Key Points
Question
What is the efficacy of evolocumab in patients with a low-density lipoprotein cholesterol level of 70 mg/dL or greater (or non–high-density lipoprotein cholesterol level of 100 mg/dL or greater) and recent (past 12 months) myocardial infarction (MI) treated with maximally tolerated high-intensity statin?
Findings
In a prespecified secondary analysis from the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial in the subgroup of 5711 patients with a recent MI, evolocumab significantly reduced the risk of the composite outcome of cardiovascular death, MI, stroke, unstable angina, or coronary revascularization by 19%, with a number needed to treat over 3 years of 27.
Meaning
Our findings support the 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol recommendations to intensify lipid-lowering treatment in patients with a recent MI.
This prespecified secondary analysis of a randomized clinical trial examines the clinical efficacy of evolocumab in patients with recent myocardial infarction.
Abstract
Importance
The 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol identified patients with recent (past 12 months) myocardial infarction (MI) as very high risk, in whom a PCSK9 inhibitor is reasonable to add to maximally tolerated statin combined with ezetimibe if their low-density lipoprotein cholesterol level is 70 mg/dL or greater or non–high-density lipoprotein cholesterol level is 100 mg/dL or greater.
Objective
To examine the clinical efficacy of evolocumab in patients with recent MI.
Design, Setting, and Participants
This was a prespecified secondary analysis of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, in which 27 564 patients with atherosclerotic cardiovascular disease treated with a statin were randomized to evolocumab vs placebo. Patients with prior MI with a known date (n = 22 320) were stratified as having a recent MI (within 12 months of randomization) or a remote MI (more than 12 months prior to randomization). Per protocol, patients with MI within 4 weeks prior to randomization were excluded from the FOURIER trial. Data were collected from February 2013 to November 2016, and data were analyzed from May 2019 to February 2020.
Main Outcomes and Measures
The primary composite end point was cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary composite end point was cardiovascular death, MI, or stroke.
Results
Of 22 320 included patients, 17 516 (78.5%) were male, and the mean (SD) age was 62.2 (9.0) years. Compared with 16 609 patients with a remote MI, 5711 patients with a recent MI were younger and more likely to be treated with high-intensity statin (77.3% [4415] vs 69.3% [11 506]). In the placebo arm, the 3-year Kaplan-Meier rate for the primary end point was 17.2% in patients with recent MI compared with 14.4% in those with remote MI (adjusted HR, 1.45; 95% CI, 1.29-1.64; P < .001). Similarly, the 3-year Kaplan-Meier rates for the key secondary end point was also higher in those with recent MI (10.9% vs 9.5%; adjusted HR, 1.45; 95% CI, 1.24-1.69; P < .001). In patients with a recent MI, evolocumab reduced the risk of the primary and key secondary end points by 19% (hazard ratio [HR], 0.81; 95% CI, 0.70-0.93) and 25% (HR, 0.75; 95% CI, 0.62-0.91), respectively. In patients with a remote MI, evolocumab reduced the risk of the primary and key secondary end points by 8% (HR, 0.92; 95% CI, 0.84-1.01; P for interaction = .13) and 15% (HR, 0.85; 95% CI, 0.76-0.96; P for interaction = .24), respectively. Given the higher event rates in patients with a recent MI, the absolute risk reductions over 3 years with evolocumab were 3.7% in those with recent MI vs 1.1% in those with remote MI for the primary end point and 3.2% vs 1.3%, respectively, for the key secondary end point.
Conclusions and Relevance
Patients with a recent MI were at higher risk of cardiovascular events and tended to experience greater absolute risk reductions with evolocumab than those with remote MIs. These findings support the concept in US and European guidelines to aggressively lower low-density lipoprotein cholesterol levels in very high-risk patients, such as those with a recent MI.
