Key Points
Question
Can systematic audio recording resolve discrepancies between systematic video recordings and narrative operative notes in laparoscopic cholecystectomy procedures?
Findings
In this multicenter study of 79 patients, video and audio recordings during laparoscopic cholecystectomy procedures significantly improved the adequacy of the depiction of essential surgical steps compared with narrative operative notes. The addition of audio recordings resolved some discrepancies between video recording and operative note.
Meaning
The study provides strong support in favor of additional audio recording during laparoscopic cholecystectomy procedures, which could lead to a better understanding of the operative procedure on review.
This multicenter observational study examines the usefulness of audio recordings during laparoscopic cholecystectomy procedures as a means to resolve discrepancies between videos and narrative operative notes.
Abstract
Importance
All events that transpire during laparoscopic cholecystectomy (LC) cannot be adequately reproduced in the operative note. Video recording is already known to add important information regarding this operation.
Objective
It is hypothesized that additional audio recordings can provide an even better procedural understanding by capturing the surgeons’ considerations.
Design, Setting, and Participants
The Simultaneous Video and Audio Recording of Laparoscopic Cholecystectomy Procedures (SONAR) trial is a multicenter prospective observational trial conducted in the Netherlands in which operators were requested to dictate essential steps of LC. Elective LCs of patients 18 years and older were eligible for inclusion. Data collection occurred from September 18, 2018, to November 13, 2018.
Main Outcomes and Measures
Adequacy rates for video recordings and operative note were compared. Adequacy was defined as the competent depiction of a surgical step and expressed as the number of adequate steps divided by the total applicable steps for all cases. In case of discrepancies, in which a step was adequately observed in the video recording but inadequately reported in the operative note, an expert panel analyzed the added value of the audio recording to resolve the discrepancy.
Results
A total of 79 patients (49 women [62.0%]; mean [SD] age, 54.3 [15.9] years) were included. Video recordings resulted in higher adequacy for the inspection of the gallbladder (note, 39 of 79 cases [49.4%] vs video, 79 of 79 cases [100%]; P < .001), the inspection of the liver condition (note, 17 of 79 [21.5%] vs video, 78 of 79 cases [98.7%]; P < .001), and the circumferential dissection of the cystic duct and the cystic artery (note, 25 of 77 [32.5%] vs video, 62 of 77 [80.5%]; P < .001). The total adequacy was higher for the video recordings (note, 849 of 1089 observations [78.0%] vs video, 1005 of 1089 observations [92.3%]; P < .001). In the cases of discrepancies between video and note, additional audio recordings lowered discrepancy rates for the inspection of the gallbladder (without audio, 40 of 79 cases [50.6%] vs with audio, 17 of 79 cases [21.5%]; P < .001), the inspection of the liver condition (without audio, 61 of 79 [77.2%] vs with audio, 37 of 79 [46.8%]; P < .001), the circumferential dissection of the cystic duct and the cystic artery (without audio, 43 of 77 cases [55.8%] vs with audio, 17 of 77 cases [22.1%]; P < .001), and similarly for the removal of the first accessory trocar (without audio, 27 of 79 [34.2%] vs with audio, 16 of 79 [20.3%]; P = .02), the second accessory trocar (without audio, 24 of 79 [30.4%] vs with audio, 11 of 79 [13.9%]; P < .001), and the third accessory trocar (without audio, 27 of 79 [34.2%] vs with audio, 14 of 79 [17.7%]; P < .001). The total discrepancy was lower with audio adjustment (without audio, 254 of 1089 observations [23.3%] vs with audio, 128 of 1089 observations [11.8%]; P < .001).
Conclusions and Relevance
Audio recording during LC significantly improves the adequacy of depicting essential surgical steps and exhibits lower discrepancies between video and operative note.
Introduction
Cholecystectomy is currently the most common abdominal surgical procedure, including more than 25 000 procedures each year in the Netherlands (a nation of 17 million inhabitants), with most performed laparoscopically.1 Since the introduction of laparoscopic cholecystectomy (LC), considerable effort has been made to improve its safety. Because LC was rapidly embraced as the gold standard for cholecystectomies, it paved the way for many surgical specialties to improve the laparoscopic approach.2,3 Nonetheless, this rapid introduction and its accompanying learning curve have led to increased biliary injury rates with rates up to 1.5%, compared with 0.2% in the open approach.4,5,6,7,8,9,10,11 The reported intraoperative detection rate of these complications differs widely, ranging from 25% up to 89%.12,13 The higher patient numbers and the increased operator experience in the last few decades have lowered the incidence of biliary injuries to rates around 0.08% to 0.30%, matching the biliary complication rates of the open approach.10,13,14,15 At first sight, these rates seem low, but because LC is a high-volume procedure, the incidence of bile duct injuries is substantial and cannot be ignored. Current understanding of these injuries attributes them mainly to misidentification of anatomical structures, which could lead to life-threatening complications, poorer surgical outcomes, prolonged hospitalization, high financial expenditures, and litigations.16,17 The most widely accepted method for identification of cystic structures is the Critical View of Safety (CVS) technique introduced by Strasberg et al,16,18,19,20 in which the cystic duct and cystic artery are circumferentially identified within the limits of the Calot hepatobiliary triangle prior to transection. For quality-control purposes, the operative note alone is not sufficient to adequately record CVS.21 Therefore, it is standard practice in the Netherlands to capture CVS either with video or image capture.22,23,24 This method, however, is not widely implemented in the rest of the world.
