Abstract
Primary HPV testing and triage of HPV-positive women is an effective cervical cancer screening strategy. Such a multi-visit screening algorithm is also promising for community-based screening in resource-poor communities, provided a robust tracking system is in place.
A cervical cancer screening campaign was conducted in a rural community in Ethiopia. All women aged 25–65 years were offered genital self-sampling using the Evalyn Brush®. Samples were HPV-DNA-tested at a central laboratory. Key indicators were captured on tablet computers and linked by a cloud-based information system. HPV-positive women were examined at the local clinic using portable colposcopy, p16/Ki-67 dual stain cytology and biopsy examination. CIN2+ women were referred for LEEP to the referral hospital.
Of 749 enumerated age-eligible women 634 (85%, (95% CI 82–88)) consented to screening, 429 samples were adequate for HPV testing, giving a total testing coverage of 57% (95% CI 53–62). The hrHPV prevalence was 14% (95% CI 5–22), 72% (95% CI 60–84) attended the clinic for a triage examination. Home-based HPV-DNA self-sampling and clinic-based triage assisted by cloud-based information technology is feasible in rural Ethiopia. Key components of such strategy are broad community awareness, high competency of community workers, and establishment of an adequate self-sampling and HPV-DNA testing platform.
Keywords: Cervical cancer screening, HPV testing, p16/ki-67 dual stain cytology, Community, Self-sampling, eHealth, Electronic data system, Ethiopia, Home-based, Rural
Abbreviations
- CI =
Confidence Interval
- CIN =
Cervical intraepithelial neoplasia
- DRC =
Dabat Research Center
- ETiCCS =
Emerging technologies in cervical cancer screening
- HDAL =
Health Development Army Leader
- HEW =
Health Extension Worker
- HEP =
Health Extension Program
- HPV =
Human Papillomavirus
- hrHPV =
High-risk Human Papillomavirus
- LBC =
Liquid-based cytology
- LEEP =
Loop electrosurgical excision procedure
- LMIC =
Low-middle income countries
- PID =
Personal identification
- RA =
Research Assistant
- SSA =
sub-Saharan Africa
- VIA =
Visual Inspection of the cervix with acetic acid
1. Introduction
Cervical cancer is the fourth most common cancer among women worldwide. Although effectively preventable [1], it is the leading cause of cancer deaths among women in low-middle income countries (LMICs) [2]. Specifically in sub-Saharan Africa (SSA), more than 80,000 women die of cervical cancer every year [3].
Low cancer awareness and poor access to screening facilities, in conjunction with inefficient screening methods and overburdened health care systems, all contribute to low screening coverage [4] and unacceptably high cervical cancer mortality in SSA [5].
Most national cervical cancer screening programs in SSA, including the one in Ethiopia, currently promote Visual Inspection with Acetic Acid (VIA), the single-visit approach of visual inspection of the cervix after application of acetic acid and cryotherapy as recommended by WHO [6]. This inexpensive and easy-to-teach approach, however, exhibits suboptimal test characteristics [7,8] and is of doubtful effectiveness when used in routine settings [9,10].
Ethiopia, a country with the second highest population in SSA and about 31 million women over 15 years of age and at risk of developing cervical cancer [3], has an estimated annual number of 7000 new cases and 4700 deaths of cervical cancer [69]. The vast majority of Ethiopia's population lives in rural and remote settings. There is a lack of cervical cancer awareness, both among the general population and among peripheral healthcare workers [12,13], all of which results in a low screening coverage [14]. Despite optimistic reports from a demonstration project in higher-level HIV clinics [13], the national screening program is experiencing difficulties in the rollout of population-wide, VIA-based screening [15].
HPV testing is increasingly recommended as a screening measure in developed countries and also considered for basic resource settings [16]. Self-collected cervico-vaginal samples for HPV testing show comparable accuracy to provider-taken sampling [[17], [18], [19], [20]]. Their great potential to include the hard-to-reach population has been shown in Europe [21] and also resource-poor communities [[22], [23], [24]]. HPV testing also provides an opportunity for community-based outreach with potentially high participation rate which is a prerequisite for an effective screening program.
HPV testing has a high sensitivity of detecting cervical intraepithelial lesions; however, the specificity is suboptimal especially among women less than 30 years of age. To avoid overtreatment several triage tests are available including visual assessment of lesions (colposcopy, VIA), cytology or molecular (biomarker) tests. In particular, HPV-DNA testing and triage using p16INK4a/Ki-67 dual stain cytology (CINTec PLUS®, Roche Diagnostics) was recently shown to be the superior cervical cancer screening algorithm in a first world setting [25].
To test whether this superior screening algorithm can be successfully implemented in resource-poor communities, we conducted a pilot study in rural Ethiopia in order to prove the feasibility of a home-based HPV-DNA-based cervical cancer screening approach conducted as a community self-sampling campaign. Triage of HPV-positive women was done at a clinic visit using portable colposcopy and dual stain cytology. For this multi-contact screening approach, it was necessary to develop a robust, app-based digital information system that linked the complete screening cascade from the household to the gynecologist.
2. Material and methods
2.1. Study design and population
This population-wide study was conducted in a representative rural kebele (neighborhood, small village in Amharic language) in the Amhara region of Ethiopia, between November 2017 and June 2018, using a community-based HPV self-sampling approach through house-to-house visits.
In the selected Chila kebele (Fig. 1) 630 households were enumerated, with 749 women who were age-eligible for the cervical cancer screening campaign.
Fig. 1.
Geographic location of study site and map of total households in study community.
Within the framework of the Ethiopian Health Extension Program (HEP), basic health care is provided through two Health Extension Workers (HEW), who are based at the kebele health post and conduct regular home visits. They liaise with Health Development Army Leaders (HDAL), who each engage with a neighborhood of about 25–100 families in health issues. The health post is operated by the health center which is located within 90 min on foot from the health post.
The Chila community is also one of 13 communities of the Dabat Research Center (DRC), which conducts regular Health and Demographic Surveillance in the Dabat district of Ethiopia [26].
All sexually active women between the ages of 25 and 65 years, permanently living in the Chila kebele, who were not currently pregnant, were capable to understand the study procedures and willing to voluntarily participate, were eligible to enroll in the study.