Trial Registration
ClinicalTrials.gov Identifier: NCT01764633
Introduction
The 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol recommend (class IIa) to add a PCSK9 inhibitor in very high-risk patients with clinical atherosclerotic cardiovascular disease (ASCVD) who have a low-density lipoprotein cholesterol (LDL-C) level of 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259) or greater or non–high-density lipoprotein cholesterol (HDL-C) level of 100 mg/dL (to convert to millimoles per liter, multiply by 0.0259) or greater despite maximally tolerated LDL-C–lowering therapy.1 Patients with a recent (past 12 months) acute coronary syndrome (ACS) represent one such very high-risk group targeted for an intensive lipid-lowering therapy.1 The European guidelines consider all patients with an ACS as a very high-risk group and recommend an LDL-C reduction of 50% or more from baseline and an LDL-C goal of less than 55 mg/dL (Class I, Level of Evidence A). The rationale for initiating lipid-lowering therapy following an ASCVD event is supported by trials showing that the initiation of statin or the addition of ezetimibe to statin soon after ACS improved clinical outcomes.2,3,4
We have previously shown in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial that patients with a myocardial infarction (MI) within the past 2 years, patients with multiple prior MIs, and patients with residual multivessel coronary disease were at significantly higher risk of cardiovascular events and tended to have greater risk reduction with evolocumab.5 The aim of this prespecified secondary analysis is to build on the prior work by (1) evaluating the risks of the major adverse cardiovascular events as a function of time from the date of the qualifying MI and (2) determining the effect of evolocumab on cardiovascular outcomes in patients with an MI within 12 months, given the 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol.
Methods
The FOURIER trial was a double-blind, placebo-controlled randomized clinical trial that enrolled 27 564 patients aged 40 to 85 years with clinically evident ASCVD, defined as prior MI, prior nonhemorrhagic stroke, or symptomatic peripheral arterial disease, LDL-C level of 70 mg/dL or greater or non–HDL-C level of 100 mg/dL or greater, and additional high-risk factors, as previously described.5,6 The trial protocol is available in Supplement 1, and the statistical analysis plan is available in Supplement 2. Relevant initial trial exclusion criteria were MI within 4 weeks of randomization, planned or expected cardiac surgery or revascularization within 3 months of randomization, New York Heart Association class III or IV heart failure, and left ventricular ejection fraction less than 30%.7 The primary end point of the FOURIER trial was the composite end point of cardiovascular death, MI, stroke, coronary revascularization, or hospitalization for unstable angina; the key secondary composite end point included cardiovascular death, MI, or stroke. Ethics committee approvals for the FOURIER trial were obtained from all relevant organizations locally or through a central institutional review board within the country. Each patient provided written informed consent.
Among 22 351 patients with prior MI, 22 320 patients with a known date of MI were stratified as having recent MI (from 1 to 12 months prior to randomization) or remote MI (more than 12 months prior to randomization) (eFigure in Supplement 3). The hazard ratios (HRs) for the risk of the primary and key secondary end points comparing recent vs remote MI in the placebo arm were adjusted for age, sex, weight, white race, stroke, history of peripheral artery disease, hypertension, diabetes, smoking, chronic kidney disease (estimated glomerular filtration rate less than 60 mL/min/1.73 m2), high-intensity statin use, region, and baseline LDL-C level.5 All efficacy analyses of evolocumab vs placebo were conducted on an intention-to-treat basis. Kaplan-Meier event rates were calculated through 3 years, and P values for time-to-event analyses were derived from log-rank tests. Hazard ratios and 95% CIs for the effect of evolocumab vs placebo in patients with recent vs remote MI were generated with a Cox proportional hazards model with stratification factors of final screening LDL-C level and region as covariates. Effect modification by subgroup on the efficacy of evolocumab was tested by incorporating interaction terms into the Cox models. In addition to Kaplan-Meier event rates, we assessed the absolute risk reduction (ARR) with evolocumab in patients with recent MI and remote MI using raw percentages and tested the differences with the Gail-Simon heterogeneity test.8 We also modeled the risks for the primary and key secondary end points by considering prior MI as a continuous time variable in cubic splines model and plotted the treatment effect (HRs) of evolocumab vs placebo. A 2-sided P value less than .05 was considered significant for all tests. Statistical analyses were done using SAS version 9.4 (SAS Institute) and Stata version 16.1 (StataCorp).