Another difficulty is that all events that transpire during surgery cannot be adequately reproduced in a postoperative setting. The only tangible source of information about the surgical procedure is the operative note, which is subjective by nature and frequently omits essential information.25 To fill this gap in procedural information, systematic video recording during surgery has proven to be feasible and useful, as has recently also been demonstrated in colorectal cancer surgery.26,27 These studies have determined that systematic video recording as a supplement to the operative note improves the availability of necessary intraoperative information and thus contributes to quality control and objectivity in reporting.
We hypothesize that by adding synchronous voice recording alongside intraoperative video recording, a new dimension could be added by capturing the surgeons’ considerations during surgery, which might provide a better understanding of the procedure on review. To our knowledge, no study has been conducted yet in which the availability of essential information during surgery has been compared without vs with the implementation of real-time voice dictation. Our aim is to investigate whether voice dictation can resolve discrepancies between videos and operative notes.
Methods
The Simultaneous Video and Audio Recording of Laparoscopic Cholecystectomy Procedures (SONAR) trial is a multicenter prospective observational trial (Netherlands Trial Registry identifier NL6822 [NTR7008]) conducted at 4 surgical centers in the Netherlands (Isala Hospital, Zuyderland Medical Center, IJsselland Hospital, and Park Medical Center). The medical research and ethics committee of the Erasmus University Medical Center exempted this study from the Research Involving Human Subjects Act. Institutional review boards of the participating centers provided separate approval of this study prior to local initiation. Informed consent was obtained from the operators for the use of their voice recordings.
Study Participants
Operators (surgeons, fellows, and resident physicians) were eligible as study participants. Endoscopic video recordings were made using a MediCap USB300 Medical Video Recorder or Epiphan Pearl (MediCapture Inc) or a Stryker Digital Capture System (SDC ULTRA HD, Stryker Corp). The operator was requested to dictate the essential steps of the procedure in real time during the procedure with a wireless and wearable microphone (Revolabs Xtag Wireless Microphone [Yamaha Unified Communications Inc]), which was attached to the operator’s scrub top. Video recordings were saved as videoLAN client (VLC) files and audio recordings as music player 3 (MP3) files using Audacity recording and editing software version version 2.3.3 (the Audacity Team) on a password-protected external hard drive. Video and audio files were synchronized using Adobe Premiere Pro CC 2018 (Adobe Systems). Elective LCs of patients 18 years or older were eligible for inclusion. Cases with incomplete video recordings, audio recordings, or unavailable operative notes were excluded.
Data Collection
The recordings were started at the moment of endoscope introduction in the abdomen and discontinued after endoscope disconnection. These recordings and notes were retrieved and anonymized for further analysis. Data regarding baseline characteristics were gathered from the patients’ medical records and anonymously entered in a database (Excel 2016 [Microsoft]).
Study Outcomes
Video recordings and corresponding operative notes were reviewed for adequacy according to predefined key steps for LC (eAppendix in the Supplement). The term adequacy was defined as the competent depiction of a surgical step. Adequacy should not be confused with surgical competence, because it only depicts whether the step could be observed in the video, was mentioned by the operator in the audio recording, or was described in the operative note.
Two researchers (Ö.E. and F.v.d.G.) analyzed the recordings based on the stepwise LC guideline of the Dutch Society for Surgery.22 The independent reviewer form can be found in the eTable in the Supplement. Steps concerning the adequate depiction of the circumferential dissection of the cystic duct and cystic artery were analyzed by an expert panel of 2 surgeons (J.F.L. and A.M.). The cumulative adequacy ratings for the video recordings were compared with those for the operative notes. In case of discrepancies between a video and an operative note in which a step was adequately observed in the video but inadequately reported in the operative note, the expert panel of surgeons would analyze the added value of audio on the discrepant steps by assessing if the aforementioned surgical steps were adequately mentioned by the operator during the procedure. The discrepancy without vs with the implementation of audio was compared. A flow diagram summarizing the steps taken to conduct this study can be found in the eFigure in the Supplement.
