This prespecified secondary analysis of a randomized clinical trial examines the variables contributing to the incidences of major or minor and vascular or nonvascular bleeding after percutaneous coronary intervention (bare metal stent or polymer-free, biolimus A9-eluting drug-coated stent) and dual antiplatelet therapy in women and men at an increased risk for bleeding.
Key Points
Question
Do women and men with a high bleeding risk have different long-term outcomes after percutaneous coronary intervention?
Findings
In this prespecified secondary analysis of patients with a high bleeding risk in the LEADERS FREE trial, women and men had a similar 2-year incidence of the primary composite safety (cardiac death, myocardial infarction, or stent thrombosis) and efficacy (target lesion revascularization) end points. Women, compared with men, experienced greater overall major bleeding within the first 30 days and greater major bleeding from vascular access sites.
Meaning
The findings suggest that women with a high bleeding risk should not be denied the benefits of percutaneous coronary intervention when indicated and that bleeding avoidance strategies should be uniformly adopted for all patients, with particular attention to women.
Abstract
Importance
Female sex has been identified as a risk factor for bleeding after percutaneous coronary intervention (PCI) and may have contributed to the underuse of drug-eluting stents in women. This risk may be further enhanced among patients with a high bleeding risk.
Objective
To assess the 2-year outcomes by sex in patients with a high bleeding risk who were enrolled in the LEADERS FREE trial.
Design, Setting, and Participants
This cohort study is a prespecified, sex-based secondary analysis of the LEADERS FREE double-blind, randomized clinical trial that was conducted at 68 sites in 20 countries from December 2012 to May 2014. Patients with a high bleeding risk who underwent PCI and met the trial eligibility criteria were enrolled at the participating sites and followed up for up to 2 years.
Interventions
Patients were randomized 1:1 to either a bare-metal stent or a polymer-free, biolimus A9-eluting drug-coated stent with 1-month of dual antiplatelet therapy.
Main Outcomes and Measures
The primary safety end point was a composite of cardiac death, myocardial infarction, or stent thrombosis. The primary efficacy end point was clinically driven target lesion revascularization. Bleeding was assessed using the Bleeding Academic Research Consortium (BARC) scale, and the source of bleeding was recorded.
Results
A total of 2432 patients with a high bleeding risk were included in the study. Of these patients, the mean (SD) age was 75 (9) years, and 1694 (69.7%) were men and 738 (30.3%) were women. Women and men had similar incidence of the 2-year primary safety (14.7% vs 13.6%; P = .37) and efficacy (9.2% vs 9.5%; P = .70) end points. The drug-coated stent was found to be superior to the bare-metal stent in both sexes, with lower target lesion revascularization (women: 6.3% vs 12.1%; men: 7.0% vs 12.0%; P for interaction = .70) and similar rates of the primary safety end point (women: 12.4% vs 17.0%; men: 12.6% vs 14.5%; P for interaction = .40). Overall, 2-year BARC types 3 to 5 major bleeding (10.2% vs 8.6%; P = .14) was not statistically different between the sexes, but women experienced greater BARC types 3 to 5 major bleeding within the first 30 days (5.1% vs 2.4%; P = .007) and greater vascular access site major bleeding than men (2.2% vs 0.5%; P < .001). In both sexes, vascular (women: hazard ratio [HR], 3.45 [95% CI, 1.51-7.87]; men: HR, 4.14 [95% CI, 1.33-12.95]) and nonvascular major bleeding (women: HR, 3.76 [95% CI, 2.17- 6.53]; men: HR, 4.62 [95% CI, 3.23-6.61]) were associated with greater 2-year mortality.
Conclusions and Relevance
This study found no sex differences in the ischemic outcomes of patients with a high bleeding risk after PCI, but women appeared to demonstrate greater early bleeding and major bleeding from the vascular access site. Both women and men with major bleeding seemed to experience worse 2-year mortality, suggesting that bleeding avoidance strategies should be uniformly adopted for all patients, with close attention dedicated to women to avoid denying them the benefits of PCI.
Trial Registration
ClinicalTrials.gov Identifier: NCT02843633
Introduction
Compared with men, women typically present with a higher risk of adverse outcomes after percutaneous coronary intervention (PCI) attributed to advanced age and greater comorbidities.1,2,3 In particular, women are at high risk for bleeding events after PCI1,2 and, consequently, may be undertreated with PCI4 or may be preferentially treated with a bare-metal stent (BMS) rather than a drug-eluting stent (DES),5 although BMS use is declining. Studies have shown that women are less likely to receive dual antiplatelet therapy (DAPT),6 potent antiplatelet therapy,1,3 or other secondary prevention medications.7 For women, these risk-treatment biases may result in not only being denied the benefits of DES PCI5 but also having higher subsequent hospitalization rates with angina.8 Compared with BMS, DES substantially reduces the risk of target lesion revascularization (TLR), but new-generation, polymer-free drug-coated stents (DCS) have been designed to accelerate healing and to mitigate the risk of stent thrombosis (ST) attributed to polymer hypersensitivity with durable polymer DES.9,10,11 The LEADERS FREE trial was the first randomized clinical trial (RCT) to demonstrate the long-term efficacy and safety of a biolimus A9-eluting DCS (BioFreedom; Biosensors International) compared with a BMS with 1 month of DAPT in patients with a high bleeding risk.12,13,14
In this prespecified secondary analysis of data from the LEADERS FREE trial, we (1) examined the baseline and trial inclusion characteristics of the enrolled patients with a high bleeding risk by sex; (2) compared the 2-year clinical end points by sex and then assessed for interaction between sex and stent type; (3) explored the differences in vascular access site and nonvascular bleeding by sex as well as by radial or femoral PCI approaches; and (4) evaluated the effect of bleeding on mortality by sex.