2.2. Procedures
2.2.1. Participant recruitment
Following consultation with and approval by the main community health stakeholders, HDAL and HEW were informed in a 1-day meeting about the project, cervical cancer and cervical cancer prevention.
The screening campaign was launched through announcement during community assemblies and church meetings by HDAL, HEW, Field Coordinator, and the coordinator of prevention and control of non-communicable diseases of the Health Bureau of the Amhara National Regional State.
Ten female research assistants (RA) aged 21–25 years living in Dabat District were recruited. They had completed secondary school education and were trained in paper sheet questionnaire data collection while working at DRC. In a 7-day protocol and skills training, RAs were capacitated about cervical cancer and prevention options, instructions how to use the self-sampling device, data collection using tablet and software, and were informed about consent procedures. The training was held in Amharic language, and included theoretical and practical hands-on training, as well as role-plays to learn culturally-appropriate conduct during home visits, management of absentees and non-participation.
The RA, equipped with a backpack containing tablet, self-sampling devices, informed consent forms and accessories for daily enrolment of up to ten women were distributed in pairs to designated gotes (neighborhoods), and visited homesteads one by one, being assisted by the respective HDAL. In a culturally appropriate way the purpose of the visit was explained and all women aged 25–65 years were invited to participate. In a quiet, private space in or nearby their home women's eligibility and willingness to participate in the study were evaluated, written informed consent was obtained and the sociodemographic and reproductive health information was collected.
Thereafter, community health workers councelled the participants about the self-sampling device and the procedure how to use it, aided by an instruction leaflet that had been translated into Amharic language. The participating women then self-collected a cervico-vaginal sample in a private place without external assistance. Brushes were securely kept in a zip bag and stored at the local clinic at the end of each working day; all study data was collected simultaneously offline on the tablet.
All self-sampling results were delivered at the local health posts for further dissemination. HPV- positive women were actively notified at their homes and invited to a temporarily set-up study clinic at the local health clinic in Dabat town.
2.2.2. Follow-up examination of HPV-positive women
The follow-up examination of HPV-positive women included portable colposcopy by a local senior gynecologist using the Gynocular® and the Swede score for documentation of findings [[27], [28], [29]]. Lesion-led biopsies using Morgan biopsy forceps were performed during colposcopy. Prior to the examination an LBC sample (ThinPrep collection kit®) was collected for dual stain cytology. Due to absence of functioning cryotherapy, women who were biopsy and/or biomarker positive were followed up at the gynecology department of the University of Gondar.
Participants with an initial inadequate sample were revisited at their home in June 2018. These women were invited to provide a resample and to answer a questionnaire on self-sampling acceptability.
2.3. Self-sampling device
The Evalyn brush™ (Rovers Medical Devices, Oss, The Netherlands) was used to collect a cervico-vaginal self-sample. In a pilot cross-over study at the gynecological department of University of Gondar Hospital, a good agreement (kappa 0.7) between self-sampling using the Evalyn brush™ and a Liquid-Based Cytology (LBC) sample (ThinPrep®, Hologic Inc., Marlborough, MA, USA) to detect high-risk (hr)HPV was found. Factors that might impede acceptance of self-sampling in the traditional, rural environment of our study had been explored in a qualitative study [30].
2.4. Sample analysis
The Evalyn brush™ sample was stored at room temperature (circa 25 °C) for a maximum of 8 weeks [31] until processing and analysis at the Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Ethiopia.
Samples were analyzed using the AID HPV-DNA array test (HPV Easy-Screening Kit from Autoimmun Diagnostika GmbH, Straßberg, Germany) [32,33]. This E1 gene-targeting PCR-reverse blot hybridization assay allows individual discrimination of 29 HPV genotypes (HPV6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, 85, and 97) including a cellularity control (GapDH) and hybridization controls in a single well of a microtiter plate in a 96 well format. The assay is thus high throughput and methodically simple combining PCR with an ELISA-type staining protocol. The automated read-out uses the AID reader and AiDot software which evaluates all wells within 10 min. The assay has been validated against Cobas [34] and BSGP5+/6+ Multiplexed Genotyping [35]. Quality control was done at the laboratory of the Clinic for Gynecology, Charité Universitätsmedizin Berlin, Germany using BSGP5+/6+ PCR followed by Luminex-MPG read-out [36].
LBC samples (ThinPrep®, Hologic Inc., Marlborough, MA, USA) of HPV-positive women, that were collected at the clinic during the triage visit, were processed using the ThinPrep® T2000 processor at Gondar pathology laboratory and slides were dual-stained with the biomarker p16INK4a/Ki-67 (CINTecPLUS p16/Ki67 kit®) according to manufacturer's protocol at Department of Applied Tumor Biology, Institute of Pathology, Heidelberg University Hospital.
Paraffin-stored cervical biopsies taken from HPV-positive women were processed at Department of Tumor Biology at Heidelberg University. Dual-stained cytology and histology slides were interpreted by an experienced cytopathologist blinded on the HPV result.
2.5. Data collection system
We used an electronic app-based data system developed for the purpose of this study with offline mode function for tablet computers, based on the earlier Emerging Technologies in Cervical Cancer Screening (ETiCCS) cloud solution used in Eldoret, Kenya, in 2014–2017 (www.eticcs.org and https://www.dmi.org/page/2016DVASAP). Six different roles specifically set with 13 different user interfaces were created to ensure a seamless data workflow between all stations involved in the screening process. The user interfaces for community data collectors were in Amharic language and the Ethiopian calendar was used. Only the study leader function has a complete overview over all user interfaces and the rights of correction.
Data security was guaranteed (i) by double password protection for the tablet and the app for every user, (ii) different access to information depending on the defined role and (iii) data encryption with a third-generation secure hash (SHA-256) plus HTTPS connection. Patient privacy was protected by randomized, unique personal identification (PID); full names were only visible for the field team, clinic nurse, and gynecologist. Data was stored in a secure central server at Heidelberg University.
2.6. Statistical analysis
Statistical analyses were conducted using SAS software, using SAS Version 9.4 (SAS Institute, Cary, NC, USA). Summary statistics and agreement of hrHPV-DNA test results between self-sample and LBC and between AID and MPG was assessed using Cohen's kappa statistics [37].