Statistical Analysis
Categorical data were presented as numbers and percentages. Data were expressed as medians (interquartile ranges) or means (SDs) for normally distributed data. Individual video recordings and operative notes were compared, assuming the probability that a specific procedural aspect was the same for both the video and the operative note. Adequacy and discrepancy between individual steps were compared with the exact McNemar test, excluding missing values.28 The total adequacy and discrepancy was compared with the paired-samples t test or Wilcoxon signed rank test, depending on normality. To reduce the probability of a type I error occurring, the Bonferroni correction was applied for multiple comparisons by multiplying the obtained P values with the number of completed tests, using P < .05 as our cutoff value for statistical significance. Data were analyzed with statistical software R, version 3.4.1, for Windows (R Foundation for Statistical Computing). Figures were created with Prism version 8.1.1 for Windows (GraphPad Software).
Sample Size
The sample size was calculated based on prior data by Wauben et al25 evaluating the quality of the narrative operative note. The step regarding CVS was selected for this calculation because this is unequivocally the most critical part of the procedure and thus most important to report adequately. The CVS technique was seen on video recordings in 99 of 125 cases (79.2%). The amount of CVS reported in the narrative operative note and seen on the video recordings was in 63 of the 125 reviewed cases (50.4%). With α = .05, power of 0.80, and δ equal to 0.10, a minimal sample size of 73 procedures was calculated. After accounting for loss of data, 90 patients were intended to be included in this trial.
Results
Study Population
Between September 18, 2018, and November 13, 2018, 90 patients who met the inclusion criteria underwent LC in the participating centers. Subsequently, 11 cases were excluded from analysis, 10 because of technical malfunctioning of the recording equipment or problems in data storage and 1 because of early termination of the procedure because of suspected liver metastases. Hence, 79 patients (49 women [62.0%]) were included for further analysis. The mean (SD) age was 54.3 (15.9) years. Study characteristics are presented in Table 1 and Table 2. Twenty-four different primary operators conducted the procedures, with a mean number of 3 cases per operator (range, 1-18 cases). Two procedures were converted to open LCs because of difficulties with identifying the anatomical structures.
Table 1. Patient Characteristics.
| Characteristic | Mean (SD) |
|---|---|
| Total, No. | 79 |
| Age, y | 54.3 (15.9) |
| Women, No. (%) | 49 (62.0) |
| Height, cm | 171.3 (9.9) |
| Weight, kg | 84.7 (16.8) |
| BMI | 28.9 (5.2) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Table 2. Surgery Characteristics (N = 79 Operations).
| Characteristic | No. (%) |
|---|---|
| Primary operator position | |
| Surgeon | 9 (11.4) |
| Fellow | 59 (74.7) |
| Resident physician | 11 (13.9) |
| Secondary operator position | |
| Surgeon | 20 (25.3) |
| Fellow | 11 (13.9) |
| Resident physician | 2 (2.5) |
| Operation assistant | 37 (46.8) |
| Medical student | 9 (11.4) |
| Surgery duration, mean (SD), min:s | 43:21 (24:52) |
| Indication for surgery | |
| Symptomatic cholelithiasis | 66 (83.5) |
| Other | 8 (10.1) |
| Acute cholecystitis | 5 (6.3) |
| Time >7 d between onset acute cholecystitis and surgery | 5 (100.0) |
| Conversion to open surgery | 2 (2.5) |
Quantitative Technical Data
A total of 57 hours and 23 minutes of footage was recorded. The mean (SD) duration per recording was 43 (25) minutes. The total required digital storage space was 590 348 megabytes, with a mean (SD) size of 7473 (4634) megabytes per case.
Adequacy
Adequacy rates are summarized in Table 3. The lowest adequacy rates in notes were with respect to the inspection of the liver condition (17 of 79 cases [21.5%]), the circumferential dissection of the cystic duct and cystic artery (25 of 77 cases [32.5%]), and the inspection of the gallbladder condition (39 of 79 cases [49.4%]). The lowest adequacy rates for the video recordings were the removal of the first accessory trocar (59 of 79 cases [74.7%]) and the circumferential dissection phase (62 of 77 cases [80.5%]). After Bonferroni correction, the video recordings resulted in significantly higher adequacy compared with the operative note for the inspection of the gallbladder (note, 39 of 79 cases [49.4%] vs video, 79 of 79 cases [100%]; P < .001), the inspection of the liver condition (note, 17 of 79 [21.5%] vs video, 78 of 79 [98.7%]; P < .001), and the circumferential dissection of the cystic duct and cystic artery (note, 25 of 77 [32.5%] vs video, 62 of 77 [80.5%]; P < .001). The total adequacy was also significantly higher in the video recordings (note, 849 of 1089 observations [78.0%] vs video, 1005 of 1089 observations [92.3%]; P < .001).