Methods
The LEADERS FREE trial was a double-blind randomized clinical trial conducted at 68 sites in 20 countries from December 2012 to May 2014.12 The protocol (Supplement 1) was developed by the executive committee, and the institutional review board at each of the 68 sites approved the study. All patients provided written informed consent. The study was conducted by Centre Européen de Recherche Cardiovasculaire, an independent research organization in Massy, France, and was sponsored by Biosensors Europe (based in Morges, Switzerland).
Study Population
In this cohort study, which is a prespecified secondary analysis of data from the LEADERS FREE trial, eligible patients with coronary artery disease and an indication for PCI met 1 or more of the inclusion criteria established to define a population of patients who had increased bleeding risk or who were considered by the LEADERS FREE investigators to be candidates for a BMS instead of a DES because of their perceived need for short, 1-month DAPT. The main eligibility criteria were individuals aged 75 years or older who had a need for chronic oral anticoagulation, kidney failure, a need for a surgical intervention within 12 months of the PCI, anemia or recent blood transfusion, or a diagnosis of non–skin cancer during the past 3 years.
Study Procedures and Follow-up
Patients were randomized 1:1 to undergo PCI with either the biolimus A9-eluting DCS or a similar BMS (Gazelle; Biosensors Interventional Technologies). Randomization was performed in blocks of 16 using either a web-based system or a telephone interactive voice-response system (both from Merge Healthcare). The patients, investigators, and members of the clinical events committee and the executive committee were blinded to group assignments. All target lesions were treated with at least 1 study stent. Staged procedures were allowed within 1 week after the index procedure, and all stents used were assigned (DCS or BMS type).
According to the study protocol, all patients received both aspirin and a P2Y12 inhibitor, preferably clopidogrel, for 30 days, followed by a single antiplatelet agent, preferably aspirin. Patients who were discharged while taking a vitamin K antagonist received either triple therapy or the vitamin K antagonist plus clopidogrel during the first 30 days of discharge.
Follow-up visits were conducted at 30 days, when the change from DAPT to single antiplatelet therapy was prescribed, and at 360 days. Further follow-up by telephone or clinic visit took place at 60, 120, and 720 days.
End Points and Definitions
The primary safety end point was a composite of cardiac death, myocardial infarction (MI), or ST. The primary efficacy end point was clinically driven TLR. Myocardial infarction was adjudicated according to the third universal definition of MI.15 All other ischemic end point events were adjudicated according to the Academic Research Consortium definitions.16,17 All ischemic and bleeding events were adjudicated by an independent clinical events committee. Bleeding was adjudicated using the Bleeding Academic Research Consortium (BARC) definitions,17 and the site of bleeding was recorded by the clinical events committee. Major bleeding was defined as BARC types 3 to 5, and major or minor bleeding was deemed as BARC types 2 to 5. BARC types 2 to 5 are any actionable bleeding events requiring prompt evaluation by a health professional with or without an increased level of care, whereas BARC types 3 to 5 are major bleeding events requiring some intervention and typically combinations of transfusion, vasoactive drugs, or a surgical procedure. The bleeding events adjudications form is shown in eFigure 1 in Supplement 2. The present analysis reports 2-year outcomes by sex.
Statistical Analysis
Patients were compared by sex. Categorical data were presented as number (%) and were compared using the χ2 test. Continuous data, presented as mean (SD), were compared using the parametric, unpaired, 2-tailed t test. Outcomes were analyzed in a time-to-event manner using the Kaplan-Meier methods and compared using the log-rank test. Hazard ratios (HRs) and 95% CIs for outcomes were calculated using Cox proportional hazards regression models. Multivariable adjustment was conducted to estimate the risk of clinical outcomes in women and men (with men as the reference group) according to imbalances in baseline characteristics. The following variables were included in the model: current smoking status, hypertension, diabetes, previous MI, previous PCI, previous coronary artery bypass graft, peripheral vascular disease, renal insufficiency, chronic obstructive pulmonary disease, multivessel disease, angina status, lesion length, and reference vessel diameter. Interaction testing was performed for outcomes by sex and stent type.
To calculate the HR for death after a major bleeding event, we divided the follow-up of each patient into time in follow-up before and after a major bleeding using the methods previously described.14 The association between major bleeding and subsequent mortality was analyzed with a Cox proportional hazards regression model using a time-updated categorical variable that enters the model on the day of the event. The calculated HR, therefore, was a comparison of the hazard of death after a major bleeding event to the hazard of death before an event (including the hazard in patients who did not have an event during follow-up).
All data were analyzed using SAS, version 9.4 (SAS Institute Inc). Two-sided P < .05 was considered statistically significant. Data analysis was conducted from August 2017 to October 2019.