2.7. Ethical considerations
This study was realized in accordance with the Helsinki Declaration. Ethical approval was obtained from the Institutional Review Board (IRB) of the University of Gondar, Ethiopia and the IRB of the Heidelberg University School of Medicine, Germany. All participants provided signature or thumbprint on informed consent form in Amharic before enrollment.
3. Results
3.1. Enrollment procedure
The community (Fig. 1) campaign lasted 3 weeks and each of the 5 pairs of RAs visited 8 households per day enrolling every day about 8–9 women. Enrolment of an eligible woman took between 30 and 45 min.
3.2. Participation
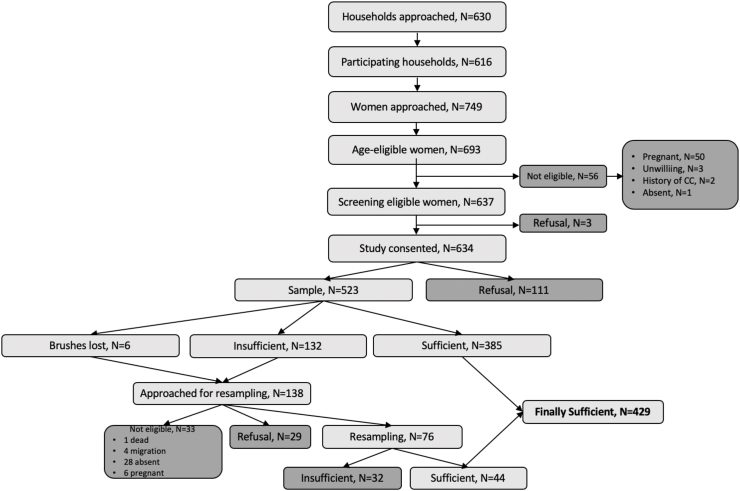
The recruitment and sampling flow is depicted in Fig. 2. 630 households with 749 age-eligible women were enumerated. In 616 visited households a total of 693 or 92.7% (95% CI 90.8 to 94.6) of age-eligible women between 25 and 65 years were approached. Fifty-six (8.1% of 693) women were deemed study-ineligible, due to pregnancy, absence of an intact uterus and/or cervical cancer history. 637 of 749 or 85.2 (95% CI 82.5 to 88) enumerated age-eligible women were eligible to participate in the screening study, and 634 of 749 or 84.7% (95% CI 81.9 to 87.5) consented to participate. Out of the total of 749 women 523 or 69.8% (95% CI 65.9 to 73.7) provided a self-sample, and 429 of 749 women or 57.2% (95% CI 52.5 to 61.9) self-collected a sample adequate for HPV-DNA analysis. Overall, 143 or 22.4% of the 637 screen-eligible women declined to participate (114 at initial visit and 29 at re-visit after initially providing a sample of poor quality).
Fig. 2.
Recruitment, eligibility, and enrolment of participants of HPV-DNA based self-sampling campaign.
At the initial household visit the sample quality was adequate for HPV-DNA testing in 385 cases, sampling material was insufficient in 132 cases. Six brushes (1% of all samples) were not received in the laboratory, but the women were easily identified and revisited. A revisit attempt was done in 138 cases to obtain a second sample including a revisit where brushes were lost. The resample was adequate in 44 cases and again insufficient in 32 cases, 29 women refused retesting and 33 women could not be reached (Fig. 2). In the questionnaire that accompanied the revisit, 80% of the women answered they would prefer self-sampling over provider-taken sample.
The baseline demographics of the enrolled population are presented in Table. 1. The mean age was 38.4 years with a large share on the younger women aged 25–39 (57.1%). Most participants were housewives (55%) or self-employed in non-farming activities (37.2%). The majority of the women were married (83.4%). Most women were parous (94%), with a median parity of 6 births and had no or no formal education (85.5%). Contraceptives were used by the minority (22.9%). In 29.8%, the women declared they had more than one sex partner in their lifetime.
Table 1.
Baseline characteristics of female residents enrolled in the HPV-based cervical cancer screening campaign.
| Age (in years) | Number (%) |
|---|---|
| <30 | 168 (26.5) |
| 30–39 | 194 (30.6) |
| 40–49 | 111 (17.5) |
| 50–59 | 115 (18.1) |
| 60–65 | 46 (7.3) |
| Education | |
| None | 461 (72.7) |
| Non-formal | 81 (12.8) |
| Primary | 47 (7.4) |
| Secondary | 30 (4.7) |
| Tertiary | 15 (2.4) |
| Employment | |
| Housewife | 349 (55) |
| Informal self-employed | 236 (37.2) |
| Farmer | 20 (3.2) |
| Full-time employed | 14 (2.2) |
| Student | 15 (2.4) |
| Marital status | |
| Married | 529 (83.4) |
| Divorced or formally separated | 43 (6.8) |
| Widowed | 49 (7.7) |
| Single/Never married | 13 (2.1) |
| Sexual debut (years) | |
| ≤14 | 271 (42.7) |
| 15–19 | 319 (50.3) |
| 20–29 | 40 (6.3) |
| ≥30 | 4 (0.6) |
| n/a | 2 (0.3) |
| Parity | |
| 0 | 38 (6.0) |
| 1 | 55 (8.7) |
| 2 | 53 (8.4) |
| 3 | 44 (6.9) |
| 4 | 57 (9.0) |
| 5 | 68 (10.7) |
| >5 | 321 (50.5) |
| Sex partners in past 12 months | |
| 0 | 72 (11.4) |
| 1 | 515 (81.2) |
| 2 | 33 (5.2) |
| >2 | 14 (2.1) |
| Sex partners in lifetime | |
| 0 | 8 (1.3) |
| 1 | 437 (68.9) |
| 2 | 151 (23.8) |
| >2 | 38 (6) |
| Contraception | |
| None | 489 (77.1) |
| Injection | 98 (15.5) |
| Loop | 22 (3.5) |
| Implant | 14 (2.2) |
| Pill | 2 (0.2) |
3.3. hrHPV prevalence
Among 429 women 58 or 13.5% (95% CI 4.7 to 22.3) had a hrHPV-DNA infection. Quality control was done on 19.5% (103/528) of samples and showed good agreement between AID and Luminex-MPG platform in detecting hrHPV-DNA, with a Cohen's kappa statistic of 0.75 (95% CI 0.58 to 0.92). Out of 103 samples 20 tested positive and 83 tested negative, with a concordant outcome in 95/103 cases.)