Table 3. Adequacy Rates in the Operative Note and the Video Recordings.
| Procedure steps (N = 79 operations) | No./total No. of steps (%) | P value for exact McNemar testa | |
|---|---|---|---|
| Note | Video | ||
| 1a. Introduction of the first accessory trocar | 79/79 (100.0) | 79/79 (100.0) | >.99 |
| 1b. Introduction of the second accessory trocar | 79/79 (100.0) | 79/79 (100.0) | >.99 |
| 1c. Introduction of the third accessory trocar | 79/79 (100.0) | 79/79 (100.0) | >.99 |
| 2a. Inspection of the gallbladder | 39/79 (49.4) | 79/79 (100.0) | <.001 |
| 2b. Inspection of the liver condition | 17/79 (21.5) | 78/79 (98.7) | <.001 |
| 3. Circumferential dissection of the cystic duct and cystic artery | 25/77 (32.5) | 62/77 (80.5) | <.001 |
| 4. Transection of the cystic artery | 71/77 (92.2) | 67/77 (87.0) | >.99 |
| 5. Transection of the cystic duct | 77/77 (100.0) | 76/77 (98.7) | >.99 |
| 6. Removal of the gallbladder from the liver bed | 76/77 (98.7) | 77/77 (100.0) | >.99 |
| 7. Inspection of liver hemostasis | 65/77 (84.4) | 65/77 (84.4) | >.99 |
| 8. Presence of spill | 32/35 (91.4) | 35/35 (100.0) | >.99 |
| 9. Saline irrigation | 27/34 (79.4) | 34/34 (100.0) | .27 |
| 10. Drain placement | 3/3 (100.0) | 3/3 (100.0) | >.99 |
| 11a. Removal of the first accessory trocar | 60/79 (75.9) | 59/79 (74.7) | >.99 |
| 11b. Removal of the second accessory trocar | 60/79 (75.9) | 68/79 (86.1) | >.99 |
| 11c. Removal of the third accessory trocar | 60/79 (75.9) | 65/79 (82.3) | >.99 |
| Total | 849/1089 (78.0) | 1005/1089 (92.3) | <.001b |
Bonferroni corrected.
Wilcoxon signed rank test (Bonferroni corrected).
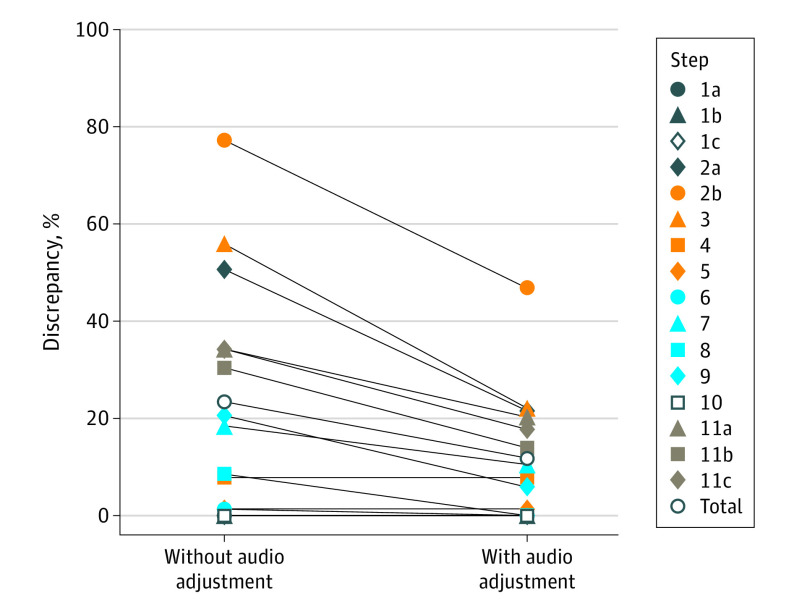
Discrepancy
Discrepancy rates are summarized in Table 4 and shown in the Figure. A discrepant step was classified as an essential surgical step that was adequately observed in the video but inadequately reported in the operative note. Discrepancies were resolved if the operator adequately mentioned the step out loud intraoperatively. After Bonferroni correction, audio adjustment of the operative note resulted in significantly lower discrepancy for the inspection of the gallbladder (without audio, 40 of 79 [50.6%] vs with audio, 17 of 79 [21.5%]; P < .001), the inspection of the liver condition (without audio, 61 of 79 [77.2%] vs with audio, 37 of 79 [46.8%]; P < .001), the circumferential dissection of the cystic duct and cystic artery (without audio, 43 of 77 cases [55.8%] vs with audio, 17 of 77 cases [22.1%]; P < .001), the removal of the first accessory trocar (without audio, 27 of 79 [34.2%] vs with audio, 16 of 79 [20.3%]; P = .02), the second accessory trocar (without audio, 24 of 79 [30.4%] vs with audio, 11 of 79 [13.9%]; P < .001), and the third accessory trocar (without audio, 27 of 79 [34.2%] vs with audio, 14 of 79 [17.7%]; P < .001). The total discrepancy was also significantly lower after audio adjustment (without audio, 254 of 1089 [23.3%] vs with audio, 118 of 1089 [11.8%]; P < .001).