Results
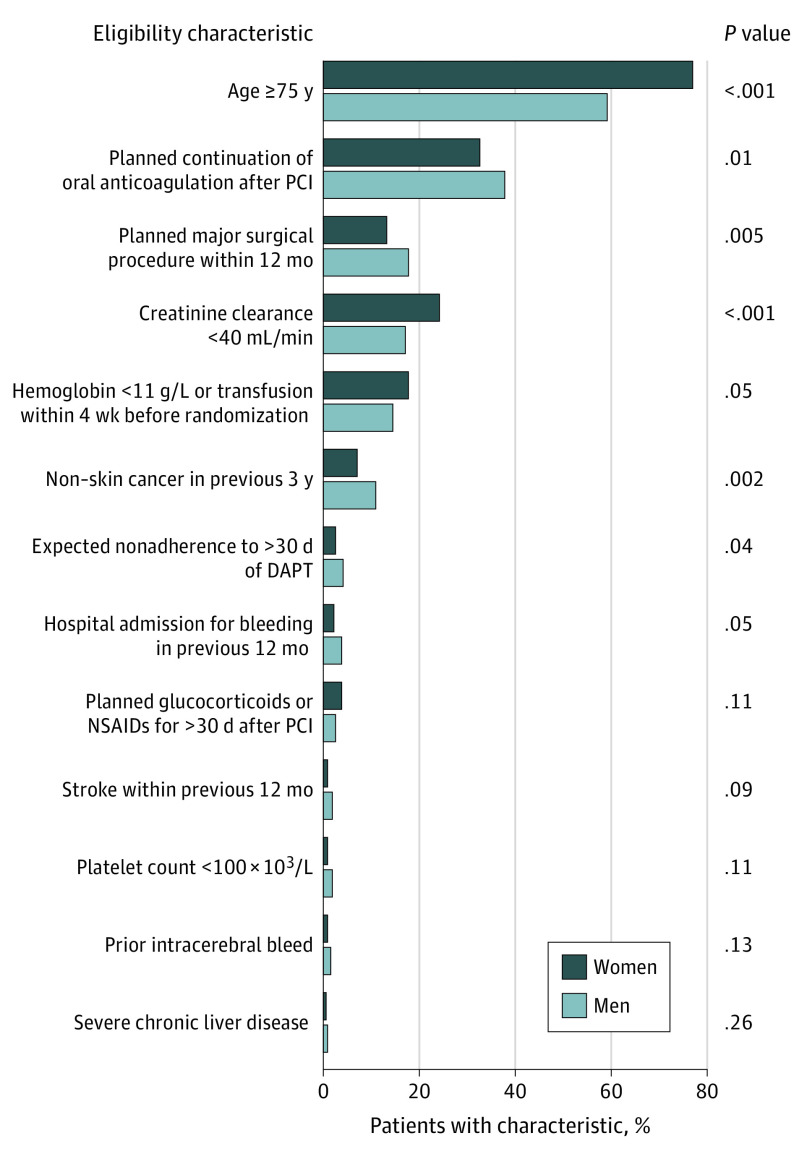
The study population included 2432 patients with a high bleeding risk. Of these patients, the mean (SD) age was 75 (9) years, and 1694 (69.7%) were men and 738 (30.3%) were women. Table 1 shows the baseline patient-, procedure- and lesion-level characteristics by sex. Women compared with men were older (mean [SD] age, 78 [8] years vs 75 [9] years; P < .001) with a higher prevalence of acute coronary syndrome but a lower prevalence of previous MI or previous PCI and current smoking status. With respect to eligibility criteria (Figure 1), more women than men were 75 years or older (76.7% vs 58.9%; P < .001) and had a higher prevalence of chronic kidney failure (creatinine clearance, <40 mL/min in 24.1% vs 16.9%; P < .001) or hemoglobin level less than 11 g/L (17.8% vs 14.6%; P = .05). Men more often than women had an indication for oral anticoagulation (37.8% vs 32.4%; P = .01), planned major surgery within the next 12 months (17.8% vs. 13.1%, P = .005), expected DAPT nonadherence within 1 year for major surgical procedures (4.1% vs 2.4%; P = .04), a history of hospital admission within the previous 12 months for bleeding (3.7% vs 2.2%; P = .05), or higher prevalence of malignant neoplasm (11.0% vs 7.0%; P = .002). According to angiographic data, fewer women than men had multivessel disease, and women had less severe site-reported stenoses. Compared with men, women had shorter mean lesion length, and more women had small vessel disease. Fewer women than men underwent radial PCI. The baseline patient and procedural characteristics by sex and stent type are shown in eTable 1 in Supplement 2.
Table 1. Baseline Patient- and Lesion-Level Characteristics .
| Characteristic | No. (%) | ||
|---|---|---|---|
| Men (n = 1694)a | Women (n = 738)a | P value | |
| Patient level | |||
| Age, mean (SD), y | 75 (9) | 78 (8) | <.001 |
| BMI, mean (SD) | 27 (4) | 27 (6) | .03 |
| Diabetes | 559 (33.1) | 246 (33.4) | .86 |
| Insulin treatment | 169 (30.2) | 93 (37.8) | .04 |
| Current smoker | 221 (13.1) | 50 (6.8) | <.001 |
| Dyslipidemia | 1027 (61.7) | 461 (63.9) | .30 |
| Hypertension | 1293 (76.5) | 620 (84.2) | <.001 |
| Previous stroke | 164 (9.7) | 78 (10.6) | .51 |
| CHF | 226 (13.4) | 99 (13.5) | .96 |
| Previous MI | 372 (22.1) | 123 (16.8) | .003 |
| Previous PCI | 404 (23.9) | 131 (17.8) | <.001 |
| Previous CABG | 193 (11.4) | 44 (6.0) | <.001 |
| PAD | 280 (16.7) | 100 (13.7) | .07 |
| Atrial fibrillation | 596 (35.3) | 246 (33.4) | .37 |
| COPD | 208 (12.4) | 64 (8.8) | .01 |
| Multivessel disease | 1082 (64.8) | 411 (56.5) | <.001 |
| PCI indication | <.001 | ||
| Stable angina | 663 (39.1) | 273 (37.0) | |
| Silent ischemia | 355 (21.0) | 112 (15.2) | |
| Unstable angina | 249 (14.7) | 121 (16.4) | |
| Acute coronary syndrome | 427 (25.2) | 232 (31.4) | |
| STEMI | 69 (16.2) | 36 (15.5) | |
| NSTEMI | 358 (83.8) | 196 (84.5) | |
| Procedure level | |||
| Radial PCI | 1107 (61.7) | 425 (55.1) | .002 |
| Lesion levelb | Men (n = 2714) | Women (n = 1123) | |
| Lesion length, mean (SD), mm | 18 (10) | 16 (8) | <.001 |
| Reference vessel diameter, mean (SD), mm | 3.0 (0.5) | 3.0 (0.5) | <.001 |
| Preprocedure stenosis, mean (SD) | 82 (12) | 81 (12) | .03 |
| Small vessel disease (≤2.5-mm stents) | 791 (29.2) | 374 (33.3) | .02 |
| Long lesion (>30 mm) | 175 (6.5) | 57 (5.1) | .10 |
| Preprocedure TIMI flow | .18 | ||
| 0 | 121 (4.5) | 34 (3.0) | |
| 1 | 95 (3.5) | 52 (4.6) | |
| 2 | 182 (6.7) | 55 (4.9) | |
| 3 | 2314 (85.3) | 981 (87.4) | |
| Lesion class (ACC/AHA class) | .002 | ||
| A | 364 (13.9) | 191 (17.7) | |
| B1 | 967 (37.0) | 418 (38.6) | |
| B2 | 680 (26.1) | 270 (25.0) | |
| C | 599 (23.0) | 203 (18.8) | |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
There were small numbers of missing data for several variables; therefore, the numbers and percentages shown are from the available data.