Results
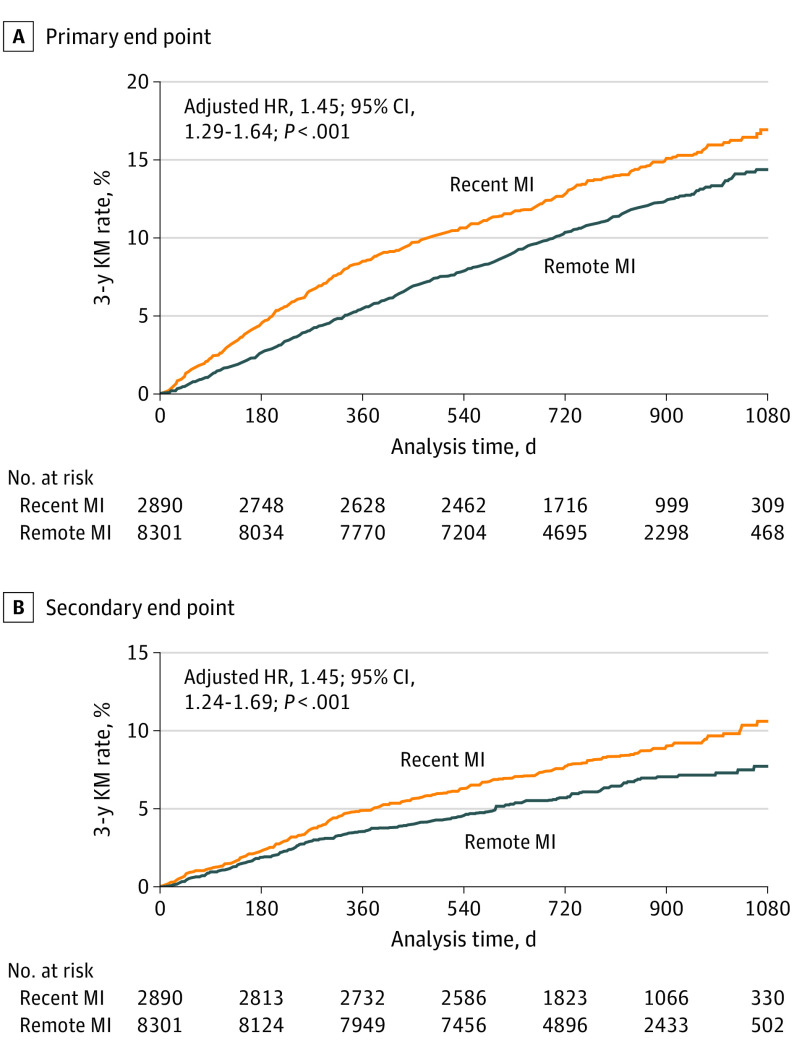
Of 22 320 included patients, 17 516 (78.5%) were male, and the mean (SD) age was 62.2 (9.0) years. Of the 5711 patients with a recent MI, the median (interquartile range) time from the qualifying MI was 4.8 (2.9-7.5) months. In contrast, for patients with a remote MI, the median (interquartile range) time from their MI was 4.9 (2.7-9.8) years. Patients with a recent MI were younger, more often treated with high-intensity statin (77.3% [4415] vs 69.3% [11 506]), and less likely to have a history of stroke, peripheral artery disease, coronary artery bypass graft, hypertension, metabolic syndrome, renal dysfunction, and diabetes compared with the 16 609 patients with a remote MI (eTable 1 in Supplement 3). In patients with a prior MI, the proportion of patients with recent and remote MI achieving LDL-C levels less than specific targets after 4 weeks of treatment with evolocumab were similar (less than 70 mg/dL: recent, 91.7% [2467 of 2690]; remote, 90.4% [7271 of 8047]; less than 55 mg/dL: recent, 83.8% [2254 of 2690]; remote, 83.3% [6700 of 8047]; less than 40 mg/dL: recent, 63.8% [1717 of 2690]; remote, 63.1% [5081 of 8047]) (eTable 2 in Supplement 3). In the placebo arm, the risk for the primary end point was 17.2% in patients with recent MI compared with 14.4% in those with remote MI (adjusted HR, 1.45; 95% CI, 1.29-1.64; P < .001). Similarly, the risk for the key secondary end point was also higher in those with recent MI (10.9% vs 9.5%; adjusted HR, 1.45; 95% CI, 1.24-1.69; P < .001) (Figure 1).