Table 4. Discrepancy Rates Between Video and Note Without and With Adjusting of the Note With Audio.
| Procedure steps (N = 79 operations) | No./total No. of steps (%) | P value for exact McNemar testd | |||
|---|---|---|---|---|---|
| Without audio adjustment | With audio adjustment | ||||
| Seen but not describeda | Described but not seenb | Seen but not described | Described but not seenc | ||
| 1a. Introduction of the first accessory trocar | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | >.99 |
| 1b. Introduction of the second accessory trocar | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | >.99 |
| 1c. Introduction of the third accessory trocar | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | 0/79 (0.0) | >.99 |
| 2a. Inspection of the gallbladder | 40/79 (50.6) | 0/79 (0.0) | 17/79 (21.5) | 0/79 (0.0) | <.001 |
| 2b. Inspection of the liver condition | 61/79 (77.2) | 0/79 (0.0) | 37/79 (46.8) | 0/79 (0.0) | <.001 |
| 3. Circumferential dissection of the cystic duct and cystic artery | 40/77 (51.9) | 3/77 (3.9) | 14/77 (18.2) | 3/77 (3.9) | <.001 |
| 4. Transection of the cystic artery | 1/77 (1.3) | 5/77 (6.5) | 0/77 (0.0) | 5/77 (6.5) | >.99 |
| 5. Transection of the cystic duct | 0/77 (0.0) | 1/77 (1.3) | 0/77 (0.0) | 1/77 (1.3) | >.99 |
| 6. Removal of the gallbladder from the liver bed | 1/77 (1.3) | 0/77 (0.0) | 0/77 (0.0) | 0/77 (0.0) | >.99 |
| 7. Inspection of liver hemostasis | 7/77 (9.1) | 7/77 (9.1) | 1/77 (1.3) | 7/77 (9.1) | .53 |
| 8. Presence of spill | 3/35 (8.6) | 0/35 (0.0) | 0/35 (0.0) | 0/35 (0.0) | >.99 |
| 9. Saline irrigation | 7/34 (20.6) | 0/34 (0.0) | 2/35 (5.9) | 0/34 (0.0) | >.99 |
| 10. Drain placement | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | >.99 |
| 11a. Removal of the first accessory trocar | 13/79 (16.5) | 14/79 (17.7) | 2/79 (2.5) | 14/79 (17.7) | .02 |
| 11b. Removal of the second accessory trocar | 16/79 (20.3) | 8/79 (10.1) | 3/79 (3.8) | 8/79 (10.1) | <.001 |
| 11c. Removal of the third accessory trocar | 16/79 (20.3) | 11/79 (13.9) | 3/79 (3.8) | 11/79 (13.9) | <.001 |
| Total | 205/1089 (18.8) | 49/1089 (4.5) | 79/1089 (7.3) | 49/1089 (4.5) | <.001e |
Steps adequately seen on video review but not adequately described in the narrative operative note.
Steps adequately described in the operative note but not adequately seen on video review.
The column labeled “described but not seen” was not adjusted with audio; the 2 columns will therefore stay similar.
Comparison of the combined discrepancies per step without audio adjustment vs with audio adjustment. Values are Bonferroni corrected.
Comparison of the combined discrepancies in total without audio adjustment vs with audio adjustment. Value is per the Wilcoxon signed rank test and is Bonferroni corrected.
Figure. Discrepancy Rates per Step and in Total Between Videos and Operative Notes, Without and With Audio Adjustment of the Operative Notes.
Discussion
Because quality control has long been an afterthought in surgery, its importance has rapidly gained recognition. However, capturing operations with multimedia to improve surgical safety and knowledge is not a new idea. In 1899, just 4 years after the introduction of the cinematograph, Argentinian surgeon Alejandro Posadas and French surgeon Eugène-Louis Doyen were the first to produce films of their operations.29,30 Doyen wrote in “The Cinematograph and the Teaching of Surgery” that cinema allowed him to improve his surgical technique and he was happy to be able to criticize himself and his operations of the previous days.29 With the introduction of the cinematograph, we could make hundreds of people follow in 1 minute what a whole lecture could not make clear to a limited number of students.