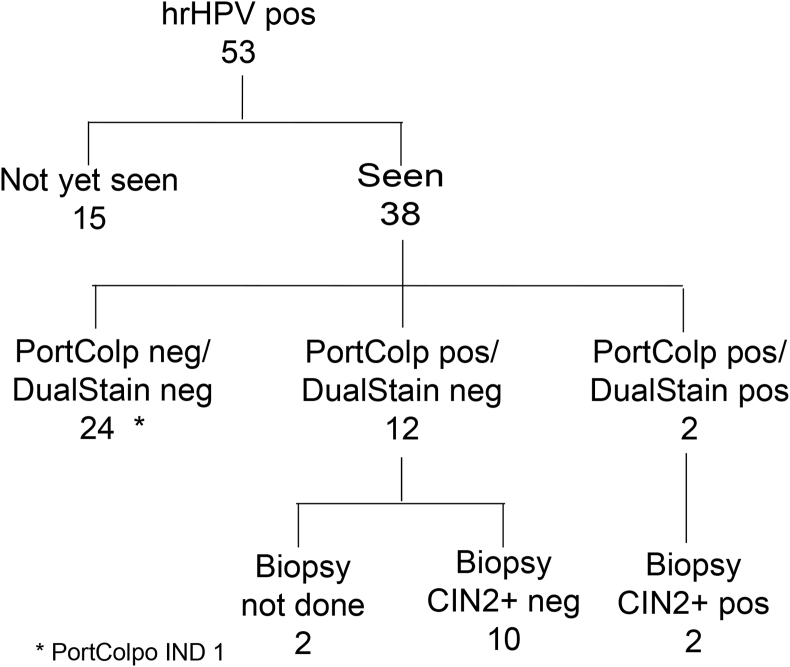
38 of the 53 or 71.7% (95% CI 59.5 to 83.9) of women with a detected hrHPV-DNA infection at the initial household visit were followed at the local clinic (Fig. 4). The remaining 20 HPV-positive women including those 5 HPV-positive women found during re-screening could only be scheduled for a date after the set-up of the temporary triage clinic.
Fig. 4.
Retention of HPV-positive women after initial household visit. Data are absolute numbers and percentages with 95% CIs.
3.4. Triage of HPV-positive women
The results of the follow-up examinations are shown in Fig. 5. Among the 38 HPV-positive women, colposcopy positive and dual-stain positive were 14 and 2 cases, respectively. Among the 14 colposcopy-positive cases, in two cases, a CIN3 histology-confirmed lesion was detected. These 2 women also had a positive dual-stain cytology and harboured a hrHPV16 infection. These women received treatment at the gynaecological department of the University of Gondar.
Fig. 5.
Triage of hrHPV positive participants. PortColp (portable colposcopy); dual stain (p16INK4a/Ki-67); CIN (cervical intraepithelial neoplasia); IND (indeterminate).
3.5. Performance of electronic data system
The app-based data system showed robust technical functionality, stability and ease-of use by health workers. Training requirements were small, also through inclusion of local language and calendar features. The user interface ensured comfortable and accurate data capturing. We did not observe any data loss and all data points were easily assessable. The functionality of off-line data collection and uploading and synchronization of data when internet access was available was error-free. Besides enabling easier data collection, timely availability of information and data quality assurance by using logic checks, the app highly enhanced the data management as compared to paper-based data retrieval.
4. Discussion
Our home-based cervical cancer screening pilot study provides encouraging information on how to improve access to high-quality cervical cancer screening also in rural and remote settings. We have demonstrated a high screening acceptance rate of 82% (523 of 637) among approached eligible women which amounts to an overall coverage of 70% (523 of 749) of enumerated age-eligible women in the community. To the best of our knowledge we have done one of the first systematic door-to-door cervical cancer screening studies using HPV-DNA-based self-sampling in SSA.
Only few similar systematic home-based screening studies have been reported from resource-poor communities [22,24,38,39] and all showed impressive coverage results. A few cervical screening studies using self-sampling on community basis have been reported from SSA [[40], [41], [42], [43], [44], [45]], and Latin America [46], that were preceded by community mobilisation, including announcement on public places, work places, churches or home visits. In these studies sampling was done in different places mostly in tents during community health campaigns or in clinics.
Community awareness
Community awareness has been a crucial component in our approach as health literacy and especially knowledge about cervical cancer and screening options are still very low in our [47,48] and similar [49] settings. Also, cultural barriers such as gender subordination and sexual taboos prevalent in our population needed to be addressed to convince women to self-collect genital samples at their home. We were able to reach out to the whole community offering screening to 85% (637 of 749) of screening-eligible women of whom 82% (523 of 637) accepted to be screened as illustrated in Fig. 3. This was largely facilitated by initial consultation with political and community leaders and health professionals followed by community sensitization through community gatherings as well as ongoing education on household level during the campaign. We realized the importance to carefully plan screening campaigns in accordance with community (farming, weather) and health activities in the region and to allow adequate time for recruitment and follow up. Also strict adherence to the announced schedule was important to maintain cooperation and trust within the community.
Fig. 3.
Cascade of community HPV screening. Data are percentages with 95%CIs and absolute numbers. Individual bars depict key steps in the screening process.
Care continuum
A key activity of the campaign-like screening approach is the competent follow-up of the identified HPV-positive women. We had established a temporary one-week screening clinic at the local health facility for the time of the follow-up, where a trained gynecologist assisted by a nurse attended all HPV-positive women who were given time slots to be seen on specific days. All women were reached and invited to the clinic through the completely available residency data from the data captured in the digital system. During the operations of the temporary screening clinic we could triage close to three quarters of HPV positive women. Nevertheless, seasonal circumstances (harvest season, rain) and non-urgency impeded compliance to follow-up appointment at the temporary screening visit. Women who missed the triage visit will be re-invited at a convenient time. Treatment acquisition is a crucial outcome of cervical cancer screening strategies. In sub-Saharan Africa most HPV-DNA-based self-sampling studies [40,41,43], but also VIA programs [50,51], report low follow-up rates. Similar compliance issues are also reported in developed countries [23,52]. For our setting we learned that active tracking and adequate time needs to be available to bring all women in need of follow-up into care.