Figure 1. Risks of the Primary and Key Secondary End Points in Patients With Recent vs Remote Myocardial Infarction (MI).
A total of 5711 patients had recent MI (ie, 1 to 12 months prior to randomization) and 16 609 patients had remote MI (ie, more than 12 months prior to randomization). The primary composite end point was cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary end point was cardiovascular death, MI, or stroke. Hazard ratios (HRs) were adjusted for age, sex, weight, white race, history of stroke, history of peripheral artery disease, hypertension, diabetes, smoking, chronic kidney disease (estimated glomerular filtration rate less than 60 mL/min/1.73 m2), high-intensity statin use, region, and baseline low-density lipoprotein cholesterol level. KM indicates Kaplan-Meier.
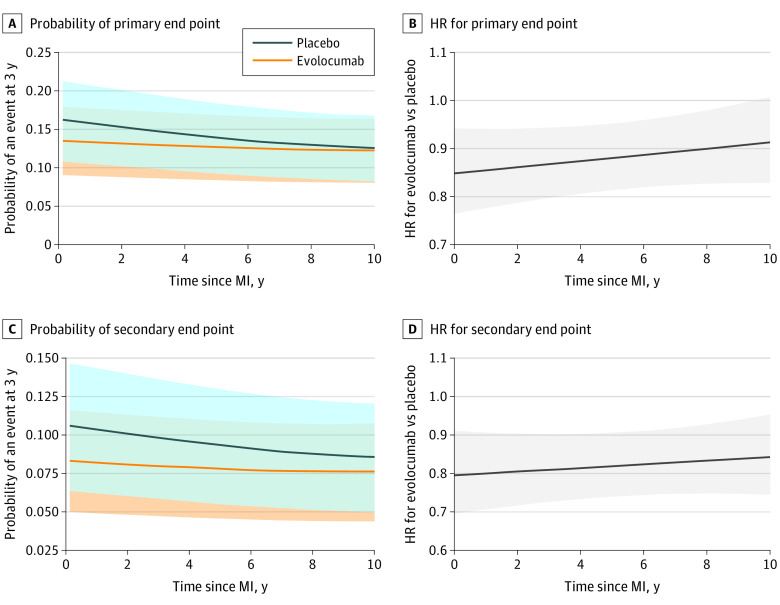
In patients with a recent MI, evolocumab reduced the relative risk of the primary end point and key secondary end point by 19% (HR, 0.81; 95% CI, 0.70-0.93) and 25% (HR, 0.75; 95% CI, 0.62-0.91), respectively (Figure 2). The event curves started to diverge at approximately 6 months. In contrast, in patients with a remote MI, the relative risk reductions for the primary and key secondary end points were 8% (HR, 0.92; 95% CI, 0.84-1.01; P for interaction = .13) and 15% (HR, 0.85; 95% CI, 0.76-0.96; P for interaction = .24), respectively (eTable 3 in Supplement 3), and the event curves did not appreciably diverge until after 12 months. Given the higher event rates in patients with a recent MI, the ARRs for the primary end point over 3 years with evolocumab were 3.7% (95% CI, 1.3-6.1) in those with recent MI and 1.1% (95% CI, −0.6 to 2.7) in those with remote MI; the ARRs for the key secondary end point over 3 years were 3.2% (95% CI, 1.2-5.2) in those with recent MI and 1.3% (95% CI, −0.1 to 2.7) in those with remote MI (Figure 2). The number needed to treat over 3 years to prevent 1 primary end point event was 27 in patients with recent MI and 91 in patients with remote MI. Testing for heterogeneity in ARRs using raw percentages, the ARRs with evolocumab were 2.7% in those with recent MI vs 0.9% in those with remote MI (P for heterogeneity = .07) for the primary end point and 2.1% vs 1.0% (P for heterogeneity = .15), respectively, for the key secondary end point. No significant treatment modification was found by baseline LDL-C level subgroup (less than 70 mg/dL vs 70 mg/dL or greater) or by use of high-intensity statin at baseline (eTable 4 in Supplement 3). The rates of the primary and key secondary end points in each treatment arm and the HR seen with evolocumab vs placebo as a function of time from qualifying MI as a continuous variable are shown in Figure 3.