As health care professionals must now meet stricter criteria for maintaining high surgical–quality standards, the availability of quality-control tools, such as real-time video recording and electronic synoptic reports, are improving. This study demonstrates that video and audio recordings during LC significantly improve the adequacy of reporting essential surgical steps, with lower discrepancies between what was captured on video and what was written in the operative note.
Of the steps in the note, 78.0% were adequately described. However, for quality-control purposes, the adequacy of the note in its current form is still insufficient. The lowest adequacy rates for the note were the inspection of the liver condition, the circumferential dissection of the cystic duct and cystic artery, and the inspection of the gallbladder condition. The inadequate description of the inspection of the gallbladder and the liver might be because operators are less likely to report normal gallbladder and liver conditions. However, by not reporting these essential steps, future readers of the operative note cannot ascertain the absence of any abnormal findings. The circumferential dissection phase is reported inadequately mainly because most operators only mention the Calot triangle, dissection of the cystic duct and cystic artery, or just CVS. Earlier findings by van de Graaf et al31 suggest that many operators are not able to adequately reproduce the definition of CVS. It simply cannot be known if the operator truly performed this technique according to the guidelines. In this respect, the description of this step should at least contain keywords describing the circumferential dissection of the cystic duct and cystic artery. Possible reasons for inaccuracy in operative notes are associated partly with practical problems. It was common that multiple elective LCs were performed clustered and subsequently after another in the centers. As a result, reporting several, nearly identical procedures at the end of the day may lead to inaccuracies. Moreover, adequacy could also be variable dependent on work experience. Some operators used self-made formats to quickly fill in operative notes. These notes were nonstandardized and often insufficient to meet current reporting standards. A standardized, preferably electronic operative note could considerably improve the adequacy of reporting.32
Of all the steps, 92.3% were adequately observed in the video recordings. The lowest adequacy rates were the removal of the first accessory trocar and the circumferential dissection phase. One important reason for the somewhat lower adequacy rate for the removal of the first accessory trocar is that the optic port is frequently used for extrication of the gallbladder; the camera would then be inserted into the first accessory trocar, after which removal of this particular trocar would frequently not be performed under vision. The circumferential dissection phase could not be adequately seen in 19.5% of the cases. This rate is substantial and should not be overlooked.
We also analyzed whether audio recordings—in which the essential steps of LC were actively dictated during the operation—could reduce discrepancies between videos and operative notes. Because video was used as the golden standard, we only adjusted the operative note with audio recordings. Discrepancies in which steps were adequately described in the note but not adequately seen in the video were thus not resolved by audio. After adjusting the notes with the audio recordings, the total discrepancy significantly declined from 23.3% to 11.8% for steps that were adequately depicted in the videos but not in the notes. The significant decline in discrepancies was again seen in the inspection of gallbladder and liver condition and the circumferential dissection phase. Additionally, discrepancies regarding the removal of the first, second, and third accessory trocars were diminished after adjustment with audio. Overall and specifically for these aforementioned steps, it can be stated that audio recordings are of additional value for the adequate description of the actions performed during surgery (Figure). These discrepancies are mainly the result of operative notes that are not written according to the guidelines. It could also be the case that steps have not been seen in the video. To reach a consensus on whether a step has been performed according to the guidelines, audio recording could be effective.
The clinical implementation of audio recordings during surgery could enable us to analyze whether this addition could lead to higher awareness among surgeons and assistants in the long term and perhaps lower complication rates. Furthermore, video with synchronous audio could be beneficial for the education of future surgeons and could also act as a tool for operators to reflect on their operations.
Limitations
A limitation of this study was that operators were not blinded for the intervention. This might have led to the Hawthorne effect, in which individuals positively modify an aspect of their behavior in response to their awareness of being observed. Because of this phenomenon, an immediate increase was expected in the operator’s awareness and prudence and thus possibly the quality of the operation. This type of autoregulation could also result in fellows and residents seeking help earlier in case of difficult situations during surgery. It is therefore expected that this phenomenon might have influenced outcomes in a positive manner, and this occurrence would also transpire in clinical practice. However, prior research introducing systematic recording using a checklist in laparoscopic colorectal cancer surgery, which was comparable with our approach, did not seem to have a significant association with operative note adequacy, and therefore the amount of bias caused by this knowledge appears to be negligible.26
Data storage requirements were higher for full-length operation recordings compared with short clips of key moments or pictures of CVS. However, with modern-day technology, the storage of these full-length video and audio recordings should be simple and inexpensive. The ultimate advantage of full-length recordings is that they will encompass possible adverse events that would have been disregarded otherwise and data regarding the technical performance of operators can be analyzed thoroughly so that they can reflect on their actions. An important disadvantage of the full-length recordings is that the density of convenient information is low, which will lead to laborious review processes, mainly in case of lengthier operations. To make information retrieval of surgical proceedings more convenient for clinical use, real-time annotation of key moments might be a solution.
