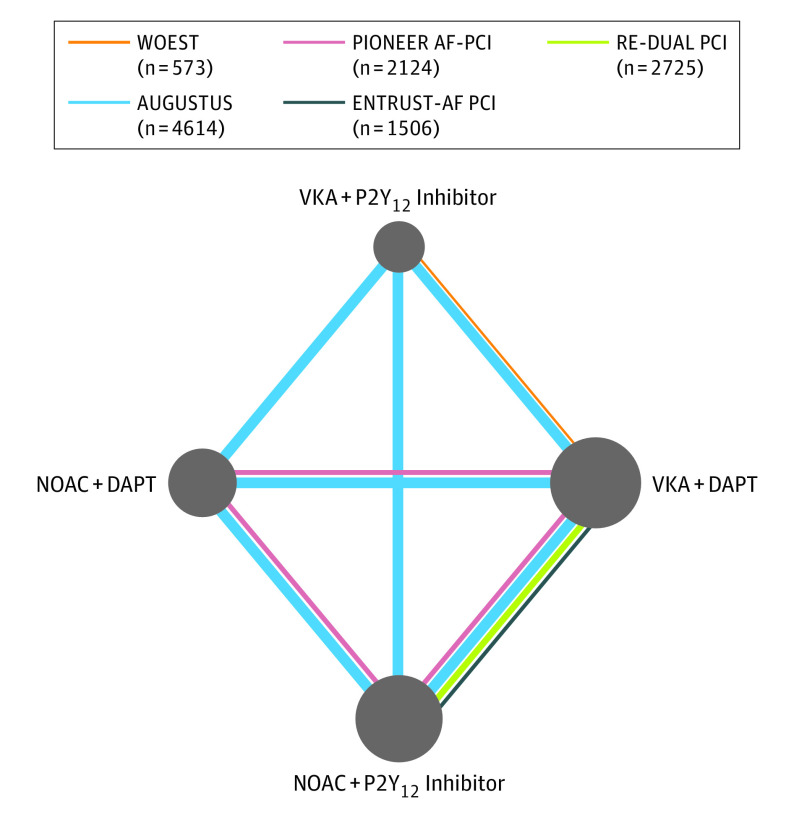
We simultaneously compared the following 4 treatment regimens: VKA plus DAPT (reference), VKA plus P2Y12 inhibitor, NOAC plus DAPT, and NOAC plus P2Y12 inhibitor (Figure 1). We assumed a class effect, that is, all 4 NOAC agents and respective doses had comparable safety and efficacy.
Figure 1. Network of 4 Antithrombotic Treatment Regimens.
The nodes represent the antithrombotic treatment regimens that were compared, and the edges represent the observed direct comparisons in the included randomized clinical trials. The size of nodes is proportional to the number of patients assigned to the treatment regimen and the thickness of edges is proportional to the sample size of each study. AF indicates atrial fibrillation; DAPT, dual antiplatelet therapy; NOAC, non-VKA oral anticoagulant; PCI, percutaneous coronary intervention; VKA, vitamin K antagonists.
Safety Outcomes
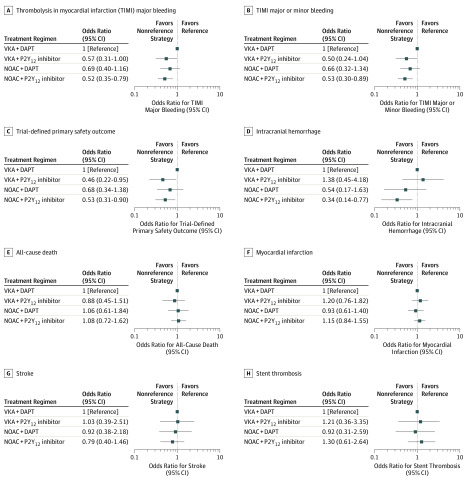
Compared with the ORs for VKA plus DAPT, those for all safety outcomes, including intracranial hemorrhage, were significantly lower for NOAC plus P2Y12 inhibitor (Figure 2A-D). Compared with VKA plus DAPT (reference), the ORs for TIMI major bleeding were 0.57 (95% CrI, 0.31-1.00) for VKA plus P2Y12 inhibitor, 0.69 (95% CrI, 0.40-1.16) for NOAC plus DAPT, and 0.52 (95% CrI, 0.35-0.79) for NOAC plus P2Y12 inhibitor. For MACE, compared with VKA plus DAPT the ORs were 0.97 (95% CrI, 0.64-1.42) for VKA plus P2Y12 inhibitor, 0.95 (95% CrI, 0.64-1.39) for NOAC plus DAPT, and 1.03 (95% CrI, 0.77-1.38) for NOAC plus P2Y12 inhibitor. Discontinuing the aspirin regimen (either with NOAC or VKA) was associated with a lower risk of trial-defined bleeding compared with regimens including aspirin. Compared with VKA plus DAPT, the ORs for trial-defined bleeding were 0.46 (95% CrI, 0.22-0.95) for VKA plus P2Y12 inhibitor and 0.53 (95% CrI, 0.31-0.90) for NOAC plus P2Y12 inhibitor.