Figure 2. Risks of the Primary and Key Secondary End Points in Patients With Recent and Remote Myocardial Infarction (MI) Randomized to Placebo vs Evolocumab.
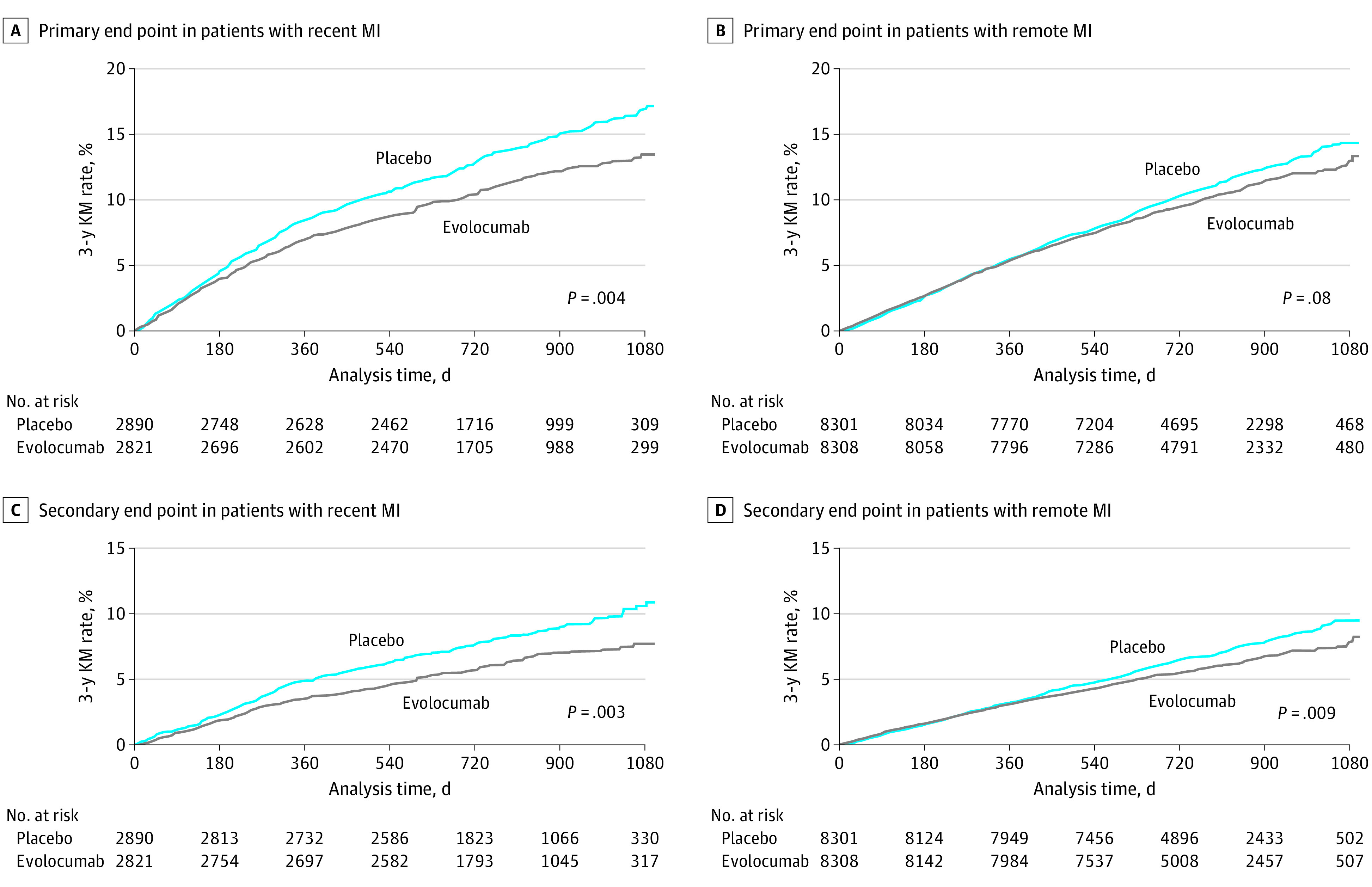
A, Primary end point among patients with recent MI randomized to placebo vs evolocumab (hazard ratio, 0.81; 95% CI, 0.70-0.93). The absolute risk reduction was 3.7% (95% CI, 1.3-6.1), and the number needed to treat over 3 years was 27. B, Primary end point among patients with remote MI randomized to placebo vs evolocumab (hazard ratio, 0.92; 95% CI, 0.84-1.01). The absolute risk reduction was 1.1% (95% CI, −0.6 to 2.7), and the number needed to treat over 3 years was 91. C, Key secondary end point among patients with recent MI randomized to placebo vs evolocumab (hazard ratio, 0.75; 95% CI, 0.62-0.91). The absolute risk reduction was 3.2% (95% CI, 1.2-5.2), and the number needed to treat over 3 years was 31. D, Key secondary end point among patients with remote MI randomized to placebo vs evolocumab (hazard ratio, 0.85; 95% CI, 0.76-0.96). The absolute risk reduction was 1.3% (95% CI, −0.1 to 2.7), and the number needed to treat over 3 years was 79. A total of 5711 patients had recent MI (ie, 1 to 12 months prior to randomization) and 16 609 patients had remote MI (ie, more than 12 months prior to randomization). The primary composite end point was cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary end point was cardiovascular death, MI, or stroke. KM indicates Kaplan-Meier.
Figure 3. Probability of an Event at 3 Years for the Primary and Key Secondary End Points and Hazard Ratio (HR) of Evolocumab vs Placebo for the Primary and Key Secondary End Points by Time Since Prior Myocardial Infarction (MI).
A, Probability of the primary end point at 3 years among patients randomized to evolocumab vs placebo by time since MI. B, Hazard ratios for evolocumab vs placebo for the primary end point by time since MI. C, Probability of the key secondary end point at 3 years among patients randomized to evolocumab vs placebo by time since MI. D, Hazard ratios for evolocumab vs placebo for the key secondary end point by time since MI. Event rates were generated using cubic splines. Hazard ratios were estimated by Cox model, which included treatment (categorical variable), time from prior MI (continuous variable), and the interaction of treatment and time from prior MI. The analysis was restricted to patients with a prior MI date up to 10 years owing to sparse data beyond that time frame. The shaded areas indicate 95% CIs.