Operator reported.
Figure 1. Sex Differences in Enrollment Characteristics .
DAPT indicates dual antiplatelet therapy; NSAID, nonsteroidal anti-inflammatory drug; and PCI, percutaneous coronary intervention.
At discharge, the rate of DAPT use was similar in women and men (96.0% vs 97.0%; P = .18). At 37 days, a higher proportion of women than men continued receiving DAPT (11.6% vs 8.6%; P = .02). However, at 12 months (8.8% vs 8.6%; P = .87) and 24 months (5.4% vs 6.9%; P = .20), no differences in the proportion of women vs men receiving DAPT were observed.
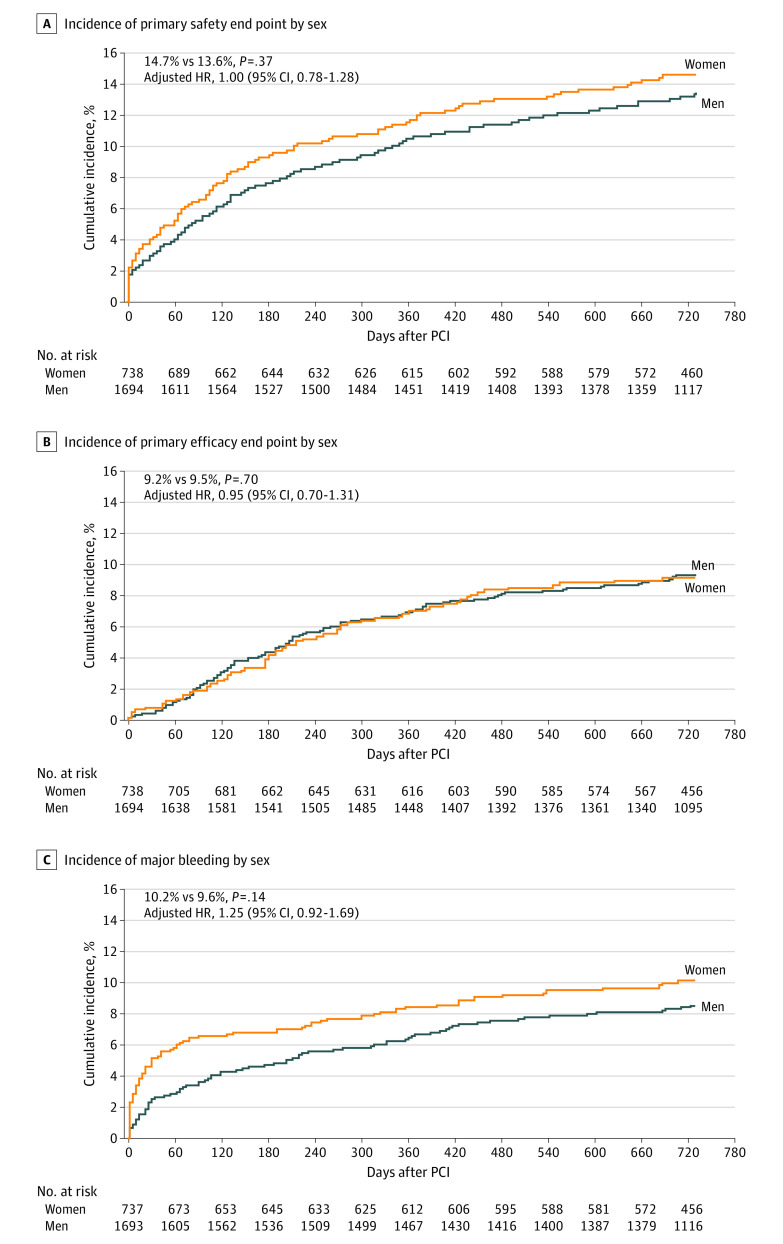
Table 2 presents the 2-year incidence and adjusted risk of clinical outcomes by sex. At the 2-year follow-up, women with a high bleeding risk compared with their male counterparts had a similar incidence of the primary safety end point (14.7% vs 13.6%; P = .37) (adjusted HR, 1.00; 95% CI, 0.78-1.28; P = .98) and efficacy end point (9.2% vs 9.5%; P = .70) (adjusted HR, 0.95; 95% CI, 0.70-1.31; P = .77) (Figure 2) as well as similar rates of MI (7.2% vs 7.2%; P = .95) and ST (2.5% vs 2.1%; P = .47). After adjustment for baseline differences, the outcomes remained similar by sex.