Cervical cancer screening using home-based HPV self-sampling is a multi-contact approach with the inherent risk of participant and/or data/material loss. Assistance of a robust digital data collection system will be crucial for such strategy both from a patient's perspective to ensure follow-up care as well as from a health system perspective [53] to provide accurate monitoring and evaluation of all screening steps. Our app-based data system proved to be a robust, flawless system. We had no data loss, the material loss (six brushes lost) was remedied by identifying and revisiting the women and all results were easily accessible to the community. Our data can be easily entered or retrofitted into a regional health database, and the system is flexible to be adapted to evolving data collection systems as the program advances. The system also allows for easy scale-up and adaption to similar health service settings.
Sampling issues
The screening cascade in Fig. 3 shows a considerable drop in participation after the consent step. Nearly every 5th women (17%) dropped out of screening before having provided a self-sample. Possible explanations for this unexpected finding are the traditional fabric of this remote and rural community, the cultural need for spousal approval, also ignorance, fear and/or shame when faced with the self-sampling brush. Given the widely reported high acceptability of self-sampling only few studies have addressed possible social harm or adverse effects related to self-sampling [45,[54], [55], [56]]. Attention to context-specific barriers in relation to self-sampling is needed when this method is newly implemented. An additional loss in screening participation was due to insufficiency of the self-sample (Fig. 3), i.e. the sample contained a too small amount of human material for HPV-DNA testing. This was a surprising finding as we had confirmed the acceptance and accuracy of the Evalyn brush™ in a small-scale survey among staff and patients of the gynaecology department. Also in the literature sample quality issues are rarely reported [24,44]. It is possible that our community health workers were not sufficiently trained to explain the self-sampling procedure and usage of the device. Also some participants could have intentionally given an improper or non-used sample while pretending to be cooperative (desirability bias).
The genital sample collection technology is rapidly advancing. In our study we used the Evalyn brush that has been widely used in Europe and has been tested favourably in sub-Saharan settings like South Africa [57] and Kenya [58]. Alternative sampling techniques including non-invasive HPV testing from a urine sample have been systematically reviewed [19,59] or are under ongoing research [60]. Further validation studies are needed to assess for different contexts which device is most appropriate and how to best assist its use.
HPV testing platforms
We used the AID HPV testing platform as at the time of our study it was one of the few HPV platforms available in Ethiopia [44]. It also has the potential to cover large testing volumes, a requirement that we consider favourable for a community-based campaign. With the availability of alternative testing platforms and also different sampling methods it will be necessary to assess the most cost-effective technique suitable for the setting, before scaling up of self-sampling campaigns.
Regarding triage testing methods, in our small sample the use of the biomarker CINtecPlus® as triage test for HPV-positive women [61] correlated well with detected precancerous lesions and warrants further evaluation in a well-powered study.
Sustainability
A key factor in the roll-out of home-based screening campaigns are the program costs. At present market prices the collection system (devices, transport medium) and molecular testing (HPV testing, dual-stain cytology) are still prohibitively expensive for resource-poor communities. It will be important to study whether the gain in diagnostic effectiveness in conjunction with competitive pricing will make HPV testing affordable, and/or whether advanced visual screening methods are cost effective alternatives to triage options such as dual stain cytology [[62], [63], [64]].
Other important program issue to be considered include human resource requirements (capacity and training), as well as program outcomes such as service uptake, service coverage, and patient follow-up. The shifting of the initial screening step to the community relieved the clinic from a potentially large screening burden and seems a cost-effective screening approach [65]. While service delivery through mobile community outreach e.g. at public meeting points [[40], [41], [42], [43], [44],66] can achieve large uptake in a labour-saving manner, our digitally assisted door-to-door approach potentially widens screening coverage, provides a platform for a screening registry with monitoring and evaluation capacity and facilitates-when needed-the retention of participants in care. In addition, such home-based approach could also be integrated into other health activities such a community-based multi-disease care and prevention programs [38,67]. The costs and effects of home-based screening approaches must further be assessed for scalability in similar settings.
Conclusion
To achieve the 2030 target of globally screening 70% of the 35–45 year old women proposed by WHO [68] on the path to the global elimination of cervical cancer within the century a broad access to high-accuracy screening tests is required. Our pilot study has provided a proof of concept to the approach of a community-based, multi-contact cervical cancer screening when assisted by a robust digital data collection system. The results of our cervical cancer screening campaign lend promise to implementation of high access and high-quality cervical cancer screening programs also in rural and resource-poor communities. Main elements are a broad community awareness and high competency of community workers as well as establishment of an adequate self-sampling and HPV-DNA testing platform.
CRediT authorship contribution statement
Felix Jede: Conceptualization, Data curation, Formal analysis, Project administration, Writing - original draft. Theresa Brandt: Conceptualization, Data curation, Formal analysis, Software, Writing - original draft, Writing - review & editing. Molla Gedefaw: Supervision, Writing - original draft. Solomon Berhe Wubneh: Investigation. Tamrat Abebe: Methodology. Brhanu Teka: Methodology. Kassahun Alemu: Methodology, Project administration, Supervision. Binyam Tilahun: Methodology, Software. Temesgen Azemeraw: Software. Abebaw Gebeyehu: Conceptualization. Dietmar Schmidt: Validation. Aleksandra Pesic: Validation. Andreas M. Kaufmann: Validation, Writing - review & editing. Bewketu Abebe: Investigation. Zelalem Ayichew: Investigation. Michael Byczkowski: Software. Timoté Vaucher: Software. Heike Sartor: Methodology. Gashaw Andargie: Project administration, Resources. Till Bärnighausen: Writing - review & editing. Magnus von Knebel Doeberitz: Conceptualization, Resources, Writing - review & editing. Hermann Bussmann: Conceptualization, Formal analysis, Project administration, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the people living in Chila community who participated in this research and for the continuous support and cooperation of the Government of Ethiopia. Very special acknowledgments to our field team at Dabat Research Center and to the Study Coordinator Masresha Baye. We thank Adam Alsegård and Shengdong Zhao for their major contribution in developing the digital application. We also thank Elena Prigge for reviewing of the manuscript. We thank AID/GenID for providing analysis kits and Hologic for the ThinPrep collection kits; both companies had no role in study design, data collection, analysis or preparation of the manuscript. The study was conducted with support of the Ladenburg Foundation, New York.