Discussion
We found that patients with recent MI as defined by the 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol were at higher risk of cardiovascular events and had a substantial clinical benefit from LDL-C−lowering treatment with evolocumab compared with those with remote MI. Although patients with recent MI had fewer baseline risk factors in the FOURIER trial, the rates of both the primary and secondary key end points were higher. Such an observation suggests that recent MI identifies patients whose pathobiology is more prone to be significantly modifiable in response to LDL-C lowering. Likewise, intensive LDL-C reduction has a favorable effect on plaque stabilization, and intracoronary vascular ultrasonography studies demonstrated that evolocumab induces coronary plaque regression.9
Based on the 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol, patients with clinical ASCVD are separated into 2 different subgroups: those at very high risk vs not.1 The management of patients at very high risk includes a recommendation for adding a PCSK9 inhibitor in patients with LDL-C levels of 70 mg/dL or greater or non–HDL-C levels of 100 mg/dL or greater in addition to maximally tolerated statin plus ezetimibe therapy.1 Likewise, the 2019 European Society of Cardiology and European Atherosclerosis Society guidelines for the management of dyslipidemias categorizes patients with MI as a very high-risk group and recommends both a reduction of LDL-C of 50% or greater and an LDL-C target of less than 55 mg/dL (1.4 mmol/L).10
Early and systematic optimization of lipid-lowering therapy is a validated process outcome for measurement of quality improvement in patients with MI.11 However, observational studies showed that underuse of high-intensity lipid-lowering therapy remains a well-known gap in secondary prevention.12 The present analysis from the FOURIER trial highlights the importance of ensuring an optimal process of care after hospital discharge within this critical first year after MI.13 In addition, the benefit of adding PCSK9 inhibition in patients with recent ACS was strengthened with data from the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY Outcomes) trial14 where the median time between the index event from the time of randomization was 2.6 months.
Limitations
Our study had limitations. We acknowledge that only 3.3% and 6.3% of patients were treated with ezetimibe prior to randomization in the recent and remote MI groups, respectively, but note that the FOURIER trial largely completed enrollment before data on the cardiovascular benefit of the Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin vs Simvastatin (IMPROVE-IT) trial was published.4 The statistical interaction between treatment effect and MI timing did not reach significance, which may have been due to the limited number of events in this subgroup (recent MI) of a subgroup (all patients with MI) and hence limited statistical power.
Conclusions
In conclusion, patients with a recent MI were at higher risk of cardiovascular events and tended to experience greater ARRs with evolocumab than those with more remote MIs. These findings support the overall concept in US and European guidelines to aggressively lower LDL-C levels in very high-risk patients, such as those with a recent MI.
Trial protocol.
Statistical analysis plan.
eFigure. CONSORT diagram.
eTable 1. Baseline characteristics of patients with recent vs remote MI in FOURIER.
eTable 2. Achievement of recommended LDL-C targets at 4 weeks in patients with recent MI (≤12 months) vs remote MI (>12 months).
eTable 3. Efficacy of evolocumab in patients with recent MI (≤12 months) vs remote MI (>12 months).
eTable 4. Efficacy of evolocumab on the primary and key secondary end points by key subgroups.
Data sharing statement.
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Olsson AG, Ezekowitz MD, et al. ; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators . Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711-1718. doi: 10.1001/jama.285.13.1711 [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from FOURIER. Circulation. 2018;138(8):756-766. doi: 10.1161/CIRCULATIONAHA.118.034309 [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Pedersen TR, Park JG, et al. ; FOURIER Investigators . Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962-1971. doi: 10.1016/S0140-6736(17)32290-0 [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk trial. Am Heart J. 2016;173:94-101. doi: 10.1016/j.ahj.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 8.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41(2):361-372. doi: 10.2307/2530862 [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373-2384. doi: 10.1001/jama.2016.16951 [DOI] [PubMed] [Google Scholar]
- 10.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 11.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Farkouh ME, Mefford M, et al. Trends in use of high-intensity statin therapy after myocardial infarction, 2011 to 2014. J Am Coll Cardiol. 2017;69(22):2696-2706. doi: 10.1016/j.jacc.2017.03.585 [DOI] [PubMed] [Google Scholar]
- 13.Gencer B, Mach F. Lipid management in ACS: should we go lower faster? Atherosclerosis. 2018;275:368-375. doi: 10.1016/j.atherosclerosis.2018.06.871 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
Statistical analysis plan.
eFigure. CONSORT diagram.
eTable 1. Baseline characteristics of patients with recent vs remote MI in FOURIER.
eTable 2. Achievement of recommended LDL-C targets at 4 weeks in patients with recent MI (≤12 months) vs remote MI (>12 months).
eTable 3. Efficacy of evolocumab in patients with recent MI (≤12 months) vs remote MI (>12 months).
eTable 4. Efficacy of evolocumab on the primary and key secondary end points by key subgroups.
Data sharing statement.