Table 2. Two-Year Clinical Outcomes by Sex.
| Clinical outcome | No. (Kaplan-Meier %) | Hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Men (n = 1694) | Women (n = 738) | Unadjusted | Adjusted | ||
| Death, MI, or ST (primary safety end point) | 222 (13.6) | 105 (14.7) | 1.11 (0.88-1.40) | 1.00 (0.78-1.28) | .98 |
| Clinically driven TLR (primary efficacy end point) | 150 (9.5) | 63 (9.2) | 0.98 (0.73-1.32) | 0.95 (0.70-1.31) | .77 |
| All death | 214 (13.0) | 106 (14.6) | 1.16 (0.92-1.47) | 1.05 (0.82-1.35) | .67 |
| Cardiac death | 101 (6.3) | 55 (7.8) | 1.28 (0.92-1.78) | 1.10 (0.77-1.57) | .59 |
| Target vessel MI | 118 (7.2) | 51 (7.2) | 1.01 (0.73-1.40) | 0.89 (0.63-1.27) | .52 |
| Definite or probable ST | 34 (2.1) | 18 (2.5) | 1.23 (0.70-2.18) | 1.23 (0.67-2.27) | .51 |
| Definite ST | 24 (1.5) | 10 (1.4) | 0.97 (0.46-2.03) | 1.08 (0.49-2.34) | .85 |
| Acute | 5 (0.3) | 3 (0.4) | 1.38 (0.33-5.77) | 1.28 (0.28 -5.88) | .75 |
| Subacute | 5 (0.3) | 4 (0.6) | 1.85 (0.50-6.88) | 2.50 (0.59 -1.61) | .21 |
| Late | 15 (0.9) | 2 (0.3) | 0.31 (0.07-1.36) | 0.35 (0.08-1.57) | .17 |
| Very late | 1 (0.1) | 1 (0.2) | 2.36 (0.15-37.71) | 3.45 (0.16-77.05) | .43 |
| Probable ST | 10 (0.6) | 8 (1.1) | 1.86 (0.73-4.71) | 1.46 (0.53-4.03) | .47 |
| Clinically indicated TVR | 169 (10.7) | 68 (9.9) | 0.94 (0.71-1.24) | 0.94 (0.70-1.27) | .69 |
| Any BARC bleeding | 372 (22.9) | 144 (20.3) | 0.90 (0.75-1.09) | 0.89 (0.73-1.10) | .28 |
| BARC types 2-5 | 292 (18.1) | 118 (16.6) | 0.96 (0.77-1.18) | 0.95 (0.75-1.20) | .68 |
| BARC types 3-5 | 138 (8.6) | 72 (10.2) | 1.24 (0.93-1.65) | 1.25 (0.92-1.69) | .16 |
Abbreviations: BARC, Bleeding Academic Research Consortium scale; MI, myocardial infarction; ST, stent thrombosis; TLR, target lesion revascularization; TVR, target vessel revascularization.
Figure 2. Kaplan-Meier Curves of 2-Year Cumulative Incidence of Clinical Events Among Women and Men With a High Bleeding Risk.
A, The primary safety end point was a composite of cardiac death, myocardial infarction, and stent thrombosis. B, The primary efficacy end point was clinically driven target lesion revascularization. C, Bleeding was assessed using the Bleeding Academic Research Consortium scale. HR indicates hazard ratio; PCI, percutaneous coronary intervention.
The 2-year clinical outcomes by stent type and sex are shown in eTable 2 in Supplement 2. Outcomes (expressed as Kaplan Meier %) were better in women and men with lower TLR in those patients who received a DCS as opposed to a BMS (women: 6.3% vs 12.1%; men: 7.0% vs 12.0%; P for interaction = .70), and a similar incidence of the primary safety end point was found in both sexes (women: 12.4% vs 17.0%; men: 12.6% vs 14.5%; P for interaction = .40) (eFigure 2 in Supplement 2). No differences in the incidence of ST were observed between the DCS and BMS in women or in men (women: 1.7% vs 3.3%; men: 2.3% vs 1.8%; P for interaction = .13).
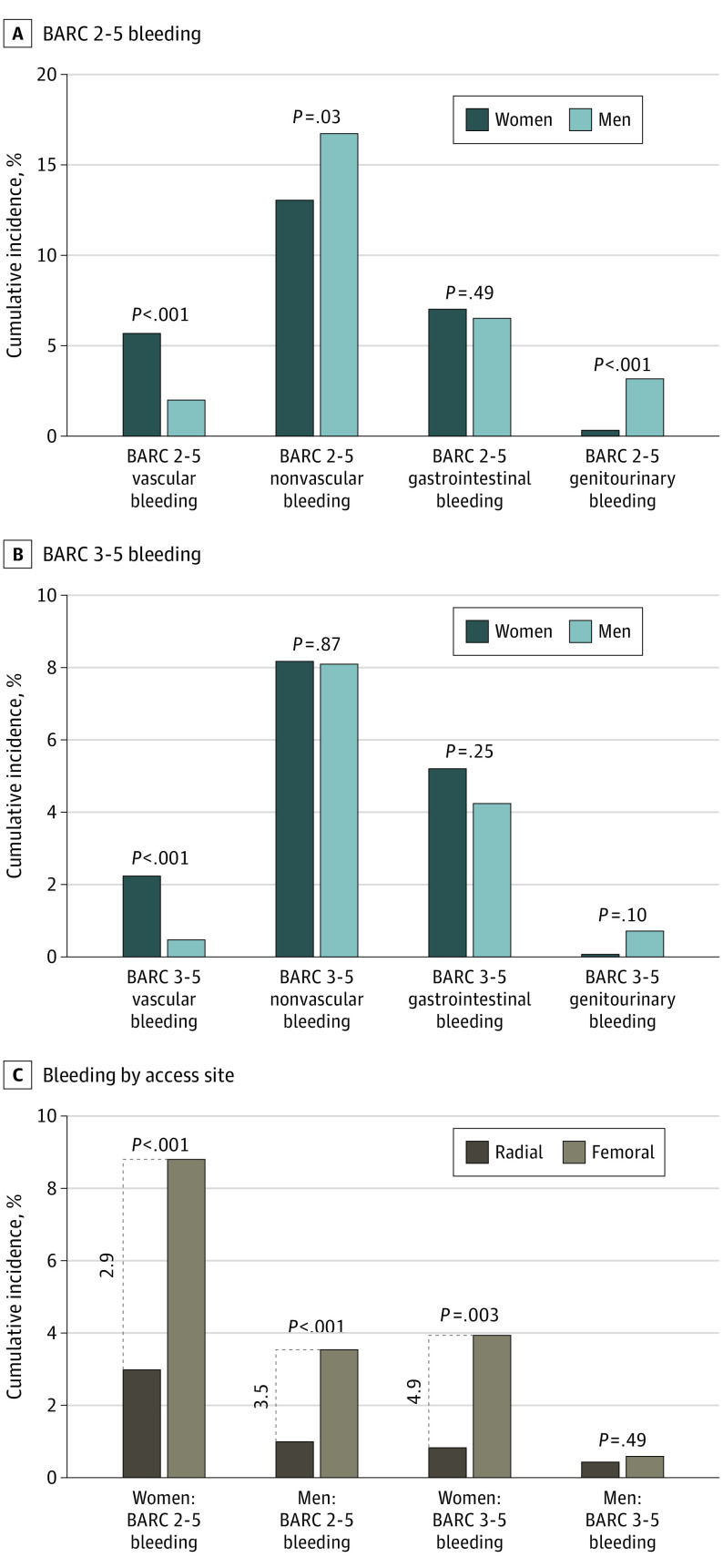