Figure 2. Forest Plots for Safety and Efficacy Outcomes.
The safety outcomes assessed were thrombolysis in myocardial infarction (TIMI) major bleeding, TIMI major or minor bleeding, trial-defined primary safety outcome, and intracranial hemorrhage. A total of 11 430 patients were included in the network meta-analyses for all safety outcomes. The efficacy outcomes assessed were all-cause death, myocardial infarction, stroke, and stent thrombosis. A total of 11 501 patients were included in the network meta-analyses for all efficacy outcomes. Odds ratios and 95% credible intervals compared with vitamin K antagonist plus dual antiplatelet therapy (VKA+DAPT) (reference) were plotted for all outcomes. NOAC indicates non-VKA oral anticoagulant.
Efficacy Outcomes
No differences were found among the antithrombotic regimens in terms of the composite of MACE as well as its individual components of (cause-specific) death, MI, stroke, or stent thrombosis (Figure 2E-H and eFigure 1 in the Supplement).
Ranking of Antithrombotic Regimens
The SUCRA values for safety and efficacy outcomes are presented in eTable 7 in the Supplement. The performance of the tested regimens is shown in a forest plot of ORs (eFigure 2 in the Supplement). Regimens in which aspirin was omitted had the best performance (ie, highest SUCRA value) for treating bleeding complications. The combination of NOAC plus P2Y12 inhibitor was the best regimen for treating major bleeding (SUCRA value, 81.9), and any regimen with NOAC (plus DAPT [SUCRA value, 67.3] or plus P2Y12 inhibitor [SUCRA value, 91.8]) was preferred to using VKA (plus DAPT [SUCRA value, 28.8] or plus P2Y12 inhibitor [SUCRA value, 12.9]) for treating intracranial hemorrhage. No treatment regimen was clearly favored overall for efficacy outcomes.
Discussion
In this updated, comprehensive network meta-analysis an antithrombotic regimen in which aspirin is discontinued a few days after PCI appears to be associated with fewer bleeding complications while preserving antithrombotic efficacy. The findings of the present study suggest that a regimen of NOAC plus P2Y12 inhibitor without aspirin had the best safety profile, with the lowest rates of intracranial bleeding and similar rates of ischemic events compared with other antithrombotic regimens that included persistent use of aspirin.
Most guideline recommendations in cardiology are based on low-quality evidence, and the field of antithrombotic therapy for AF and ACS and PCI is no exception.10,11 The recommendation for traditional antithrombotic triple therapy mostly relied on extrapolation and findings from observational studies.1,2,12,13,14,15 Remarkable advances have been made over the past few years, starting with the initial findings from WOEST followed by PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS, and ENTRUST-AF PCI.3,4,5,6,8 Combined, these studies encompass high-quality data from more than 11 000 patients that allow for meaningful observations with regard to bleeding and ischemic outcomes.
Limitations
A question that remains is whether this network meta-analysis provides definitive answers for infrequent outcomes, such as stent thrombosis. Although we did not observe a statistically significant difference in the rates of stent thrombosis between regimens with and without aspirin, the numerical excess of stent thrombosis in patients when aspirin therapy was discontinued may be important, particularly for patients at high risk of stent thrombosis and those in whom the consequences of this condition would be severe. Clear guidance on how to identify such patients based on the available evidence is lacking, and we do not foresee the data to do so becoming available in the future.11 Although the use of a network meta-analysis allows for simultaneous comparisons and evidence-based grading to facilitate overall conclusions, we believe that it does not have the granularity to address specific subgroups of patients. Future studies with individual patient-level data analyses, whether trial-specific or pooled, may help to further refine which patients would benefit most from longer-term aspirin use in combination with a NOAC and a P2Y12 inhibitor.