A pitfall in the logistics of audio recording was the absence of routine use of audio-recording devices in the participating centers. Multiple devices (a microphone with charging base, laptop, and external hard drive [all in a nonsterile zone]) were required to record the operator’s voice. One researcher (Ö.E.) was responsible for the storage of the recordings to minimize technical failures. Loss of data mainly occurred in the starting phase of the study, when video recording devices would be inadvertently switched off before storage was completed. Today, as multimedia equipment is being integrated into smart operating rooms, operations can be recorded with the touch of a button.
Conclusions
Video and audio recordings during LC significantly improve the adequacy of the depiction of essential surgical steps compared with the narrative operative note. The addition of audio leads to lower discrepancies between the video recording and the narrative operative note, which could lead to a better understanding of the operative procedure on review.
eAppendix. Requirements for an adequate video recording, audio recording, and operative note.
eTable. Independent reviewer form.
eFigure. Flow diagram summarizing the steps taken to conduct this study.
References
- 1.Centraal Bureau voor de Statistiek—StatLine Operaties in het ziekenhuis; soort opname leg, 1995-2010. 05 februari 2014. 2014. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/80386ned/table?dl=20724
- 2.Nagy AG, Poulin EC, Girotti MJ, Litwin DE, Mamazza J. History of laparoscopic surgery. Can J Surg. 1992;35(3):271-274. [PubMed] [Google Scholar]
- 3.Begos DG, Modlin IM. Laparoscopic cholecystectomy: from gimmick to gold standard. J Clin Gastroenterol. 1994;19(4):325-330. doi: 10.1097/00004836-199412000-00015 [DOI] [PubMed] [Google Scholar]
- 4.Girard RM, Morin M. Open cholecystectomy: its morbidity and mortality as a reference standard. Can J Surg. 1993;36(1):75-80. [PubMed] [Google Scholar]
- 5.Bernard HR, Hartman TW. Complications after laparoscopic cholecystectomy. Am J Surg. 1993;165(4):533-535. doi: 10.1016/S0002-9610(05)80956-0 [DOI] [PubMed] [Google Scholar]
- 6.Caputo L, Aitken DR, Mackett MC, Robles AE. Iatrogenic bile duct injuries: the real incidence and contributing factors—implications for laparoscopic cholecystectomy. Am Surg. 1992;58(12):766-771. [PubMed] [Google Scholar]
- 7.Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141(12):1207-1213. doi: 10.1001/archsurg.141.12.1207 [DOI] [PubMed] [Google Scholar]
- 8.Morgenstern L, McGrath MF, Carroll BJ, Paz-Partlow M, Berci G. Continuing hazards of the learning curve in laparoscopic cholecystectomy. Am Surg. 1995;61(10):914-918. [PubMed] [Google Scholar]
- 9.Voitk AJ, Tsao SG, Ignatius S. The tail of the learning curve for laparoscopic cholecystectomy. Am J Surg. 2001;182(3):250-253. doi: 10.1016/S0002-9610(01)00699-7 [DOI] [PubMed] [Google Scholar]
- 10.Halbert C, Pagkratis S, Yang J, et al. Beyond the learning curve: incidence of bile duct injuries following laparoscopic cholecystectomy normalize to open in the modern era. Surg Endosc. 2016;30(6):2239-2243. doi: 10.1007/s00464-015-4485-2 [DOI] [PubMed] [Google Scholar]
- 11.Huang ZQ, Huang XQ. Changing patterns of traumatic bile duct injuries: a review of forty years experience. World J Gastroenterol. 2002;8(1):5-12. doi: 10.3748/wjg.v8.i1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau WY, Lai EC, Lau SH. Management of bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg. 2010;80(1-2):75-81. doi: 10.1111/j.1445-2197.2009.05205.x [DOI] [PubMed] [Google Scholar]
- 13.Rystedt J, Lindell G, Montgomery A. Bile duct injuries associated with 55,134 cholecystectomies: treatment and outcome from a national perspective. World J Surg. 2016;40(1):73-80. doi: 10.1007/s00268-015-3281-4 [DOI] [PubMed] [Google Scholar]
- 14.Mangieri CW, Hendren BP, Strode MA, Bandera BC, Faler BJ. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 2019;33(3):724-730. doi: 10.1007/s00464-018-6333-7 [DOI] [PubMed] [Google Scholar]
- 15.Barrett M, Asbun HJ, Chien HL, Brunt LM, Telem DA. Bile duct injury and morbidity following cholecystectomy: a need for improvement. Surg Endosc. 2018;32(4):1683-1688. doi: 10.1007/s00464-017-5847-8 [DOI] [PubMed] [Google Scholar]