Contributor Information
Felix Jede, Email: felix@jede.name.
Theresa Brandt, Email: theresa-brandt@gmx.net.
Molla Gedefaw, Email: bdfam72@gmail.com.
Solomon Berhe Wubneh, Email: solomonbe2310@gmail.com.
Tamrat Abebe, Email: tamrat.abebe@aau.edu.et.
Brhanu Teka, Email: brhanu.teka@aau.edu.et.
Kassahun Alemu, Email: kassalemu@gmail.com.
Binyam Tilahun, Email: binigcms@gmail.com.
Temesgen Azemeraw, Email: tazemeraw@gmail.com.
Abebaw Gebeyehu, Email: gabebaw2worku@gmail.com.
Dietmar Schmidt, Email: dischmi57@gmail.com.
Aleksandra Pesic, Email: aleksandra.pesic@charite.de.
Andreas M. Kaufmann, Email: andreas.kaufmann@charite.de.
Bewketu Abebe, Email: dischmi57@gmail.com.
Zelalem Ayichew, Email: zelalem538@yahoo.com.
Michael Byczkowski, Email: michael.byczkowski@sap.com.
Timoté Vaucher, Email: timote.vaucher@epfl.ch.
Heike Sartor, Email: heike.sartor@med.uni-heidelberg.de.
Gashaw Andargie, Email: gashawab@gmail.com.
Till Bärnighausen, Email: till.baernighausen@uni-heidelberg.de.
Magnus von Knebel Doeberitz, Email: magnus.knebel-doeberitz@med.uni-heidelberg.de.
Hermann Bussmann, Email: Hermann.Bussmann@med.uni-heidelberg.de.
References
- 1.Nour N.M. Cervical cancer: a preventable death. Rev Obstet Gynecol. 2009;2(4):240–244. [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Bruni L., Albero G., Serrano B., Mena M., Gómez D., Muñoz J. 2018. Human Papillomavirus and Related Diseases in Africa. [Google Scholar]
- 4.Gakidou E., Nordhagen S., Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny L., Anorlu R. Cervical cancer in Africa. Cancer Epidemiol. Biomark. Prev. 2012;21(9):1434–1438. doi: 10.1158/1055-9965.EPI-12-0334. [DOI] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R., Wesley R.S. 2003. A Practical Manual on Visual Screening for Cervical Neoplasia. Lyon, France. [Google Scholar]
- 7.Ajenifuja K.O., Gage J.C., Adepiti A.C., Wentzensen N., Eklund C., Reilly M. A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria. Int. J. Gynecol. Canc. 2013;23(3):507–512. doi: 10.1097/IGC.0b013e318280f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustafa R.A., Santesso N., Khatib R., Mustafa A.A., Wiercioch W., Kehar R. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int. J. Gynaecol. Obstet. 2016;132(3):259–265. doi: 10.1016/j.ijgo.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Fokom Domgue J., Valea F.A. Is it relevant to keep advocating visual inspection of the cervix with acetic acid for primary cervical cancer screening in limited-resource settings? J Glob Oncol. 2018;4:1–5. doi: 10.1200/JGO.17.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silkensen S., Schiffman M., Sahasrabuddhe V., Flanigan J. Is it time to move beyond visual inspection with acetic acid for cervical cancer screening? Glob. Health: Science and Practice. 2018;6(2) doi: 10.9745/GHSP-D-18-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kress C., Sharling L., Owen-Smith A., Desalegn D., Blumberg H., Goedken J. Knowledge, attitudes, and practices regarding cervical cancer and screening among Ethiopian health care workers. Int. J. Wom. Health. 2015;7:765–772. doi: 10.2147/IJWH.S85138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiferaw N., Salvador-Davila G., Kassahun K., Brooks M.I., Weldegebreal T., Tilahun Y. The single-visit approach as a cervical cancer prevention strategy among women with HIV in Ethiopia: successes and lessons learned. Glob Health Sci Pract. 2016;4(1):87–98. doi: 10.9745/GHSP-D-15-00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie K.S., de Sanjose S., Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Trop. Med. Int. Health. 2009;14(10):1287–1302. doi: 10.1111/j.1365-3156.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 15.Tadesse S. Preventive mechanisms and treatment of cervical cancer in Ethiopia. Cervical Cancer. 2015;1(101) [Google Scholar]
- 16.Jeronimo J., Castle P., Temin S., Denny L., Gupta V., Kim J. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2017;3(5):635–657. doi: 10.1200/JGO.2016.006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F.H., Lewkowitz A.K., Chen F., Lin M.J., Hu S.Y., Zhang X. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012;104(3):178–188. doi: 10.1093/jnci/djr532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbyn M., Verdoodt F., Snijders P.J.F., Verhoef V.M.J., Suonio E., Dillner L. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 19.Arbyn M., Smith S.B., Temin S., Sultana F., Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. Bmj. 2018;363 doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketelaars P.J.W., Bosgraaf R.P., Siebers A.G., Massuger L., van der Linden J.C., Wauters C.A.P. High-risk human papillomavirus detection in self-sampling compared to physician-taken smear in a responder population of the Dutch cervical screening: results of the VERA study. Prev. Med. 2017;101:96–101. doi: 10.1016/j.ypmed.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Verdoodt F., Jentschke M., Hillemanns P., Racey C.S., Snijders P.J., Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur. J. Canc. 2015;51(16):2375–2385. doi: 10.1016/j.ejca.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Arrossi S., Thouyaret L., Herrero R., Campanera A., Magdaleno A., Cuberli M. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015;3(2):e85–94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 23.Crosby R.A., Hagensee M.E., Vanderpool R., Nelson N., Parrish A., Collins T. Community-based screening for cervical cancer: a feasibility study of rural appalachian women. Sex. Transm. Dis. 2015;42(11):607–611. doi: 10.1097/OLQ.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandigo M., Frett B., Laurent J.R., Bishop I., Raymondville M., Marsh S. Pairing community health workers with HPV self-sampling for cervical cancer prevention in rural Haiti. Int. J. Gynaecol. Obstet. 2015;128(3):206–210. doi: 10.1016/j.ijgo.