With respect to bleeding outcomes, no statistically significant differences were seen in the incidence of overall 2-year major BARC types 3 to 5 bleeding by sex (10.2% in women vs 8.6% in men; P = .14) (adjusted HR, 1.25; 95% CI, 0.92-1.69; P = .16) (Figure 2) or by stent type (women: 9.8%with DCS vs 10.6% with BMS, P = .71; men: 8.6% with DCS vs 8.5% with BMS, P = .84; P for interaction = .64) (eFigure 2 in Supplement 2). However, women compared with men experienced statistically significant greater rates of major bleeding between 0 and 30 days (5.1% vs 2.4%; P = .007) (adjusted HR, 2.22; 95% CI, 1.38-3.62; P = .001) and 0 and 60 days (5.9% vs 2.9%; P < .001) (adjusted HR, 2.22; 95% CI, 1.42-3.47; P < .001) after the PCI. Furthermore, women experienced higher rates of major and major or minor bleeding from the vascular access site compared with men (BARC types 3-5: 2.2% in women vs 0.5% in men [P < .001]; BARC types 2-5: 5.6% in women vs 2.0% in men [P < .001]) (Figure 3; eTable 3 in Supplement 2). Men compared with women had greater rates of nonvascular major or minor bleeding (BARC types 2-5: 16.4% vs 12.8%; P = .03), mainly genitourinary bleeding (BARC types 2-5: 3.1% vs 0.3%; P < .001). The rates of gastrointestinal bleeding were similar in women and men (BARC types 2-5: 6.9% vs 6.3%; P = .49) (BARC types 3-5: 5.2% vs 4.2%; P = .25). In a comparison of PCI access route, radial (vs femoral) access was associated with 2- to 3-fold lower bleeding event rates from the vascular access site in both sexes (BARC types 2-5 women: 3.0% vs 8.8%; P < .001; men: 1.0% vs 3.5%; P < .001) without interaction (P for interaction = .72) (Figure 3, eTable 4 in Supplement 2).
Figure 3. Sex Differences in Vascular and Nonvascular Bleeding.
A and B, Bleeding was assessed using the Bleeding Academic Research Consortium (BARC) scale. C, The percutaneous coronary intervention access site was either radial or femoral.
In both sexes, vascular (women: HR, 3.45 [95% CI, 1.51-7.87]; men: HR, 4.14 [95% CI, 1.33-12.95]) and nonvascular (women: HR, 3.76 [95% CI, 2.17- 6.53]; men: HR, 4.62 [95% CI, 3.23-6.61]) major bleeding events were associated with greater 2-year mortality. The associations between vascular and nonvascular major bleeding and 2-year mortality in women and men are shown in eTable 5 in Supplement 2.
Discussion
The main findings of the present prespecified secondary analysis were as follows. First, women and men enrolled in the LEADERS FREE trial were deemed to be at high bleeding risk for considerably different reasons. Second, compared with men, women had a lower prevalence of previous MI or revascularization and smoking but presented more often with acute coronary syndrome. Procedurally, women had shorter lesion lengths, more small vessel disease, less multivessel disease, and less severe stenoses. They underwent radial PCI less often than men. Third, at the 2-year follow-up, women and men had similar incidence of the primary safety and efficacy end points as well as MI and ST. With respect to stent type, both women and men had lower risk of TLR with the DCS compared with the BMS along with similar incidence of the primary safety end point and ST. Fourth, although women and men had similar rates of any BARC types 3 to 5 major bleeding, women experienced a higher incidence of bleeding from the vascular access site than did men. Radial access for PCI resulted in substantially lower vascular bleeding events in both sexes without interaction. Fifth, patients who experienced vascular or nonvascular major bleeding had worse 2-year mortality.