- 16.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180(1):101-125. [PubMed] [Google Scholar]
- 17.Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237(4):460-469. doi: 10.1097/01.SLA.0000060680.92690.E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanford DE, Strasberg SM. A simple effective method for generation of a permanent record of the Critical View of Safety during laparoscopic cholecystectomy by intraoperative “doublet” photography. J Am Coll Surg. 2014;218(2):170-178. doi: 10.1016/j.jamcollsurg.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 19.de Mestral C, Rotstein OD, Laupacis A, et al. Comparative operative outcomes of early and delayed cholecystectomy for acute cholecystitis: a population-based propensity score analysis. Ann Surg. 2014;259(1):10-15. doi: 10.1097/SLA.0b013e3182a5cf36 [DOI] [PubMed] [Google Scholar]
- 20.Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211(1):132-138. doi: 10.1016/j.jamcollsurg.2010.02.053 [DOI] [PubMed] [Google Scholar]
- 21.Plaisier PW, Pauwels MM, Lange JF. Quality control in laparoscopic cholecystectomy: operation notes, video or photo print? HPB (Oxford). 2001;3(3):197-199. doi: 10.1080/136518201753242208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Association of Surgeons of the Netherlands (NVvH) Evidence based guideline: diagnosis and treatment of cholelithiasis. Published 2016. Accessed April 14, 2020. https://heelkunde.nl/sites/heelkunde.nl/files/richtlijnen-definitief/Richtlijn_Galsteenlijden_09032016.pdf
- 23.Buddingh KT, Hofker HS, ten Cate Hoedemaker HO, van Dam GM, Ploeg RJ, Nieuwenhuijs VB. Safety measures during cholecystectomy: results of a nationwide survey. World J Surg. 2011;35(6):1235-1241. doi: 10.1007/s00268-011-1061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wauben LS, Goossens RH, van Eijk DJ, Lange JF. Evaluation of protocol uniformity concerning laparoscopic cholecystectomy in the Netherlands. World J Surg. 2008;32(4):613-620. doi: 10.1007/s00268-007-9323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wauben LS, van Grevenstein WM, Goossens RH, van der Meulen FH, Lange JF. Operative notes do not reflect reality in laparoscopic cholecystectomy. Br J Surg. 2011;98(10):1431-1436. doi: 10.1002/bjs.7576 [DOI] [PubMed] [Google Scholar]
- 26.van de Graaf FW, Lange MM, Spakman JI, et al. Comparison of systematic video documentation with narrative operative report in colorectal cancer surgery. JAMA Surg. 2019;154(5):381-389. doi: 10.1001/jamasurg.2018.5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Graaf FW, Lange MM, Menon AG, O’Mahoney PR, Milsom JW, Lange JF. Imaging for quality control: comparison of systematic video recording to the operative note in colorectal cancer surgery: a pilot study. Ann Surg Oncol. 2016;23(suppl 5):798-803. doi: 10.1245/s10434-016-5563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fay MP. Exact McNemar’s test and matching confidence intervals. Published 2016. Accessed May 13, 2019. https://cran.r-project.org/web/packages/exact2x2/vignettes/exactMcNemar.pdf
- 29.Doyen EL. The cinematograph and the teaching of surgery. Brit Gynaec J. 1899;579-586.26205573 [Google Scholar]
- 30.Venturini AH. The earliest-known extant motion picture of anesthesia in the world was filmed in Buenos Aires. J Anesth Hist. 2015;1(2):55-57. doi: 10.1016/j.janh.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 31.van de Graaf FW, van den Bos J, Stassen LPS, Lange JF. Lacunar implementation of the critical view of safety technique for laparoscopic cholecystectomy: results of a nationwide survey. Surgery. 2018;S0039-6060(18)30032-1. [DOI] [PubMed] [Google Scholar]
- 32.Eryigit Ö, van de Graaf FW, Lange JF. A systematic review on the synoptic operative report versus the narrative operative report in surgery. World J Surg. 2019;43(9):2175-2185. doi: 10.1007/s00268-019-05017-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Requirements for an adequate video recording, audio recording, and operative note.
eTable. Independent reviewer form.
eFigure. Flow diagram summarizing the steps taken to conduct this study.