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Wentzensen N., Clarke M.A., Bremer R., Poitras N., Tokugawa D., Goldhoff P.E. Clinical evaluation of human papillomavirus screening with p16/ki-67 dual stain triage in a large organized cervical cancer screening program. JAMA Intern Med. 2019;179(7):881–888. doi: 10.1001/jamainternmed.2019.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abebe S.M., Andargie G., Shimeka A., Alemu K., Kebede Y., Wubeshet M. The prevalence of non-communicable diseases in northwest Ethiopia: survey of Dabat Health and Demographic Surveillance System. BMJ Open. 2017;7(10) doi: 10.1136/bmjopen-2016-015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nessa A., Wistrand C., Begum S.A., Thuresson M., Shemer I., Thorsell M. Evaluation of stationary colposcope and the Gynocular, by the Swede score systematic colposcopic system in VIA positive women: a crossover randomized trial. Int. J. Gynecol. Canc. 2014;24(2):339–345. doi: 10.1097/IGC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman H., Hu J., Li X., He J., Bradford L., Shan S. Evaluation of portable colposcopy and human papillomavirus testing for screening of cervical cancer in rural China. Int. J. Gynecol. Canc. 2019;29(1):23–27. doi: 10.1136/ijgc-2018-000006. [DOI] [PubMed] [Google Scholar]
- 29.Taghavi K., Banerjee D., Mandal R., Kallner H.K., Thorsell M., Friis T. Colposcopy telemedicine: live versus static swede score and accuracy in detecting CIN2+, a cross-sectional pilot study. BMC Wom. Health. 2018;18(1) doi: 10.1186/s12905-018-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandt A.M. How AIDS invented global health. N. Engl. J. Med. 2013;368(23):2149–2152. doi: 10.1056/NEJMp1305297. [DOI] [PubMed] [Google Scholar]
- 31.Ejegod D.M., Pedersen H., Alzua G.P., Pedersen C., Bonde J. Time and temperature dependent analytical stability of dry-collected Evalyn HPV self-sampling brush for cervical cancer screening. Papillomavirus Res. 2018;5:192–200. doi: 10.1016/j.pvr.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesic A., Krings A., Hempel M., Mathewos A., Komp J., Höfler D. 2017. Validation Of Hpv Dna Array Genotyping Assay With Cervical Cancer Samples. [Google Scholar]
- 33.Pesic A., Krings A., Schreckenberger C., Hempel M., Preyer R., Kaufmann A.M. Analytical evaluation of the human papillomavirus HPV DNA array E1-based genotyping assay. Intervirology. 2019;62(3–4):124–133. doi: 10.1159/000502207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesic A., Krings A., Hempel M., Preyer R., Chatzistamatiou K., Agorastos T. CIN2+ detection of the HPV DNA Array genotyping assay in comparison with the Cobas 4800 HPV test and cytology. Virol. J. 2019;16(1):92. doi: 10.1186/s12985-019-1197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pesic A., Krings A., Hempel M., Preyer R., Kaufmann A.M. Clinical performance of the HPV DNA Array genotyping assay in detection of CIN2+ lesions with BS GP5+/6+ MPG Luminex tested cervical samples. J. Med. Virol. 2020;92(1):113–118. doi: 10.1002/jmv.25583. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt M., Dondog B., Waterboer T., Pawlita M. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J. Clin. Microbiol. 2008;46(3):1050–1059. doi: 10.1128/JCM.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman D.G. Chapman and Hall; London: 1991. Practical Statistics for Medical Research. [Google Scholar]
- 38.Basu P., Mahajan M., Patira N., Prasad S., Mogri S., Muwonge R. A pilot study to evaluate home-based screening for the common non-communicable diseases by a dedicated cadre of community health workers in a rural setting in India. BMC Publ. Health. 2019;19(1) doi: 10.1186/s12889-018-6350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazcano-Ponce E., Lorincz A.T., Cruz-Valdez A., Salmerón J., Uribe P., Velasco-Mondragón E. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378(9806):1868–1873. doi: 10.1016/S0140-6736(11)61522-5. [DOI] [PubMed] [Google Scholar]
- 40.Ogilvie G.S., Mitchell S., Sekikubo M., Biryabarema C., Byamugisha J., Jeronimo J. Results of a community-based cervical cancer screening pilot project using human papillomavirus self-sampling in Kampala, Uganda. Int. J. Gynaecol. Obstet. 2013;122(2):118–123. doi: 10.1016/j.ijgo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Moses E., Pedersen H.N., Mitchell S.M., Sekikubo M., Mwesigwa D., Singer J. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop. Med. Int. Health. 2015;20(10):1355–1367. doi: 10.1111/tmi.12549. [DOI] [PubMed] [Google Scholar]
- 42.Swanson M., Ibrahim S., Blat C., Oketch S., Olwanda E., Maloba M. Evaluating a community-based cervical cancer screening strategy in Western Kenya: a descriptive study. BMC Wom. Health. 2018;18(1):116. doi: 10.1186/s12905-018-0586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huchko M.J., Ibrahim S., Blat C., Cohen C.R., Smith J.S., Hiatt R.A. Cervical cancer screening through human papillomavirus testing in community health campaigns versus health facilities in rural western Kenya. Int. J. Gynecol. Obstet. 2018;141(1):63–69. doi: 10.1002/ijgo.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gizaw M., Teka B., Ruddies F., Abebe T., Kaufmann A.M., Worku A. Uptake of cervical cancer screening in Ethiopia by self-sampling HPV DNA compared to visual inspection with acetic acid: a cluster randomized trial. Cancer Prev Res (Phila). 2019;12(9):609–616. doi: 10.1158/1940-6207.CAPR-19-0156. [DOI] [PubMed] [Google Scholar]
- 45.Fokom Domgue J., Futuh B., Ngalla C., Kakute P., Manjuh F., Manga S. Feasibility of a community-based cervical cancer screening with “test and treat” strategy using self-sample for an HPV test: experience from rural Cameroon, Africa. Int. J. Canc. 2020;1:128–138. doi: 10.1002/ijc.32746. [DOI] [PubMed] [Google Scholar]