Sex-based studies have consistently shown that women have higher crude incidences of ischemic events after PCI.1,2,3 This finding has been considered to be the result of the differences in baseline comorbidities rather than biological factors, especially with older age in women, higher prevalence of diabetes, renal insufficiency, and high platelet reactivity.18 Nevertheless, previous studies have suggested that women are undertreated with PCI4 and potent antiplatelet therapies1,3 and are more likely to undergo earlier DAPT cessation.19 Part of the reason for this finding may be that women demonstrate higher bleeding event rates because of greater vascular complications and some possible differences in non–access site–related bleeding.1,2
Drug-eluting stents have substantially evolved since their conception, and polymer-free DES offers the advantage of reducing the risk of TLR without increasing the ST associated with polymer hypersensitivity.9,10,11,20,21 Yet, physicians have typically been biased in treating patients with a high bleeding risk, avoiding DES owing to the need for longer-term DAPT. To our knowledge, the LEADERS FREE trial is the first RCT to specifically enroll a population with a high bleeding risk and to demonstrate the long-term safety and efficacy of polymer-free DCS compared with BMS with only 1 month of DAPT after PCI.12,13 These results are important given that patients with a high bleeding risk, who may represent up to one-fifth of patients who undergo PCI, constitute a special group at elevated risk of both bleeding and ischemic events.22,23,24 Although the most frequent eligibility criteria for enrollment in the LEADERS FREE trial were age 75 years or older and the need for anticoagulation,12 we observed considerable differences by sex in the eligibility criteria of enrolled patients. Women with a high bleeding risk were older, had more chronic kidney disease, and had hemoglobin levels less than 11 g/L, whereas men with a high bleeding risk were more likely to be on chronic oral anticoagulation; have expected DAPT cessation within 12 months of the PCI for a major surgical procedure; and have a history of bleeding or malignant neoplasm. Other baseline sex differences were aligned with those found in past PCI studies, with a lower prevalence of previous revascularization and smoking3 as well as shorter lesion length and more small vessel disease in women than in men.25
Despite these baseline differences, women and men with a high bleeding risk had similar crude outcomes at the 2-year follow-up. However, women experienced greater rates of vascular access site bleeding, whereas men suffered more nonvascular bleeding, particularly genitourinary actionable bleeding. Other studies involving patients who underwent PCI with primarily femoral access have shown women to have a substantially higher risk of bleeding.1,2 Although radial access for PCI is an important bleeding avoidance strategy, the Study of Access Site for Enhancement of PCI for Women trial did not demonstrate considerable benefits in bleeding outcomes with radial vs femoral access in women.26 Nevertheless, the practice of radial access for PCI has evolved in the past decade, allowing for increased uptake and improved operator skills. In the present trial, although radial access for PCI was performed in more than 50% of patients, more men than women underwent successful radial PCI. Radial PCI was associated with at least 3-fold lower event rates compared with femoral PCI in both sexes, whereas women who underwent radial PCI had almost similar vascular bleeding rates as men who underwent femoral PCI, emphasizing the high bleeding risk in women.
With respect to ischemic outcomes, we found that, although event rates in patients with a high bleeding risk were higher than in other comparatively unselected contemporary PCI trials,27,28 no substantial differences by sex were found. These results remained unchanged even after adjustment for baseline differences. The polymer-free DCS performed better than the BMS in both women and men. Although no interaction was noted by sex, women had a considerably lower incidence of the composite efficacy end point (clinically driven TLR) compared with men. These data suggest that patients of both sexes with a high bleeding risk benefit from DCS PCI with 1-month DAPT, thus eligible women with a high bleeding risk should not be denied the benefits of DCS technology.
To our knowledge, the LEADERS FREE trial is the first RCT to specifically compare outcomes between polymer-free DCS and BMS in patients with a high bleeding risk. However, other PCI studies have suggested low ST event rates with 1 month of DAPT after a second-generation DES PCI.29,30,31
Limitations
This analysis has some limitations. We did not collect information on adherence to medications other than antiplatelet therapy during follow-up. Although the trial protocol recommended low-dose aspirin, we did not record data on the use of low- or high-dose aspirin. We used a broad definition of high bleeding risk, but this analysis was the first sex-based subanalysis in patients with a high bleeding risk enrolled in an RCT. Thus, it provides detailed sex-based descriptions of baseline demographic characteristics and long-term outcomes up to 2 years with a DCS or BMS PCI and 1 month of DAPT.
Conclusions
Women at increased risk of bleeding had similar 2-year ischemic outcomes to men, suggesting that, when indicated, women should not be denied the benefits of PCI. In both women and men at increased risk of bleeding, the polymer-free biolimus A9-eluting DCS performed better than the BMS in terms of TLR and should be favored regardless of sex. Women underwent radial access PCI less often and experienced higher incidences of vascular bleeding compared with men; however, in both sexes, vascular and nonvascular major bleeding appeared to be associated with worse 2-year mortality. Bleeding avoidance strategies during PCI should be uniformly adopted in all patients, especially women.
Trial Protocol
eTable 1. Baseline Patient and Procedural Characteristics by Stent Type in Women and Men
eTable 2. Two-Year Clinical Outcomes by Stent Type in Women and Men With Interaction Testing
eTable 3. Vascular Access Site or Non-Vascular Bleeding Location in Women and Men
eTable 4. Bleeding Sources After Radial or Femoral Access Site PCI in Women and Men
eTable 5. Two-Year Mortality in Women and Men Following a Major BARC 3-5 Bleed
eFigure 1. Bleeding Events Adjudication Form
eFigure 2. Kaplan-Meier Curves Indicating the 2-Year Cumulative Incidence of Events by Stent Type Among Women and Men
Data Sharing Statement
References
- 1.Hess CN, McCoy LA, Duggirala HJ, et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J Am Heart Assoc. 2014;3(1):e000523. doi: 10.1161/JAHA.113.000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Mehran R, Grinfeld L, et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85(3):359-368. doi: 10.1002/ccd.25630 [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekhar J, Baber U, Sartori S, et al. Sex-related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: results from the PROMETHEUS study. Catheter Cardiovasc Interv. 2017;89(4):629-637. doi: 10.1002/ccd.26606 [DOI] [PubMed] [Google Scholar]