Conclusions
Selecting the optimal antithrombotic regimen for patients with AF undergoing PCI presents an important unmet clinical need. We believe that the findings of this study support the use of regimens in which aspirin therapy is discontinued a few days after PCI. A regimen that includes a NOAC plus a P2Y12 inhibitor seems to be the most favorable treatment option and may be the preferred antithrombotic regimen for most of these patients.
eTable 1. Definitions of Trial-defined Primary Bleeding and MACE Outcomes in Each Study
eTable 2. Search Code (PubMed/MEDLINE Search)
eTable 3. Risk of Bias of Included Trials Using the Cochrane Risk Assessment Tool
eTable 4. Characteristics of Included Trials
eTable 5. Treatment Strategies in Each Study
eTable 6. Updated Network Meta-Analysis Data: Sample Size and the Number of Participants Who Had Each Outcome in Each Study
eTable 7. SUCRA Values for Each Treatment Regimen and Outcomes
eFigure 1. Forest Plots for Efficacy Outcomes: (A) Trial-defined MACE, (B) Cardiovascular Death
eFigure 2. Odds Ratios for TIMI Major Bleeding and MACE
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with Atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 3.Dewilde WJM, Oirbans T, Verheugt FWA, et al. ; WOEST Study Investigators . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Bhatt DL, Oldgren J, et al. ; RE-DUAL PCI Steering Committee and Investigators . Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513-1524. doi: 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 5.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423-2434. doi: 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]
- 6.Lopes RD, Heizer G, Aronson R, et al. ; AUGUSTUS Investigators . Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509-1524. doi: 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
- 7.Lopes RD, Hong H, Harskamp RE, et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019;4(8):747-755. doi: 10.1001/jamacardio.2019.1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394(10206):1335-1343. doi: 10.1016/S0140-6736(19)31872-0 [DOI] [PubMed] [Google Scholar]
- 9.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76(1):142-154. doi: 10.1161/01.CIR.76.1.142 [DOI] [PubMed] [Google Scholar]
- 10.Fanaroff AC, Califf RM, Windecker S, Smith SC Jr, Lopes RD. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology guidelines, 2008-2018. JAMA. 2019;321(11):1069-1080. doi: 10.1001/jama.2019.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harskamp RE, Alexander JH, Lopes RD. Navigating the treacherous waters of antithrombotic therapies in patients with atrial fibrillation and coronary artery disease: lessons from AUGUSTUS. Eur J Intern Med. 2019;65:4-5. doi: 10.1016/j.ejim.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 12.Cho JR, Angiolillo DJ. Percutaneous coronary intervention and atrial fibrillation: the triple therapy dilemma. J Thromb Thrombolysis. 2015;39(2):203-208. doi: 10.1007/s11239-014-1132-z [DOI] [PubMed] [Google Scholar]
- 13.Nikolsky E, Mehran R, Dangas GD, et al. Outcomes of patients treated with triple antithrombotic therapy after primary percutaneous coronary intervention for ST-elevation myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction [HORIZONS-AMI] trial). Am J Cardiol. 2012;109(6):831-838. doi: 10.1016/j.amjcard.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 14.Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J. 2013;166(5):864-870. doi: 10.1016/j.ahj.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 15.Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126(10):1185-1193. doi: 10.1161/CIRCULATIONAHA.112.114967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Trial-defined Primary Bleeding and MACE Outcomes in Each Study
eTable 2. Search Code (PubMed/MEDLINE Search)
eTable 3. Risk of Bias of Included Trials Using the Cochrane Risk Assessment Tool
eTable 4. Characteristics of Included Trials
eTable 5. Treatment Strategies in Each Study
eTable 6. Updated Network Meta-Analysis Data: Sample Size and the Number of Participants Who Had Each Outcome in Each Study
eTable 7. SUCRA Values for Each Treatment Regimen and Outcomes
eFigure 1. Forest Plots for Efficacy Outcomes: (A) Trial-defined MACE, (B) Cardiovascular Death
eFigure 2. Odds Ratios for TIMI Major Bleeding and MACE