- 46.Abuelo C.E., Levinson K.L., Salmeron J., Sologuren C.V., Fernandez M.J., Belinson J.L. The Peru Cervical Cancer Screening Study (PERCAPS): the design and implementation of a mother/daughter screen, treat, and vaccinate program in the Peruvian jungle. J. Community Health. 2014;39(3):409–415. doi: 10.1007/s10900-013-9786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Getahun F., Mazengia F., Abuhay M., Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in Northwest Ethiopia. BMC Canc. 2013;13:2. doi: 10.1186/1471-2407-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasa A.S., Tesfaye T.D., Temesgen W.A. Knowledge, attitude and practice towards cervical cancer among women in finote selam city administration, west gojjam zone, Amhara region, north west Ethiopia. Afr. Health Sci. 2017;18(3) doi: 10.4314/ahs.v18i3.20. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orang’o E.O., Wachira J., Asirwa F.C., Busakhala N., Naanyu V., Kisuya J. Factors associated with uptake of visual inspection with acetic acid (VIA) for cervical cancer screening in western Kenya. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0157217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adefuye P.O., Broutet N.J., de Sanjose S., Denny L.A. Trials and projects on cervical cancer and human papillomavirus prevention in sub-Saharan Africa. Vaccine. 2013;31(Suppl 5):F53–F59. doi: 10.1016/j.vaccine.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 51.Khozaim K., Orang'o E., Christoffersen-Deb A., Itsura P., Oguda J., Muliro H. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int. J. Gynaecol. Obstet. 2014;124(1):12–18. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Sancho-Garnier H., Tamalet C., Halfon P., Leandri F.X., Le Retraite L., Djoufelkit K. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int. J. Canc. 2013;133(11):2681–2687. doi: 10.1002/ijc.28283. [DOI] [PubMed] [Google Scholar]
- 53.Drummond J.L., Were M.C., Arrossi S., Wools-Kaloustian K. Cervical cancer data and data systems in limited-resource settings: challenges and opportunities. Int. J. Gynaecol. Obstet. 2017;138(Suppl 1):33–40. doi: 10.1002/ijgo.12192. [DOI] [PubMed] [Google Scholar]
- 54.Rahman R., Clark M.D., Collins Z., Traore F., Dioukhane E.M., Thiam H. Cervical cancer screening decentralized policy adaptation: an African rural-context-specific systematic literature review. Glob. Health Action. 2019;12(1) doi: 10.1080/16549716.2019.1587894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braz N.S., Lorenzi N.P., Sorpreso I.C., Aguiar L.M., Baracat E.C., Soares J. The acceptability of vaginal smear self-collection for screening for cervical cancer: a systematic review. Clinics. 2017;72(3):183–187. doi: 10.6061/clinics/2017(03)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh P.T., Kennedy C.E., de Vuyst H., Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health. 2019;4(3) doi: 10.1136/bmjgh-2018-001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahomed K., Evans D., Sauls C., Richter K., Smith J., Firnhaber C. Human papillomavirus (HPV) testing on self-collected specimens: perceptions among HIV positive women attending rural and urban clinics in South Africa. Pan Afr Med J. 2014;17:189. doi: 10.11604/pamj.2014.17.189.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manguro G.O., Masese L.N., Mandaliya K., Graham S.M., McClelland R.S., Smith J.S. Preference of specimen collection methods for human papillomavirus detection for cervical cancer screening: a cross-sectional study of high-risk women in Mombasa, Kenya. Reprod. Health. 2018;15(1):206. doi: 10.1186/s12978-018-0651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Othman N.H., Mohamad Zaki F.H. Self-collection tools for routine cervical cancer screening: a review. Asian Pac. J. Cancer Prev. APJCP. 2014;15(20):8563–8569. doi: 10.7314/apjcp.2014.15.20.8563. [DOI] [PubMed] [Google Scholar]
- 60.Arbyn M., Depuydt C., Benoy I., Bogers J., Cuschieri K., Schmitt M. VALGENT: a protocol for clinical validation of human papillomavirus assays. J. Clin. Virol. 2016;76(Suppl 1):S14–S21. doi: 10.1016/j.jcv.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Wright T.C., Jr., Behrens C.M., Ranger-Moore J., Rehm S., Sharma A., Stoler M.H. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol. Oncol. 2017;144(1):51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 62.Basu P., Banerjee D., Mittal S., Mandal R., Ghosh I., Das P. Evaluation of a compact, rechargeable, magnifying device to triage VIA and HPV positive women in a cervical cancer screening program in rural India. Cancer Causes Control. 2016;27(10):1253–1259. doi: 10.1007/s10552-016-0805-7. [DOI] [PubMed] [Google Scholar]
- 63.Cholli P., Bradford L., Manga S., Nulah K., Kiyang E., Manjuh F. Screening for cervical cancer among HIV-positive and HIV-negative women in Cameroon using simultaneous co-testing with careHPV DNA testing and visual inspection enhanced by digital cervicography: findings of initial screening and one-year follow-up. Gynecol. Oncol. 2018;148:118–125. doi: 10.1016/j.ygyno.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Hu L., Bell D., Antani S., Xue Z., Yu K., Horning M.P. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J. Natl. Cancer Inst. 2019;111(9):923–932. doi: 10.1093/jnci/djy225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mezei A.K., Pedersen H.N., Sy S., Regan C., Mitchell-Foster S.M., Byamugisha J. Community-based HPV self-collection versus visual inspection with acetic acid in Uganda: a cost-effectiveness analysis of the ASPIRE trial. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2017-020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma M., Ying R., Tarr G., Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–S85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzpatrick M.B., El-Khatib Z., Katzenstein D., Pinsky B.A., Chirenje Z.M., McCarty K. Community-based self-collected human papillomavirus screening in rural Zimbabwe. BMC Publ. Health. 2019;19(S1) doi: 10.1186/s12889-019-6810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.WHO . DRAFT; 2019. Global Strategy towards the Elimination of Cervical Cancer as a Public Health Problem. [Google Scholar]
- 69.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARCInformation Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and RelatedDiseases in Ethiopia. Summary Report 17 June 2019. [1 July 2019].