- 4.Udell JA, Koh M, Qiu F, et al. Outcomes of women and men with acute coronary syndrome treated with and without percutaneous coronary revascularization. J Am Heart Assoc. 2017;6(1):6. doi: 10.1161/JAHA.116.004319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chieffo A, Hoye A, Mauri F, et al. ; WIN Group . Gender-based issues in interventional cardiology: a consensus statement from the Women in Innovations (WIN) initiative. EuroIntervention. 2010;5(7):773-779. doi: 10.4244/EIJV5I7A130 [DOI] [PubMed] [Google Scholar]
- 6.Batchelor W, Kandzari DE, Davis S, et al. Outcomes in women and minorities compared with white men 1 year after everolimus-eluting stent implantation: insights and results from the PLATINUM Diversity and PROMUS Element plus post-approval study pooled analysis. JAMA Cardiol. 2017;2(12):1303-1313. doi: 10.1001/jamacardio.2017.3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfors B, Angerås O, Råmunddal T, et al. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish Web System for Enhancement of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). J Am Heart Assoc. 2015;4(7):4. doi: 10.1161/JAHA.115.001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess CN, Kaltenbach LA, Doll JA, Cohen DJ, Peterson ED, Wang TY. Race and sex differences in post-myocardial infarction angina frequency and risk of 1-year unplanned rehospitalization. Circulation. 2017;135(6):532-543. doi: 10.1161/CIRCULATIONAHA.116.024406 [DOI] [PubMed] [Google Scholar]
- 9.Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56(10)(suppl):S43-S78. doi: 10.1016/j.jacc.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Otsuka F, Yahagi K, Ladich E, et al. Hypersensitivity reaction in the US Food and Drug Administration-approved second-generation drug-eluting stents: histopathological assessment with ex vivo optical coherence tomography. Circulation. 2015;131(3):322-324. doi: 10.1161/CIRCULATIONAHA.114.012658 [DOI] [PubMed] [Google Scholar]
- 11.Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129(2):211-223. doi: 10.1161/CIRCULATIONAHA.113.001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urban P, Meredith IT, Abizaid A, et al. ; LEADERS FREE Investigators . Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373(21):2038-2047. doi: 10.1056/NEJMoa1503943 [DOI] [PubMed] [Google Scholar]
- 13.Jensen CJ, Naber CK, Urban P, et al. Two-year outcomes of high bleeding risk patients with acute coronary syndrome after Biolimus A9 polymer-free drug-coated stents: a LEADERS FREE substudy. EuroIntervention. 2018;13(16):1946-1949. doi: 10.4244/EIJ-D-17-00720 [DOI] [PubMed] [Google Scholar]
- 14.Garot P, Morice MC, Tresukosol D, et al. ; LEADERS FREE Investigators . 2-Year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017;69(2):162-171. doi: 10.1016/j.jacc.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging Subcommittee; Classification Subcommittee; Intervention Subcommittee; Trials & Registries Subcommittee; ESC Committee for Practice Guidelines (CPG); Document Reviewers . Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-1598. doi: 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 17.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Mehran R, Baber U, et al. Sex differences in the clinical impact of high platelet reactivity after percutaneous coronary intervention with drug-eluting stents: results from the ADAPT-DES Study (Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents). Circ Cardiovasc Interv. 2017;10(2):10. doi: 10.1161/CIRCINTERVENTIONS.116.003577 [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Baber U, Mastoris I, et al. Sex-based differences in cessation of dual-antiplatelet therapy following percutaneous coronary intervention with stents. JACC Cardiovasc Interv. 2016;9(14):1461-1469. doi: 10.1016/j.jcin.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10)(suppl):S1-S42. doi: 10.1016/j.jacc.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 21.Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare metal stents? late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation. 2007;115(11):1433-1439. doi: 10.1161/CIRCULATIONAHA.106.666826 [DOI] [PubMed] [Google Scholar]
- 22.Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials. JACC Cardiovasc Interv. 2011;4(6):654-664. doi: 10.1016/j.jcin.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar J, Mehran R, Dangas G, et al. Effect of thrombotic and bleeding risk according to platelet reactivity on 2-year mortality: the ADAPT-DES study. J Am Coll Cardiol. 2017;69(11)(suppl): 1012. doi: 10.1016/S0735-1097(17)34401-7 [DOI] [Google Scholar]
- 24.Sorrentino S, Claessen B, Mehran R, et al. Validation of PARIS risk scores in patients treated with everolimus-eluting stents for left main coronary artery disease: analysis from the EXCEL Trial. J Am Coll Cardiol. 2018;72(13) (suppl): B333. doi: 10.1016/j.jacc.2018.08.2078 [DOI] [Google Scholar]
- 25.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3)(suppl):S62-S72. doi: 10.1016/j.jcmg.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 26.Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv. 2014;7(8):857-867. doi: 10.1016/j.jcin.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Mauri L, Kereiakes DJ, Yeh RW, et al. ; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155-2166. doi: 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607-2617. doi: 10.1016/S0140-6736(16)31920-1 [DOI] [PubMed] [Google Scholar]
- 29.Silber S, Kirtane AJ, Belardi JA, et al. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur Heart J. 2014;35(29):1949-1956. doi: 10.1093/eurheartj/ehu026 [DOI] [PubMed] [Google Scholar]
- 30.Généreux P, Rutledge DR, Palmerini T, et al. Stent thrombosis and dual antiplatelet therapy interruption with everolimus-eluting stents: insights from the Xience V Coronary Stent System Trials. Circ Cardiovasc Interv. 2015;8(5):8. doi: 10.1161/CIRCINTERVENTIONS.114.001362 [DOI] [PubMed] [Google Scholar]
- 31.Varenne O, Cook S, Sideris G, et al. ; SENIOR investigators . Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391(10115):41-50. doi: 10.1016/S0140-6736(17)32713-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Patient and Procedural Characteristics by Stent Type in Women and Men
eTable 2. Two-Year Clinical Outcomes by Stent Type in Women and Men With Interaction Testing
eTable 3. Vascular Access Site or Non-Vascular Bleeding Location in Women and Men
eTable 4. Bleeding Sources After Radial or Femoral Access Site PCI in Women and Men
eTable 5. Two-Year Mortality in Women and Men Following a Major BARC 3-5 Bleed
eFigure 1. Bleeding Events Adjudication Form
eFigure 2. Kaplan-Meier Curves Indicating the 2-Year Cumulative Incidence of Events by Stent Type Among Women and Men
Data Sharing Statement